Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3805
Peer-review started: January 27, 2017
First decision: March 3, 2017
Revised: March 13, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: June 7, 2017
Processing time: 134 Days and 21.5 Hours
To investigate protective effects and molecular mechanisms of green tea polyphenols (GTP) on non-alcoholic fatty liver disease (NAFLD) in Zucker fatty (ZF) rats.
Male ZF rats were fed a high-fat diet (HFD) for 2 wk then treated with GTP (200 mg/kg) or saline (5 mL/kg) for 8 wk, with Zucker lean rat as their control. At the end of experiment, serum and liver tissue were collected for measurement of metabolic parameters, alanine aminotransferase (ALT) and aspartate aminotransferase (AST), inflammatory cytokines and hepatic triglyceride and liver histology. Immunoblotting was used to detect phosphorylation of AMP-activated protein kinase (AMPK) acetyl-CoA carboxylase (ACC), and sterol regulatory element-binding protein 1c (SREBP1c).
Genetically obese ZF rats on a HFD presented with metabolic features of hepatic pathological changes comparable to human with NAFLD. GTP intervention decreased weight gain (10.1%, P = 0.052) and significantly lowered visceral fat (31.0%, P < 0.01). Compared with ZF-controls, GTP treatment significantly reduced fasting serum insulin, glucose and lipids levels. Reduction in serum ALT and AST levels (both P < 0.01) were observed in GTP-treated ZF rats. GTP treatment also attenuated the elevated TNFα and IL-6 in the circulation. The increased hepatic TG accumulation and cytoplasmic lipid droplet were attenuated by GTP treatment, associated with significantly increased expression of AMPK-Thr172 (P < 0.05) and phosphorylated ACC and SREBP1c (both P < 0.05), indicating diminished hepatic lipogenesis and triglycerides out flux from liver in GTP treated rats.
The protective effects of GTP against HFD-induced NAFLD in genetically obese ZF rats are positively correlated to reduction in hepatic lipogenesis through upregulating the AMPK pathway.
Core tip: The aim of this study was to examine the protective effects and molecular mechanism of green tea polyphenols (GTP) on non-alcoholic fatty liver disease (NAFLD)-induced by high fat diet (HFD) in genetically obese Zucker fatty (ZF) rats. The data of the study has demonstrated: (1) HFD-ZF rats is an optimal rodent model of NAFLD for testing novel/natural agents for this disease; (2) GTP treatment ameliorated metabolic and histopathological abnormalities in HFD-ZF rats with NAFLD; and (3) GTP exerted a protective effect against hepatic steatosis and liver injury by attenuating inflammatory cytokines and inhibiting lipogenesis through upregulating AMPK activation. Therefore, GTP proves to be an effective natural agent for preventing NAFLD and reducing the risk of its severe complications such as nonalcoholic steatohepatitis, cirrhosis and hepatic cellular carcinoma.
- Citation: Tan Y, Kim J, Cheng J, Ong M, Lao WG, Jin XL, Lin YG, Xiao L, Zhu XQ, Qu XQ. Green tea polyphenols ameliorate non-alcoholic fatty liver disease through upregulating AMPK activation in high fat fed Zucker fatty rats. World J Gastroenterol 2017; 23(21): 3805-3814
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3805.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3805
Non-alcoholic fatty liver disease (NAFLD), characterised by excess accumulation of fat in the liver, has a dramatically rising incidence parallel with the increasing population of metabolic syndrome worldwide[1,2]. NAFLD is emerging as a potential health burden in society because hepatic lipid accumulation can trigger hepatocyte damage, inflammation and fibrogenesis leading to more serious liver disorders such as nonalcoholic steatohepatitis (NASH), cirrhosis and hepatic cellular carcinoma (HCC)[3]. In addition, increased liver lipids [triglyceride (TG), diglyceride, and ceramides] exacerbate hepatic insulin resistance subsequently leading to type 2 diabetes and cardiovascular complications[4,5]. Therefore, identifying strategies to reduce excessive fat accumulation in the liver, especially to decrease hepatic TG, is critical for treatment of NAFLD and prevention of its potential consequences such as cardiometabolic disease.
Currently, no pharmacological therapy is approved for NAFLD. Several drug treatments for the management of NAFLD have been proposed, but none have shown considerable efficacy on the whole spectrum of liver damage[6]. Lifestyle interventions involving exercise and weight loss are the only accepted treatments for this disease but are often difficult to maintain for NAFLD sufferers. Thus, there is an urgent need to discover agents targeted at elevated hepatic lipids and safe for long-term administration.
Green tea (Camellia sinensis) contains natural catechins that is a heterogeneous class of polyphenolic compounds, mainly (-)-epigallocatechin-3-gallate (EGCG), with beneficial effects related to their antioxidant and anti-inflammatory properties[7]. Recently, green tea polyphenols (GTP) has received considerable attention for its metabolic effects against metabolic syndrome and type 2 diabetes[8]. Several lines of studies suggest that the anti-diabetic effects of GTP are probably due to suppressing appetite, modifying dietary fat emulsification in the gastrointestinal tract, inhibiting gastrointestinal lipolysis and reducing nutrient absorption[9-11]. Our previous studies have demonstrated that GTP has direct effects on glucose and lipid metabolism through enhancing glycogen synthesis and inhibiting lipogenesis in hepatocytes[12]. Based on the results of this in vitro study, we hypothesis that GTP can reduce hepatic lipid accumulation against fatty liver in vivo.
NAFLD is a complex metabolic disease. Many epidemiological studies have demonstrated that overnutrition or inappropriate diet leads to insulin resistance, dyslipidaemia and chronic inflammation, which significantly contribute to the development of NAFLD[13,14], in particular for those who have tendency for obesity genetically[15]. To evaluate effects of GTP on a rodent model that mimics most pathophysiological features of NAFLD in humans, Zucker fatty (ZF) rat, a genetic model of metabolic syndrome with obesity[16] was used in the study and fed with a high fat diet (HFD). There is increasing evidence that increased liver TG content is highly correlated to increased de novo lipogenesis[17]. AMP-activated protein kinase (AMPK) activity plays a vital role in mediating hepatic lipogenesis[18]. Recent studies have shown that AMPK activity is reduced by the factors implicated in the development of NAFLD, such as inflammation and obesity[19,20]. Therefore, inhibition of hepatic lipogenesis by AMPK activation is envisaged as a viable therapeutic strategy to prevent the initiation and progression of NAFLD[21]. Whether GTP improves both genetic and dietary factors implicated in NAFLD through activation of AMPK pathway, however, has not previously been addressed. In the current study, we tested the hypothesis that GTP would confer an effective therapy against obese- and HFD-induced NAFLD in rats through upregulating the AMPK pathway. Thus, protein molecules involved in AMPK-mediated hepatic lipogenesis were detected followed by a metabolic study in HFD-ZF rats with or without GTP treatment.
The polyphenols extracted from green tea (98% purity) were analyzed using an Agilent 1200 series liquid chromatograph/mass selective detector equipped with QTOF 6510 mass spectrometer (Agilent Technologies). Mass spectrometry data were acquired and quantified with an EGCG standard. The concentration of other polyphenols were calculated using the EGCG standard (CAS No: 989-51-5, MF: C22H18O11, MW: 458.378 and Purity: ≥ 99% (HPLC).
Male Zucker fatty (fa/fa) rats (ZF) and their lean littermates, aged 5-7 wk and approximately 160 g body weight, were supplied by the Monash Animal Research Platform (Monash University, Vic, Australia). The animal protocol designed to minimize pain or discomfort to the animals was approved by the Animal Care and Ethics Committee (ACEC # 2009-325A) of the University of Technology Sydney and was in accordance with the National Health and Medical Research Council of Australia Guidelines on Animal Experimentation. Rats were acclimatised in communal cages at 22 °C, with a 12 h light-dark cycle (lights on 0700 h) for 1 wk and had ad libitum access to a standard chow diet (Gordon’s Specialty Stock Feed, Sydney, Australia) and water. ZF rats (n = 24) were fed a high-fat diet (HFD) for 2 wk. The energy percentage composition of the HFD was 59% fat, 20% carbohydrate, and 21% protein, with equal quantities of fibre, vitamins, and minerals to the standard chow diet. The HFD was replenished daily. After two weeks of HFD feeding, ZF rats were divided into two groups. The control group (ZF-Con; n = 12) was administered saline (5 mL/kg of body weight) and ZF rats in the GTP group (ZF-GTP; n = 12) was administered GTP at 200 mg/kg of body weight daily for 8 wk via oral gavage. HFD feeding was continued throughout the 8 wk treatment period. Lean Zucker rats (Lean, n = 12) were fed a standard laboratory chow diet throughout the treatment period.
At the end of treatment, blood samples were collected from the tail of rats after an overnight fast (12 h). Fasting serum total cholesterols (TC), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG) and non-esterified fatty acids (NEFAs) were analysed using enzymatic colorimetric kits obtained from Roche Diagnostic (Mannheim, Germany) and Wako Pure Chemical Industries (Osaka, Japan) respectively. Low-density lipoprotein cholesterol (LDL-C) concentrations were calculated by Friedewald's formula: LDL-C (mmol/L) = TC - HDL-C - TG/2.2. Fasting serum insulin concentration was measured using a RIA kit (Linco Diagnostic Services Inc, St. Charles). Fasting serum glucose, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were determined by spectrophotometric analysis using commercial kits (Dialab, Vienna, Austria). All experimental assays were carried out according to the manufacturer’s instruction.
At the end of the experiment, animals were anesthetized using inhalant aesthetic gas (isoflurane and nitrous oxide) after 12 h of fasting. Liver and visceral fat (epididymal and peri-rental adipose tissues) were quickly excised, washed by ice-cold PBS then stored at -80 °C for subsequent histological and molecular assays.
Approximately 50 mg of liver tissues was homogenized at 4 °C in RIPA lysis buffer (Sigma-Aldrich, St. Luis, MO, United States). Lipids from the liver homogenate were extracted using chloroform/methanol method (2:1), evaporated, and dissolved in 1 mL ethanol (Sigma-Aldrich, St. Luis, MO, United States). TG concentration was assayed using kits from Roche Diagnostic (Mannheim, Germany) following the manufacturers’ instructions.
A small portion of frozen liver tissue was cut and embedded with pre-cooled optimal cutting compound (Torrance, CA, United States) for cryostat sectioning at 6 μm. The sections were mounted on microscope slides then fixed with 10% formaldehyde solution for 48 h. The samples were then stained with Haematoxylin and Eosin (H&E) or Oil Red O (ORO, Sigma-Aldrich, St. Luis, MO, United States) to investigate architecture of the liver and hepatic lipid droplets. Stained ORO slides were visualized with the Olympus microscope and images were captured with an Olympus digital camera (DP70, Tokyo, Japan) using Image-Pro6.2 software (Media Cybernetics, Inc. MD, United States). Lipid droplets were quantified at least 5 different high-power fields in a blinded way. For each group, liver samples from 6-8 rats were prepared and stained and six fragments from each liver were further analysed. All slides were scanned at an absolute magnification of 400X using Image-Pro6.2 software (Media Cybernetics, Inc. MD, United States) under a light microscopy (Olympus, BX51 microscope, Tokyo, Japan). Morphometric results are presented as area fractions-the percentage of specific counts in relation to total number of counted points.
Serum levels of TNF-α, interleukins (IL-6 and IL-10) and interferon gamma (IFNγ) were measured with a standard sandwich enzyme immunoassay method using the Bio-Plex Pro mouse cytokine assay kit (Bio-rad Laboratories, Hercules, CA, United States) for the simultaneous quantification of TNF-α, ILs and IFNγ according to the manufacturer’s instructions. Resulting TNF-α, ILs and IFNγ levels were determined using a Bio-Plex MAGPIX array reader, which identified and quantified each specific reaction based on bead colour and fluorescent signal intensity and expressed as pictogram per millilitre.
One hundred milligram of frozen liver samples was homogenized in 1 mL lysis buffer (Roche Diagnostics, Indianapolis, IN, United States). Then, the homogenates were centrifuged at 3000 g for 10 min at 4 °C. The supernatants were collected and the protein concentration of each supernatant was determined by the Bradford method. Total protein (20 μg) was separated to 7.5% SDS-polyacrylamide gel electrophoresis then transferred to 0.45 μmol/L polyvinyldenedifluoride (PVDF) membrane and immunoblotted with primary antibodies against phospho-AMPKα (Thr172), AMPKα, phospho-ACC (Ser79) and ACC (Cell Signaling Technology Inc, MA), SREBP-1c and β-actin (Santa Cruz, CA, United States) at 1:1000 dilution and secondary antibodies (Santa Cruz, CA, United States) at 1:10000 dilution. Blots were then developed with enhanced chemiluminescence (ECL) (Pierce, IL, United States) according to manufacturer’s instructions. The protein bands were visualized by ChemiDoc XRS systems (Bio-Rad Laboratories, CA, United States). The density of bands was quantified with Quality One 4.6.1 software (Bio-Rad, CA, United States) and the quantified results from 8 rats of each group were calculated as percentage of control lean rats for statistical analysis.
All values are expressed as mean ± SEM. Comparisons across the three groups were performed using one-way ANOVA followed by post-hoc analysis of Tukey’s test to determine significant differences between the two groups using Prism version 6 (GraphPad Inc, San Diego, CA, United States). P value < 0.05 was considered statistically significant.
Average food intake remained consistent and similar between ZF control rats and GTP treatment at approximately 17 g/rat per day for the duration of the study period. The body weight and visceral fat of ZF rat were significantly higher than their lean control, associated with hyperglycaemia and hyperlipidaemia (Table 1). No significant differences in liver weight were noted between ZF control and GTP-ZF rats. GTP treatment prevented HFD-induced weight gain by 10.1% (P = 0.052) and significantly decreased visceral fat by 31.0% (P < 0.01) and lowered fasting serum glucose and insulin levels (Table 1, ZF-Con vs ZF-GTP group, both P < 0.05). GTP treatment also significantly reduced the elevated serum levels of cholesterol, TG and NEFAs by 25.4% (P < 0.05), 34.6% (P < 0.01), and 20.9% (P < 0.05), respectively, when compared with the ZF-Con rat. HFD feeding caused a 48.1% reduction of serum HDL level (ZF-Con vs Lean group, P < 0.01) while HDL level was significantly higher in GTP treated ZF rats than ZF-Con rats (Table 1, P < 0.05).
| Paradigm parameter | Lean | ZF-Con | ZF-GTP |
| Initial body weight (g) | 258.6 ± 9.5 | 369.8 ± 12.8b | 370.4 ± 13.7 |
| 344.8 ± 8.4 | 449.6 ± 17.6b | 404.1 ± 10.8 | |
| Body weight (g) | |||
| Food intake (g/d/rat) | 23.13 ± 0.04 | 17.01 ± 0.33a | 16.52 ± 0.51 |
| Liver weight (g) | 11.84 ± 0.41 | 13.51 ± 0.82a | 12.73 ± 0.74 |
| Visceral adipose (g) | 1.96 ± 0.57 | 9.16 ± 0.17e | 6.32 ± 0.21d |
| Glucose (mmol/L) | 4.38 ± 0.54 | 7.54 ± 1.70a | 6.60 ± 1.22c |
| Insulin (μU/L) | 0.55 ± 0.04 | 77.7 ± 14.3e | 38.64 ± 11.2c |
| SHBG (nmol/L) | 102.2 ± 6.9 | 82.6 ± 4.8a | 91.2 ± 5.6c |
| Serum lipids levels | |||
| Total cholesterol (mmol/L) | 2.90 ± 0.07 | 5.42 ± 0.21b | 4.04 ± 0.23c |
| Triglyceride (mmol/L) | 0.11 ± 0.01 | 0.75 ± 0.02e | 0.49 ± 0.04d |
| HDL (mmol/L) | 2.64 ± 0.22 | 1.37 ± 0.21b | 2.03 ± 0.07c |
| LDL (mmol/L) | 1.37 ± 0.2 | 4.28 ± 0.42e | 2.46 ± 0.36c |
| NEFAs (mmol/L) | 0.76 ± 0.04 | 1.29 ± 0.03a | 1.02 ± 0.07c |
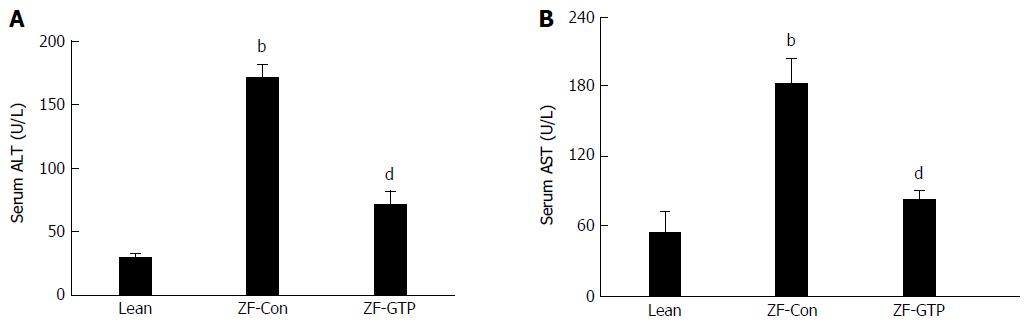
When compared with the lean control, serum hepatic enzyme (ALT and AST) levels in HFD-fed ZF rats were increased by 6.2 fold (Figure 1A; 29.20 ± 4.01 U/L vs 189.89 ± 20.69 U/L, P < 0.001) and 3 fold (Figure 1B; 54.71 ± 17.88 U/L vs 183.14 ± 13.91 U/L, P < 0.001), respectively. GTP treatment significantly attenuated the elevated ALT and AST values (both P < 0.01) when compared with control ZF rats (Figure 1).
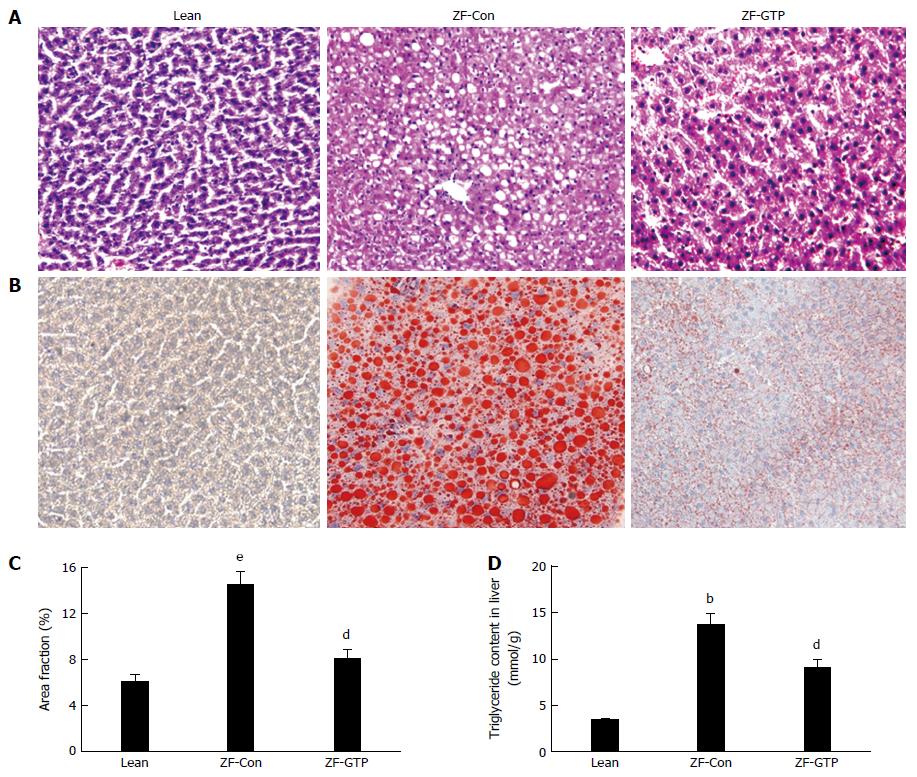
The macrovesicular steatosis and fat droplets were assessed in H&E and ORO-stained sections by light microscopy and digital image analysis (DAI). More microvesicular steatosis was observed on H&E stained sections of ZF control rats than ZF-GTP rats and no macrovesicular steatosis in the lean control (Figure 2A). Fat filtration and hepatic steatosis were further confirmed with ORO-stained cryosections because ORO staining analysis is much higher sensitivity and specificity for hepatic steatosis. The elevated lipid accumulation was revealed by many lipid droplets in ORO-stained cryosections of ZF control rats compared to the lean control (Figure 2B). DIA results (Figure 2C) showed significant reduction in lipid droplets with GTP treated ZF rats, indicating that GTP greatly prevented lipid infiltrations and hepatic steatosis.
Consistent with ORO-staining results, measurement of TG content in the liver showed that HFD ZF rats presented significantly higher hepatic TG by 4-fold compared to lean rats (13.65 ± 4.51 μmol/g vs 3.36 ± 0.72 μmol/g; P < 0.01). Results in Figure 2D showed that GTP treatment significantly reduced hepatic TG content in HFD fed ZF rats (9.05 ± 2.87 μmol/g vs 13.65 ± 4.51 μmol/g; P < 0.01).
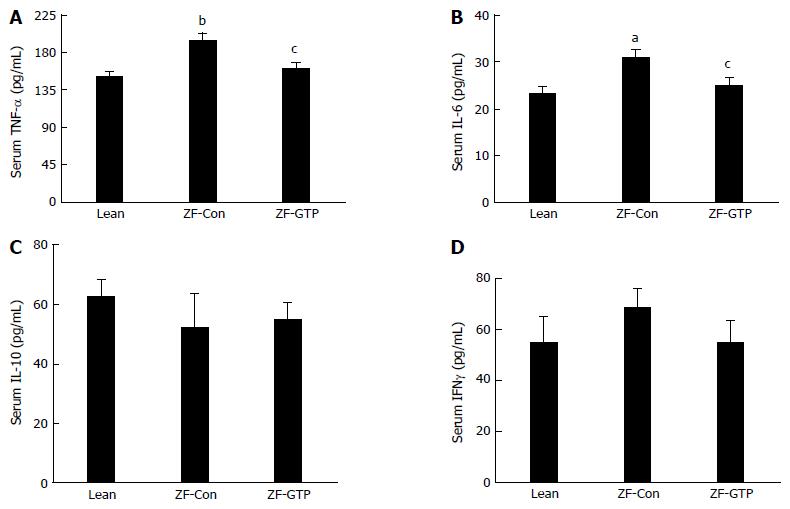
Circulating inflammatory cytokines were assessed by enzyme immunoassay. Figure 3 shows significantly increased TNF-α and IL-6 in HFD-ZF control rats compared with the Lean control (P < 0.01 and P < 0.05 respectively), whereas, GTP treatment markedly decreased the elevation of TNF-α and IL-6 (Figure 3A and B, both P < 0.05). IL-10 and IFNγ levels were no significant difference among three groups (Figure 3C and D).
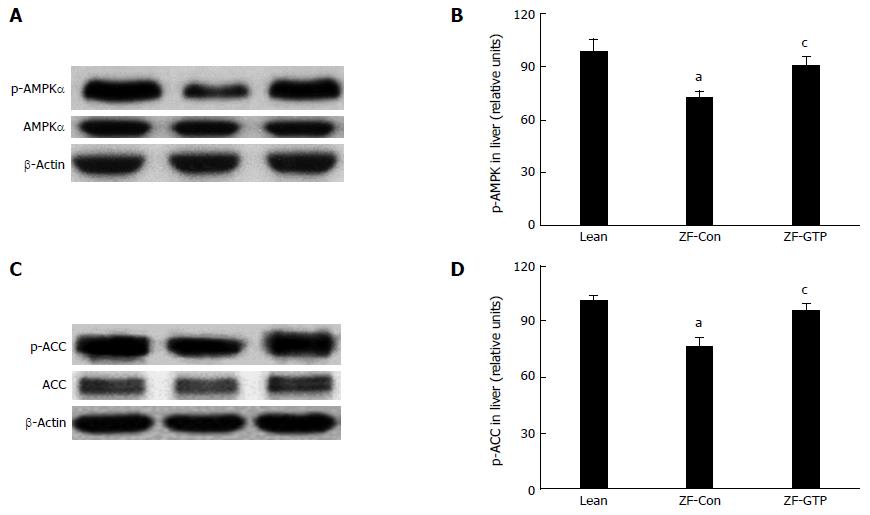
AMPK and ACC are key enzymes that regulate de novo lipogenesis in the liver and contribute considerably to overall metabolism of lipids[15-16]. Results in Figure 4 show that expression of phospho-AMPKα (Thr172) was significantly lower in HFD fed ZF rats compared with the lean control rats (P < 0.05), and GTP administration significantly enhanced expression of phospho-AMPKα (Thr172) (P < 0.05) in HFD ZF rats. A reduction of ACC (Ser79) phosphorylation was observed in the liver of HFD ZF rats, and this was reversed by GTP treatment in a similar manner to that phospho-AMPKα (Thr172) expression.
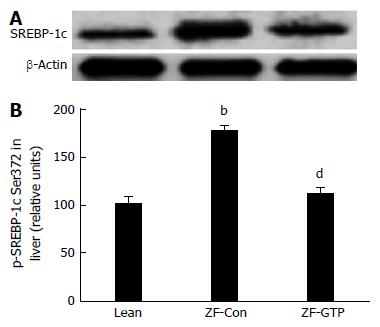
SREBP-1c, a key transcription factor regulating the gene expression of key enzymes implicated in upregulating de novo lipogenesis in the liver[22] was also detected by immunoblotting. HFD-fed ZF rats presented an approximate 1.75 fold increase in SREBP-1c expression in the liver compared to the lean control (Figure 5B; P < 0.01). GTP treatment abolished overexpression of SREBP-1c protein compared to ZF control rats (Figure 5B; P < 0.01).
Hepatic steatosis is the first stage of NAFLD, which is mostly associated with increased de novo lipogenesis and obesity, however, it can progress to a more advanced form of the disease, NASH, hepatic necrosis, fibrosis and cirrhosis and hepatocellular carcinoma. Therefore, it is important to control NAFLD at the initial stage. Here, we report for the first time that GTP can protect against HFD-induced NAFLD in genetically obese rats. HFD-fed Zucker fatty rats recapitulated the key metabolic features seen in humans with NAFLD, including central obesity, hyperlipidemia, and hyperinsulinemia and elevated fasting glucose levels. Hepatic manifestations of NAFLD in the rats were evidenced by the elevated ALT and AST levels, increased lipids droplets and TG content in the liver. The parameters associated with obesity and metabolisms shown in Table 1 indicate that HFD-fed ZF rat is an optimal model of NAFLD to test preventive and therapeutic agents against NAFLD.
The key finding of this study is that GTP, as a natural agent, protected liver damage by HFD feeding and genetic obese, evidenced by improved hepatic enzymes (AST and ALT) levels and significantly lowered TG content in the liver of HFD-ZF rats. Results attained from histological analysis showed that GTP also markedly reduced lipid droplets in the liver of HFD-ZF rats. Elevated serum ALT and AST levels are the primary abnormality seen in patients with NAFLD and other chronic liver disease in human[23]. These finding indicate that GTP can protect hepatocytes against HFD- and obesity-induced hepatic steatosis. Traditionally, beneficial effects of GTP on the liver have been attributed to their antioxidant properties[8]. Accumulating evidence has revealed that NAFLD is strongly related to inflammation. For example, IL-6, as a proinflammatory cytokine, has been demonstrated as a potential mediator leading to NAFLD. TNFα has been implicated in liver fibrosis and advanced stages of NAFLD in human[24,25]. In this study, HFD fed obese rats presented systemic inflammation by markedly raised circulating TNFα and IL-6 levels. Although no change was found in anti-inflammatory cytokine IL-10, raised TNFα and IL-6 were attenuated by GTP treatment. This funding is consistent with a recent study in which Xiao et al[26] reported EGCG, a major active compound in GTP, against HFD-induced liver fibrosis associated with inflammation in rats through deregulation of TGF/SMAD, PI3 K/Akt/FoxO1, and NF-κB pathways. Taken together, intervention with GTP and EGCG can effectively prevent and reverse inflammation-mediated liver damage and fibrosis in rats, suggesting that GTP is beneficial in humans with NAFLD-induced by dietary factors and genetic obesity.
In addition to anti-inflammatory and antioxidant action, protective effects of GTP on NAFLD may be related to improved hyperinsulinemia and hyperlipidaemia. In NAFLD, there is excess of (free fatty acids) FFAs/NEFAs delivered to the liver due to increased adipose lipolysis under insulin resistant status, which lead to hepatic steatosis by over conversion of FFAs into TG and cause hepatocytes injury-induced by lipotoxicity[27]. In this study, serum NEFAs level was significantly higher in HFD-ZF rats than the lean control, whereas, GTP treatment significantly attenuated the elevated NEFAs levels, which in turn, could reduce substrate influx to the liver for hepatic TG synthesis and also may diminish unoxidized fatty acids accumulation, therefore, prevent lipoperoxidative stress-induced hepatic injury[27,28]. Furthermore, reduction of hepatic lipids in GTP treated rats may be also attributed for improved hyperglycemia. Consequently, glucose influx to the liver was reduced and ultimately decreased de novo lipogenesis (DNL) in the liver.
Hepatic lipogenesis is the metabolic pathway by which acetyl-CoA carboxylase (ACC) converts acetyl-CoA derived from glucose to fats, which involves sub-processes of fatty acid synthesis and subsequent triglyceride synthesis utilizing carbohydrate in the liver[17]. In healthy individuals, DNL contributes < 5% to liver TG content, whereas in individuals with NAFLD, DNL contributes > 26% of liver TG[17]. The significant increase (approximately five fold) in DNL suggests that this pathway may play an important role in the development of NAFLD. Thus, controlling hepatic DNL highlights a potential therapeutic utility for alleviating NAFLD and preventing its potential consequences (NASH and HCC). DNL is regulated by complex enzyme cascades/pathways. Among these, AMPK-mediated phosphorylation of target substrates plays a critical role in the regulation of hepatic lipogenesis[21]. The activity of AMPK is stimulated by insulin through promoting its phosphorylation at Thr172[29], subsequently, AMPK activation leads to phosphorylation of ACC1 at Ser79 and ACC2 at Ser221, causing a reduction in ACC activity that lowers malonyl-CoA from acetyl-CoA, which leads to decreased hepatic TG synthesis[30,31].
We have previously reported that GTP inhibits lipogenesis associated with enhanced expression of phosphorylated AMPKα and ACC proteins in hepatocytes[12]. To elucidate molecular mechanisms by which GTP improves hepatic steatosis in vivo, AMPK-ACC pathway was examined by immunoblotting analysis. We found that obese rats exposed to HFD expressed less AMPK Thr172 associated with reduced phosphorated ACC-Ser79 protein in the liver than the lean control rats. GTP intervention significantly enhanced protein expression of AMPK Thr172 and ACC Ser79 compared to saline-treated ZF rats, indicating that decreased hepatic TG and reversed liver histopathology are likely through diminishing hepatic lipogenesis in GTP-ZF rats. In addition to suppression of de novo lipogenesis, increased AMPK activity associated with inactivation of ACC by GTP may prevent hepatic injury by increasing fatty acid oxidation. Several lines of studies have demonstrated that reduction in malonyl-CoA secondary to AMPKα1-mediated ACC inactivation enhance carnitine palmitoyltransferase I (CPT I) activity, which in turn, increases in mitochondrial fatty acid oxidation by increasing CPT I flux[29,32]. Thus, by enhancing AMPK activation, reduced hepatic TG in GTP treated rats is due, at least in part, to suppression of hepatic lipogenesis and fatty acid oxidation in the liver through upregulating AMPK activation.
SREBP-1c is a key transcription factor regulating the gene expression of key enzymes implicated in hepatic lipogenesis and triglyceride-rich lipoprotein secretion[33]. In this study, SREBP1c levels were lower in HFD-ZF rats and GTP enhanced expression SREBP-1c, which is likely to reduce hepatic triglyceride biosynthesis and out flux. This finding indicates that GTP inhibited lipogenesis also through inactivation of SREBP-1c. This finding explained the molecular mechanism of decreased serum cholesterol and TG levels in GTP-treated ZF rats.
Significant reduction of serum ALT and AST levels and hepatic TG content in GTP-treated HFD ZF rats strongly supports that GTP can protect HFD-induced liver damage. Mechanisms are likely to include anti-inflammation and suppression de novo lipogenesis through enhancing AMPK activation and promoting fatty-acid oxidation thereby reducing hepatic lipotoxicity. In summary, we provide the first evidence that consistent administration of GTP protect genetically obese rodent against HFD induced hepatic steatosis. This protection was associated with increased activation of AMPK pathway to control hepatic de novo lipogenesis and hepatic TG secretion. Our results encourage further study into the effects and safety for GTP conferring protection against NAFLD in human.
We are grateful to Zuyi Lushen Kangyuan Co (Meitan, Guizhou, China) for supporting this study by providing GTP. The authors also would like to thank Dr Yali Sun’s advice for chemical analysis of GTP.
Non-alcoholic fatty liver disease (NAFLD) has a dramatically rising incidence and is emerging as a potential health burden in society because hepatic lipid accumulation can trigger hepatocyte damage, inflammation and fibrogenesis leading to more serious liver disorders. There is an urgent need to discover agents for NAFLD treatment and prevention. Green tea polyphenols (GTP) has received considerable attention for its metabolic effects against metabolic syndrome and type 2 diabetes. The previous studies have demonstrated that GTP has direct effects on glucose and lipid metabolism in vitro study. Thus, we hypothesize that GTP can reduce hepatic lipid accumulation against fatty liver in vivo. The authors tested this hypothesis in high fat diet (HFD) fed genetically Zucker obese rat.
Currently, no pharmacological therapy is approved for NAFLD and there is also a recent surge in interest in naturally derived products for the treatment of metabolic disorders. There have been no previous studies into GTP on animal models of NAFLD from both dietary and genetic factors.
The authors provide the first evidence that consistent administration of GTP protect genetically obese rodent against HFD induced hepatic steatosis. This protection was associated with increased activation of AMP-activated protein kinase pathway to control hepatic de novo lipogenesis and hepatic TG secretion.
These results encourage further study into the effects and safety for GTP conferring protection against NAFLD in human.
In this article, the authors showed beneficially effects of GTP on NAFLD, including improved lipid profiles and drastically reduced visceral fat, improved liver function and reduced TG accumulation in the liver. The overall study is solid and well designed. The results are consistent with the proposed hypothesis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Liagre B, Zhang JD S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1619] [Article Influence: 115.6] [Reference Citation Analysis (1)] |
| 2. | Wong VW, Chu WC, Wong GL, Chan RS, Chim AM, Ong A, Yeung DK, Yiu KK, Chu SH, Woo J. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 390] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 3. | Pais R, Charlotte F, Fedchuk L, Bedossa P, Lebray P, Poynard T, Ratziu V. A systematic review of follow-up biopsies reveals disease progression in patients with non-alcoholic fatty liver. J Hepatol. 2013;59:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Samuel VT, Liu ZX, Qu X, Elder BD, Bilz S, Befroy D, Romanelli AJ, Shulman GI. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J Biol Chem. 2004;279:32345-32353. [PubMed] |
| 5. | Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 833] [Cited by in RCA: 772] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 6. | Rotman Y, Sanyal AJ. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut. 2017;66:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 336] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 7. | Sabu MC, Smitha K, Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 2002;83:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 214] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Zhao B. Antioxidant effects of green tea polyphenols. Kexue Tongbao. 2003;48:315-319. |
| 9. | Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003;14:326-332. [PubMed] |
| 10. | Shishikura Y, Khokhar S, Murray BS. Effects of tea polyphenols on emulsification of olive oil in a small intestine model system. J Agric Food Chem. 2006;54:1906-1913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Cha KH, Song DG, Kim SM, Pan CH. Inhibition of gastrointestinal lipolysis by green tea, coffee, and gomchui (Ligularia fischeri) tea polyphenols during simulated digestion. J Agric Food Chem. 2012;60:7152-7157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Kim JJ, Tan Y, Xiao L, Sun YL, Qu X. Green tea polyphenol epigallocatechin-3-gallate enhance glycogen synthesis and inhibit lipogenesis in hepatocytes. Biomed Res Int. 2013;2013:920128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Luo Y, Burrington CM, Graff EC, Zhang J, Judd RL, Suksaranjit P, Kaewpoowat Q, Davenport SK, O’Neill AM, Greene MW. Metabolic phenotype and adipose and liver features in a high-fat Western diet-induced mouse model of obesity-linked NAFLD. Am J Physiol Endocrinol Metab. 2016;310:E418-E439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 14. | Kanwar P, Nelson JE, Yates K, Kleiner DE, Unalp-Arida A, Kowdley KV. Association between metabolic syndrome and liver histology among NAFLD patients without diabetes. BMJ Open Gastroenterol. 2016;3:e000114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | Mollard RC, Sénéchal M, MacIntosh AC, Hay J, Wicklow BA, Wittmeier KD, Sellers EA, Dean HJ, Ryner L, Berard L. Dietary determinants of hepatic steatosis and visceral adiposity in overweight and obese youth at risk of type 2 diabetes. Am J Clin Nutr. 2014;99:804-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Okamoto T, Kanemoto N, Ohbuchi Y, Okano M, Fukui H, Sudo T. Characterization of STZ-Induced Type 2 Diabetes in Zucker Fatty Rats. Exp Anim. 2008;57:335-345. [PubMed] |
| 17. | Lambert JE, Ramos-Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 2014;146:726-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 756] [Cited by in RCA: 798] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
| 18. | Steinberg GR, Kemp BE. AMPK in Health and Disease. Physiol Rev. 2009;89:1025-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1285] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 19. | Hasanvand A, Amini-Khoei H, Hadian MR, Abdollahi A, Tavangar SM, Dehpour AR, Semiei E, Mehr SE. Anti-inflammatory effect of AMPK signaling pathway in rat model of diabetic neuropathy. Inflammopharmacology. 2016;24:207-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Xu XJ, Gauthier MS, Hess DT, Apovian CM, Cacicedo JM, Gokce N, Farb M, Valentine RJ, Ruderman NB. Insulin sensitive and resistant obesity in humans: AMPK activity, oxidative stress, and depot-specific changes in gene expression in adipose tissue. J Lipid Res. 2012;53:792-801. [PubMed] |
| 21. | Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730-E740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 378] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 22. | Ferré P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12 Suppl 2:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 536] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 23. | Arora A, Sharma P. Non-invasive Diagnosis of Fibrosis in Non-alcoholic Fatty Liver Disease. J Clin Exp Hepatol. 2012;2:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Kishimoto T. IL-6: from its discovery to clinical applications. Int Immunol. 2010;22:347-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 579] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 25. | Lesmana CR, Hasan I, Budihusodo U, Gani RA, Krisnuhoni E, Akbar N, Lesmana LA. Diagnostic value of a group of biochemical markers of liver fibrosis in patients with non-alcoholic steatohepatitis. J Dig Dis. 2009;10:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 26. | Xiao J, Ho CT, Liong EC, Nanji AA, Leung TM, Lau TY, Fung ML, Tipoe GL. Epigallocatechin gallate attenuates fibrosis, oxidative stress, and inflammation in non-alcoholic fatty liver disease rat model through TGF/SMAD, PI3 K/Akt/FoxO1, and NF-kappa B pathways. Eur J Nutr. 2014;53:187-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Guturu P, Duchini A. Etiopathogenesis of nonalcoholic steatohepatitis: role of obesity, insulin resistance and mechanisms of hepatotoxicity. Int J Hepatol. 2012;2012:212865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Jones JG. Hepatic glucose and lipid metabolism. Diabetologia. 2016;59:1098-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 192] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 29. | Chiu CY, Chan IL, Yang TH, Liu SH, Chiang MT. Supplementation of chitosan alleviates high-fat diet-enhanced lipogenesis in rats via adenosine monophosphate (AMP)-activated protein kinase activation and inhibition of lipogenesis-associated genes. J Agric Food Chem. 2015;63:2979-2988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 30. | Cho YS, Lee JI, Shin D, Kim HT, Jung HY, Lee TG, Kang LW, Ahn YJ, Cho HS, Heo YS. Molecular mechanism for the regulation of human ACC2 through phosphorylation by AMPK. Biochem Biophys Res Commun. 2010;391:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Choi S, Choi Y, Choi Y, Kim S, Jang J, Park T. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem. 2013;141:3627-3635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | McGarry JD, Takabayashi Y, Foster DW. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978;253:8294-8300. [PubMed] |
| 33. | Gorgani-Firuzjaee S, Meshkani R. SH2 domain-containing inositol 5-phosphatase (SHIP2) inhibition ameliorates high glucose-induced de-novo lipogenesis and VLDL production through regulating AMPK/mTOR/SREBP1 pathway and ROS production in HepG2 cells. Free Radic Biol Med. 2015;89:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |