Published online Jun 7, 2017. doi: 10.3748/wjg.v23.i21.3797
Peer-review started: February 20, 2017
First decision: March 16, 2017
Revised: March 22, 2017
Accepted: May 4, 2017
Article in press: May 4, 2017
Published online: June 7, 2017
Processing time: 109 Days and 15.1 Hours
Spontaneous tumor regression is an extremely rare phenomenon in the oncology field. However, there are several case reports resulted in the regression of hepatocellular carcinoma (HCC) and the accumulation of clinical information and analyses of the mechanism can contribute to the development of a novel therapy. For this purpose, we have carefully reviewed 23 cases of spontaneously regressed HCC published in recent 5 years and our case. The information regarding the tumor size, tumor marker, treatments, etc., have been summarized. The mechanism of spontaneous regression has been discussed to date and presumed to be due to many factors, including hypoxia and immunological reactions. In this careful review of the 24 cases based on the clinical information, hypoxia, systemic inflammation, and both upon spontaneous regression were seen in 3, 8, and 4 cases, respectively among the 15 cases for which the information regarding the proposed mechanisms are available. Recent development of immunotherapeutic approaches in oncology shows promising results, therefore, accumulation of additional cases and analysis of mechanisms underlying the spontaneous regression of HCC are essential and could lead to the development of a new generation of immunotherapies including antibodies directed against immune reactions.
Core tip: Spontaneous tumor regression of hepatocellular carcinoma (HCC) have been reported in several reports although rare. The analyses of the mechanisms underlying this phenomenon is the potential target for the novel therapeutic methods for HCC. For this purpose, we have carefully reviewed 24 cases of spontaneously regressed HCC including cases published in recent 5-years and our case. The minute clinical information and clinical courses are reviewed and summarized. Based on the information, hypoxia and/or systemic inflammation are involved in all cases for which the information regarding the proposed mechanism is available. An accumulation of additional cases and the analysis of mechanism underlying the spontaneous regression of HCC could lead to the development of new therapeutic strategy for HCC.
- Citation: Sakamaki A, Kamimura K, Abe S, Tsuchiya A, Takamura M, Kawai H, Yamagiwa S, Terai S. Spontaneous regression of hepatocellular carcinoma: A mini-review. World J Gastroenterol 2017; 23(21): 3797-3804
- URL: https://www.wjgnet.com/1007-9327/full/v23/i21/3797.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i21.3797
Hepatocellular carcinoma (HCC) is the most common histological type of primary liver cancer. It is the fifth most prevalent type of cancer and the second most common cause of cancer-associated mortality in males worldwide; it is also the seventh most commonly diagnosed cancer and the sixth greatest cause of cancer-associated mortality in females worldwide[1]. Approximately 70%-80% of HCCs develop in patients with liver cirrhosis[2]. Although many treatment options are available, the prognosis of patients with advanced HCC remains poor. Patients diagnosed at an early stage may achieve a 5-year survival rate of approximately 50%; however, those diagnosed at an intermediate to advanced stage have a 20%-50% survival at 3 years, and those diagnosed at the terminal stage usually die within 6 mo[3].
The therapeutic options for HCC include resection, radiation, radiofrequency ablation, transarterial chemoembolization (TACE), and systemic chemotherapy including molecular targeted medicine, sorafenib. TACE showed anti-tumor effect by reducing the arterial blood supply to the tumor[4,5]. The resected specimen of HCC after TACE had a liquefied necrotic region with focal hemorrhage, accompanied by non-specific inflammatory infiltrates containing foamy histiocytes and lymphocytes at the tumor periphery as reported in a study[6].
Spontaneous tumor regression was firstly reported by Cole and Everson in 1956[7], and was defined as a partial or complete involution of a malignant tumor in the absence of the application of a specific therapy. Spontaneous tumor regression is a very rare phenomenon, with an incidence rate of one out of every 60000-100000 cases[8]. Conventionally, malignant melanoma, neuroblastoma, and cancer of the kidney are the most frequent types of tumors presenting this phenomenon[8]; however, in recent reports, lung and liver cancers are also considered to be the frequent types of cancer[9]. Spontaneous regression of HCC was defined as an involution of HCC without any therapies and first described in 1972[10]. The mechanism of spontaneous regression may include hypoxia and immunological reaction which we often find in the liver tissue treated with TACE, however, the clear evidences remain unclear and the accumulation of additional cases is essential to develop a novel treatment approach for HCC. However, there are only a few reports summarizing these information and therefore, we have carefully reviewed 23 cases of spontaneously regressed HCC recently published in 5-years, and additional our case to summary information.
A PubMed (National Center for Biotechnology Information at the National Institutes of Health in Bethesda, Maryland, United States) was used for performing search using the key words “spontaneous regression” or “spontaneous remission” and “hepatocellular carcinoma” to extract studies published in recent 5 years. Fifty four reports were included in the initial search; reports that were not published in English literature and not describing spontaneous regression were excluded. Finally, a total of 20 reports and 24 cases including our recent case that matched the definition of spontaneous regression of HCC were reviewed (Table 1)[11-30]. Written informed consent was obtained from the patient for reporting their clinical information.
| Ref. | Age | Sex | Country | Etiology | Background liver | Size (mm) | Location | Impaired blood flow | Pre AFP | Post AFP | Pre DCP | Post DCP | Histological Diagnosis | Following therapy | Recurrence | Intrahepatic metastasis | Extrahepatic metastasis | Proposed mechanism | Cause |
| Jianxin et al[11] | 64 | M | China | HBV | CH | 92 | S6 | N/A | 9022 | 2516 | N/A | N/A | N/A | N | N | N | Omentum | Immunological | Herbal medicine |
| Saito et al[12] | 74 | M | Japan | Alc | CH | 36 | S8 | N/A | 31 | 3 | 17 | N/A | N/A | Resection | N | N | N | Immunological | Cessation of drinking and smoking |
| Pectasides et al[13] | 53 | M | United States | HCV/Alc | LC | 90 | Left | PV, HA | 602410 | 2177 | N/A | N/A | N/A | N | Remnant | Y | Lung | Combine | Portal vein thrombosis and immunological reaction |
| Alam et al[14] | 65 | M | United Kingdom | HCV | LC | N/A | S5/6 | N | 2893 | 6 | N/A | N/A | N/A | N | Y | N | LNs | Combine | Rapid tumor growth and immunological reaction |
| Kumar et al[15] | 40 | M | United States | N/A | N/A | 26 | S2/3/5 | N/A | 832 | 2 | N/A | N/A | Poor | Radiation | N | Y | Lung | Immunological | Cessation of immunosuppressive Therapy |
| Kumar et al[15] | 74 | M | United States | N/A | N/A | 56 | Right | N/A | 97932 | 722 | N/A | N/A | Poor | N | Remnant | Y | N | Immunological | Cessation of immunosuppressive Therapy |
| Yang et al[16] | 59 | M | China | HBV | N/A | 40 | S6 | N/A | 2100 | N/A | N/A | N/A | N/A | N | Y | Y | N | Immunological | Seroconversion of HBV |
| Okano et al[17] | 73 | M | Japan | HBV | N/A | 15 | S8 | N/A | 748 | 5 | 20 | N/A | N/A | N | N | N | N | N/A | Angiography |
| Takeda et al[18] | 68 | M | Japan | Alc | CH | 30 | S4 | Y | 2 | 2 | 427 | 41 | N/A | Resection | N | N | N | Hypoxia | Hepatic arterioportal shunts and vessel thrombosis |
| Kim et al[19] | 57 | M | South Korea | HBV | LC | 37 | S6 | N | 4778 | 50 | 22 | N/A | N/A | Resection | N | N | N | Immunological | Unknown |
| Matsuoka et al[20] | 67 | M | Japan | NASH | CH | 43 | S6 | PV, HA | 11 | N/A | 6 | N/A | Moderate | Resection | N | N | N | Hypoxia | Hepatic arterial and portal vein thromboses |
| Wang et al[21] | 50 | M | China | HBV | CH | 100 | S7/8 | N | 22592 | N/A | N/A | N/A | N/A | Resection | N | Y | N | Immunological | Unknown |
| Parks et al[22] | 69 | M | United States | HCV | N/A | 22 | S8 | N/A | 4077 | 37 | N/A | N/A | Well | N | Y | Y | LNs | N/A | N/A |
| Parks et al[22] | 63 | M | United States | HCV | N/A | N/A | S7 | N/A | 91 | 27 | N/A | N/A | Combined | N | Y | Y | LNs | N/A | N/A |
| Parks et al[22] | 67 | M | United States | HCV | LC | N/A | N/A | PV | 35689 | 8 | N/A | N/A | Moderate | N | N | Y | N | N/A | N/A |
| Saito et al[23] | 75 | M | Japan | HCV | CH | 200 | Right | PV | 452100 | 107 | 596000 | 34 | N/A | TACE | N | Y | Lung | Combine | Portal vein thrombosis and immunological reaction |
| Bhardwaj et al[24] | 74 | F | Australia | Unknown | N/A | 90 | Left | N/A | WNL | N/A | N/A | N/A | Well | N | N | Y | N | N/A | Unknown |
| Lim et al[25] | 64 | M | South Korea | HBV | CH | N/A | Right | N/A | 17 | 2 | 12900 | 23 | N/A | N | N | N/A | Lung, adrenal glands, LNs | Immunological | Herbal medicine |
| Tomino et al[26] | 77 | M | Japan | Alc | N/A | 30 | S1 | PV, HA | 6 | N/A | 19 | N/A | Moderate | Resection | N | N | N | Hypoxia | Hepatic arterial and portal vein thromboses |
| Tsai et al[27] | 74 | M | Taiwan | HCV | LC | 80 | Left | N/A | 810 | WNL | N/A | N/A | N/A | N | N | N/A | N/A | N/A | N/A |
| Okano et al[28] | 77 | M | Japan | Alc | LC | 50 | S8 | N/A | 1825 | 51 | 3043 | 411 | N/A | TACE | Y | Y | N | Combine | Stenosis of hepatic artery and cessation of drinking |
| Sasaki et al[29] | 79 | M | Japan | Alc | N/A | 20 | S2 | N/A | 8 | N/A | N/A | N/A | N/A | Resection | N/A | N | N | N/A | Unknown |
| Tomishige et al[30] | 76 | F | Japan | Unknown | NL | 12 | S6 | N/A | WNL | N/A | N/A | N/A | N/A | Resection | N | N | N | N/A | Unknown |
| Our case | 78 | M | Japan | NASH | CH | 20 | S8 | PV | 2201 | < 1 | 46 | 21 | Poor | N | Y | Y | LN | Immunological | Hemodialysis |
The minute report regarding the epidemiology of spontaneous HCC regression has not been reported. The review of 24 patients showed that the median age of the patients with spontaneous regression was 68.5 years (range: 40-79 years). Of the 24 patients, 91.7% (22/24) were male, 10 patients were reported from Japan (41.7%), and 66.7% of the total cases were from East Asia (Table 1); the result consist with the previously reported fact that the spontaneous regression of HCC was observed with a high frequency in individuals of Asian origin[14,31]. Oquiñena et al[32] reported the partial response (over 50% of tumor regression) rate of spontaneous regression as 0.4% based on the meta-analysis of the control arms for HCC treatment in a randomized clinical trial. They and others also reported the regional difference in the world and the higher frequency of spontaneous regression in the Asian countries although its reason remains unclear[14,31,32].
Among the cases for which the information is available, the etiologies of liver disease of these cases were alcoholic abuse in 5 patients, hepatitis B viral infection in 6 patients, hepatitis C viral infection in 6 patients, non-alcoholic steatohepatitis (NASH) in 2 patients, and hepatitis C viral infection plus alcoholic abuse in 1 patient. The background liver conditions were chronic hepatitis in 8 patients, liver cirrhosis in 6 patients, and normal liver (without hepatitis and fibrosis) in 1 patient (Table 1). Oquiñena et al[33] also reported 3 patients of who expressed wide range of tumor markers, as well as various liver diseases and background conditions and suggested the possibility that the spontaneous regression of HCC may not be associated with the level of tumor marker expression or the background liver disease. These results suggest that the spontaneous regression of HCC can frequently be seen in male in East Asian countries although various patterns were seen in tumor size, etiology, background of liver condition.
The median size of HCC before spontaneous regression was 38.5 mm with a wide ranges of 12-200 mm. Among these, tumors less than 30 mm were diagnosed accidentally (30.0%) which is higher than that of the previously published literatures[12,18,23] , 0%-11.1%, and this change is based on the advances in imaging modalities and are not associated with subjective symptoms. The main tumor was found in the right lobe of the liver in 16 patients and left lobe in 7 patients, and no significant tendency regarding the location of the tumor was seen. The metastatic tumors were found in extrahepatic lesions, for e.g., omentum, lung, lymphnodes, adrenal glands, in 9 patients and intrahepatic in 12 patients; most of these patients showed the reduction or disappearance of the metastatic regions as well as the primary tumor in the liver. Impairment of blood flow of the portal vein and/or hepatic artery were observed in 8 patients (Table 1). Tumor markers including alpha-fetoprotein (AFP) and/or des-γ-carboxy prothrombin (DCP) showed a significant decrease upon the spontaneous regression in 16 patients (Table 1).
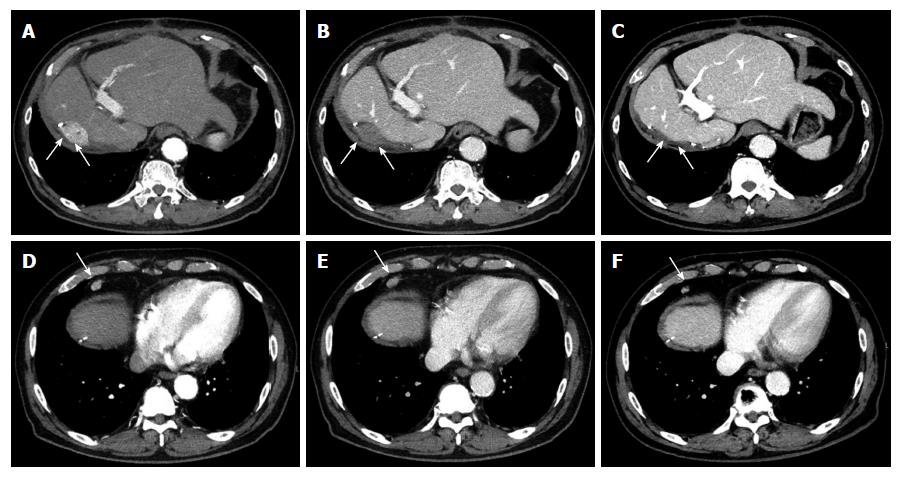
In our case, a 78-year-old Japanese man with chronic hepatitis with a background of NASH and chronic renal failure on hemodialysis. Following an extended right hepatectomy, a 20 mm stump recurrence of HCC (Figure 1A and B) and a metastasis of surrounding lymph nodes (Figure 1D and E) was detected on dynamic computed tomography (CT). Tumor marker levels were increased (AFP, 2204 ng/mL; DCP, 46 mAU/mL) at that point. The patient was admitted to our hospital for interventional therapy three months after the CT and was found for the spontaneous regression of HCC and metastatic tumor in the lymphnodes without any therapy (Figure 1C and F) with significant reduction of AFP to a level below the measurement sensitivity (Table 1).
The mechanism of spontaneous HCC regression has been discussed and proposed to be mediated by many factors (Table 2) and several reports have categorized the factors into two major types: (1) tumor hypoxia; and (2) systemic immunological reactions[17,28,34]. These changes can also be seen in the liver tissue after TACE treatment suggesting the mechanism of spontaneous regression of the tumor. In addition, it is likely that the mechanism of spontaneous regression is complex and multiple factors may be involved in each case. Several cases suggest that both factors are involved in spontaneous regression, tumor hypoxia, and immunological reactions; the initial necrosis of the primary tumor led to immune-activation that then caused a secondary regression of the metastasis[13,35].
| Tumor hypoxia | Immunological reactions |
| Tumor thrombosis of hepatic artery[13,20,36] | Abstinence from alcohol[12,46,47] |
| Tumor thrombosis of portal vein[13,20,23] | Abstinence from smoking[12] |
| Hepatic angiography[37] | Herbal medicines[11,25] |
| Tumor rapid growth[14,38,39] | Prolonged fever[26,48,49] |
| Hepatic arterioportal shunts[18] | Antidiabetics[50] |
| Massive gastrointestinal hemorrhage[40,41] | Vitamin K administration[51] |
| Hemodialysis[42,43] | |
| Surgical invasion[44] |
Our literature review also showed hypoxia in 3 patients, systemic inflammatory response in 9 patients, and combination of both tumor hypoxia and the inflammatory response in 4 patients among 16 patients for whom the potential mechanisms were reported (Table 1). Spontaneous HCC regression may occur in association with a single factor or several complex factors.
In our case, immunological reaction and hypoxia can be the reason for spontaneous regression because the size and contrast enhancement of extrahepatic lymphnode metastasis significantly reduced upon the regression of intrahepatic lesions (Figure 1D-F).
Tumor hypoxia is an intuitively appealing mechanism as it mirrors the established treatment modalities for HCC (TACE, sorafenib, etc.)[34]. Tumor thromboses of the hepatic artery[13,20,36] and portal vein[13,20,23], hepatic angiography[37], rapid tumor growth[14,38,39], and hepatic arterioportal shunts[18] are considered to directly induce an ischemic change leading to hypoxia, nutrition impairment, and dehydration in tumor. Interestingly, there are a few patients experienced profound systemic hypoperfusion associated with a massive gastrointestinal hemorrhage[40,41], hemodialysis[42,43], and surgical invasion[44], which also resulted in the spontaneous regression of the tumor. Uenishi et al[45] reported that the tumor was completely necrotic and had a thick fibrous capsule, in the tissue resected following the spontaneous regression of HCC; multiple inflammatory cells had also infiltrated into the tumor which is similar pattern with those found following embolization of HCC. Moreover, Matsuoka et al[20] reported the spontaneous regression of tumor with portal vein tumor thrombosis confirmed by the histopathological analyses of surgically removed tissues. These results indicate that the hypoxia in the tumor tissue may contribute to the spontaneous regression of tumor.
It is well known that immunological reactions inhibit tumor growth, including HCC. Immunological reactions are reported to be the main mechanism of spontaneous regression of HCC[17,34]. Abstinence from alcohol[12,46,47] and smoking[12], herbal medicines[11,25], prolonged fever[26,48,49], antidiabetics[50], and vitamin K administration[51] are considered to induce systemic immunological reaction with various patterns. Evdokimova et al[52] reported that APC-specific cluster of differentiation (CD) 4 positive T lymphocytes exhibit an anti-tumor capacity for HCC. In addition, Wada et al[53] reported that patients of HCC with marked inflammatory cell infiltration are associated with a better prognosis compared to without infiltration, which can be attributed to the anti-tumor effect induced by cellular immunity of CD8 positive and CD4 positive T lymphocytes. Furthermore, in spontaneous HCC regression case reports, an increase in interleukin (IL)-18[54], tumor necrosis factor (TNF)-alpha[55], and CD163 positive macrophages[21] were also reported and suggested the involvement of immunological reactions. These reports indicate that antitumor immunity can improve the prognosis for HCC under some conditions and the minute analyses of the mechanisms of spontaneous tumor regression can contribute to develop the novel therapeutic strategy.
Among 24 reports, histological characteristics were available in 9 patients. Well differentiated, moderately differentiated, poorly differentiated, and combined tumors were seen in 2, 3, 3, and 1 patients, respectively. Although several cases described no relapses during the long-term follow-up without any additional therapies after the spontaneous regression[17,25]; however, tumor remnant[29] and recurrences[14,56] were also observed in some cases.
In our reviewed cases, additional therapies following spontaneous regression were performed in 11 patients: hepatic resection in 8 patients, TACE in 2 patients, and conventional radiation therapy in 1 patient. Histopathological findings of the resected hepatic specimens showed necrotic tissue; several cases also revealed inflammatory cell infiltration at the tissue periphery[12,21]. Remnant tumor, persistence of tumor cell, was confirmed by the imaging modalities in 2 patients after the spontaneous regression. In contrast, local tumor recurrence, relapse of HCC was seen in 6 patients (25%), 5 of them had received no additional therapy and 1 patient had underwent TACE as an additional therapy (Table 1). Interestingly, among 11 patients underwent additional therapies, recurrence was seen in only 1 patient. Based on the information summarized from our review, spontaneous regression can reduce the volume of HCC and improves the patient’s prognosis[56]; however, the additional therapies including surgical treatment, radiation, TACE, etc. are recommended to be performed after the spontaneous regression of HCC if the hepatic function of the patient permitted[12] and if the imaging modalities suggest the remnant or relapse of the tumor.
The minute review for spontaneous regression of HCC showed that the spontaneous regression of HCC can frequently be seen in males in East Asian countries. Their tumors varies in terms of histological types, size, and clinical courses. Additional therapies following the spontaneous regression should be considered. The mechanisms might include hypoxia and immunological responses as previously reported. Since immunotherapeutic approaches in oncology may be most efficacious for cancers susceptible to intrinsic immune modulation[57,58] and recently, an increasing understanding of tumor immunogenicity spurred the development of novel classes of immune-targeted therapies, including the inhibition of co-stimulatory pathways mediated by cytotoxic T lymphocyte-associated antigen-4, as well as the programmed death-1 (PD-1) receptor and its ligand PD-L1[22]. Based on these progress, the analyses of mechanisms involved in the spontaneous tumor regression might contribute to develop a novel therapies for HCC. Therefore, it is clear that the accumulation of additional cases and analyses are necessary.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chiu KW, Mihaila R, Wakui N, Wang LT S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 2. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2881] [Cited by in RCA: 3088] [Article Influence: 220.6] [Reference Citation Analysis (0)] |
| 3. | Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:7-11. [PubMed] |
| 4. | Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2010;40:943-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Xie B, Wang DH, Spechler SJ. Sorafenib for treatment of hepatocellular carcinoma: a systematic review. Dig Dis Sci. 2012;57:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 6. | Higuchi T, Kikuchi M, Okazaki M. Hepatocellular carcinoma after transcatheter hepatic arterial embolization. A histopathologic study of 84 resected cases. Cancer. 1994;73:2259-2267. [PubMed] |
| 7. | Cole WH, Everson TC. Spontaneous regression of cancer: preliminary report. Ann Surg. 1956;144:366-383. [PubMed] |
| 8. | Cole WH. Efforts to explain spontaneous regression of cancer. J Surg Oncol. 1981;17:201-209. [PubMed] |
| 9. | Iwanaga T. [Studies on cases of spontaneous regression of cancer in Japan in 2011, and of hepatic carcinoma, lung cancer and pulmonary metastases in the world between 2006 and 2011]. Gan To Kagaku Ryoho. 2013;40:1475-1487. [PubMed] |
| 10. | Johnson FL, Lerner KG, Siegel M, Feagler JR, Majerus PW, Hartmann JR, Thomas ED. Association of androgenic-anabolic steroid therapy with development of hepatocellular carcinoma. Lancet. 1972;2:1273-1276. [PubMed] |
| 11. | Jianxin C, Qingxia X, Junhui W, Qinhong Z. A Case of Recurrent Hepatocellular Carcinoma Acquiring Complete Remission of Target Lesion With Treatment With Traditional Chinese Medicine. Integr Cancer Ther. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Saito R, Amano H, Abe T, Fujikuni N, Nakahara M, Yonehara S, Teramen K, Noriyuki T. Complete spontaneous necrosis of hepatocellular carcinoma confirmed on resection: A case report. Int J Surg Case Rep. 2016;22:70-74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Pectasides E, Miksad R, Pyatibrat S, Srivastava A, Bullock A. Spontaneous Regression of Hepatocellular Carcinoma with Multiple Lung Metastases: A Case Report and Review of the Literature. Dig Dis Sci. 2016;61:2749-2754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Alam MA, Das D. Spontaneous Regression of Hepatocellular Carcinoma-a Case Report. J Gastrointest Cancer. 2017;48:194-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Kumar A, Le DT. Hepatocellular Carcinoma Regression After Cessation of Immunosuppressive Therapy. J Clin Oncol. 2016;34:e90-e92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Yang SZ, Zhang W, Yuan WS, Dong JH. Recurrence of Hepatocellular Carcinoma With Epithelial-Mesenchymal Transition After Spontaneous Regression: A Case Report. Medicine (Baltimore). 2015;94:e1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Okano A, Ohana M. Spontaneous regression of hepatocellular carcinoma: its imaging course leading to complete disappearance. Case Rep Oncol. 2015;8:94-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Takeda Y, Wakui N, Asai Y, Dan N, Yamauchi Y, Ueki N, Otsuka T, Oba N, Nishinakagawa S, Minagawa M. Spontaneous complete necrosis of hepatocellular carcinoma: A case report and review of the literature. Oncol Lett. 2015;9:1520-1526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Kim SB, Kang W, Shin SH, Lee HS, Lee SH, Choi GH, Park JY. Spontaneous neoplastic remission of hepatocellular carcinoma. Korean J Gastroenterol. 2015;65:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Matsuoka S, Tamura A, Moriyama M, Fujikawa H, Mimatsu K, Oida T, Sugitani M. Pathological evidence of the cause of spontaneous regression in a case of resected hepatocellular carcinoma. Intern Med. 2015;54:25-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Wang Z, Ke ZF, Lu XF, Luo CJ, Liu YD, Lin ZW, Wang LT. The clue of a possible etiology about spontaneous regression of hepatocellular carcinoma: a perspective on pathology. Onco Targets Ther. 2015;8:395-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 22. | Parks AL, McWhirter RM, Evason K, Kelley RK. Cases of spontaneous tumor regression in hepatobiliary cancers: implications for immunotherapy? J Gastrointest Cancer. 2015;46:161-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Saito T, Naito M, Matsumura Y, Kita H, Kanno T, Nakada Y, Hamano M, Chiba M, Maeda K, Michida T. Spontaneous regression of a large hepatocellular carcinoma with multiple lung metastases. Gut Liver. 2014;8:569-574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Bhardwaj N, Li M, Price T, Maddern GJ. Spontaneous regression of a biopsy confirmed hepatocellular carcinoma. BMJ Case Rep. 2014;2014:pii: bcr2014204897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Lim DH, Park KW, Lee SI. Spontaneous complete regression of multiple metastases of hepatocellular carcinoma: A case report. Oncol Lett. 2014;7:1225-1228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Tomino T, Yamashita Y, Iguchi T, Itoh S, Ninomiya M, Ikegami T, Yoshizumi T, Soejima Y, Kawanaka H, Ikeda T. Spontaneous massive necrosis of hepatocellular carcinoma with narrowing and occlusion of the arteries and portal veins. Case Rep Gastroenterol. 2014;8:148-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Tsai SC, Kao JL, Shiao CC. Spontaneous regression of a hepatoma with ring calcification. Acta Clin Belg. 2014;69:130-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Okano A, Ohana M, Kusumi F, Nabeshima M. Spontaneous Regression of Hepatocellular Carcinoma due to Disruption of the Feeding Artery. Case Rep Oncol. 2013;6:180-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Sasaki T, Fukumori D, Yamamoto K, Yamamoto F, Igimi H, Yamashita Y. Management considerations for purported spontaneous regression of hepatocellular carcinoma: a case report. Case Rep Gastroenterol. 2013;7:147-152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Tomishige H, Morise Z, Mizoguchi Y, Kawabe N, Nagata H, Ohshima H, Kawase J, Arakawa S, Yoshida R, Isetani M. A case of solitary necrotic nodule treated with laparoscopic hepatectomy: spontaneous regression of hepatocellular carcinoma? Case Reports Hepatol. 2013;2013:723781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | van Halteren HK, Salemans JM, Peters H, Vreugdenhil G, Driessen WM. Spontaneous regression of hepatocellular carcinoma. J Hepatol. 1997;27:211-215. [PubMed] |
| 32. | Oquiñena S, Guillen-Grima F, Iñarrairaegui M, Zozaya JM, Sangro B. Spontaneous regression of hepatocellular carcinoma: a systematic review. Eur J Gastroenterol Hepatol. 2009;21:254-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 33. | Oquiñena S, Iñarrairaegui M, Vila JJ, Alegre F, Zozaya JM, Sangro B. Spontaneous regression of hepatocellular carcinoma: three case reports and a categorized review of the literature. Dig Dis Sci. 2009;54:1147-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 34. | Huz JI, Melis M, Sarpel U. Spontaneous regression of hepatocellular carcinoma is most often associated with tumour hypoxia or a systemic inflammatory response. HPB (Oxford). 2012;14:500-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Heianna J, Miyauchi T, Suzuki T, Ishida H, Hashimoto M, Watarai J. Spontaneous regression of multiple lung metastases following regression of hepatocellular carcinoma after transcatheter arterial embolization. A case report. Hepatogastroenterology. 2007;54:1560-1562. [PubMed] |
| 36. | Imaoka S, Sasaki Y, Masutani S, Ishikawa O, Furukawa H, Kabuto T, Kameyama M, Ishiguro S, Hasegawa Y, Koyama H. Necrosis of hepatocellular carcinoma caused by spontaneously arising arterial thrombus. Hepatogastroenterology. 1994;41:359-362. [PubMed] |
| 37. | Takayasu K, Muramatsu Y, Shima Y, Moriyama N, Yamada T, Yoshida T, Makuuchi M, Kishi K. Necrosis of hepatocellular carcinoma as a result of subintimal injury incurred by hepatic angiography: report of two cases. Am J Gastroenterol. 1986;81:979-983. [PubMed] |
| 38. | Nakajima T, Moriguchi M, Watanabe T, Noda M, Fuji N, Minami M, Itoh Y, Okanoue T. Recurrence of hepatocellular carcinoma with rapid growth after spontaneous regression. World J Gastroenterol. 2004;10:3385-3387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Iwasaki M, Furuse J, Yoshino M, Moriyama N, Kanemoto H, Okumura H. Spontaneous regression of hepatocellular carcinoma: a case report. Jpn J Clin Oncol. 1997;27:278-281. [PubMed] |
| 40. | Kondo S, Okusaka T, Ueno H, Ikeda M, Morizane C. Spontaneous regression of hepatocellular carcinoma. Int J Clin Oncol. 2006;11:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Tocci G, Conte A, Guarascio P, Visco G. Spontaneous remission of hepatocellular carcinoma after massive gastrointestinal haemorrhage. BMJ. 1990;300:641-642. [PubMed] |
| 42. | Sato Y, Fujiwara K, Nakagawa S, Kanishima S, Ohta Y, Oka Y, Hayashi S, Oka H. A case of spontaneous regression of hepatocellular carcinoma with bone metastasis. Cancer. 1985;56:667-671. [PubMed] |
| 43. | Harimoto N, Shirabe K, Kajiyama K, Gion T, Takenaka M, Nagaie T, Maehara Y. Spontaneous regression of multiple pulmonary recurrences of hepatocellular carcinoma after hepatectomy: report of a case. Surg Today. 2012;42:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Iijima H, Moriwaki Y, Yamamoto T, Takahashi S, Nishigami T, Hada T. Spontaneous regression of hepatic adenoma in a patient with glycogen storage disease type I after hemodialysis: ultrasonographic and CT findings. Intern Med. 2001;40:891-895. [PubMed] |
| 45. | Uenishi T, Hirohashi K, Tanaka H, Ikebe T, Kinoshita H. Spontaneous regression of a large hepatocellular carcinoma with portal vein tumor thrombi: report of a case. Surg Today. 2000;30:82-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Lee HS, Lee JS, Woo GW, Yoon JH, Kim CY. Recurrent hepatocellular carcinoma after spontaneous regression. J Gastroenterol. 2000;35:552-556. [PubMed] |
| 47. | Grossmann M, Hoermann R, Weiss M, Jauch KW, Oertel H, Staebler A, Mann K, Engelhardt D. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1995;90:1500-1503. [PubMed] |
| 48. | Markovic S, Ferlan-Marolt V, Hlebanja Z. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1996;91:392-393. [PubMed] |
| 49. | Stoelben E, Koch M, Hanke S, Lossnitzer A, Gaertner HJ, Schentke KU, Bunk A, Saeger HD. Spontaneous regression of hepatocellular carcinoma confirmed by surgical specimen: report of two cases and review of the literature. Langenbecks Arch Surg. 1998;383:447-452. [PubMed] |
| 50. | Kato H, Nakamura M, Muramatsu M, Orito E, Ueda R, Mizokami M. Spontaneous regression of hepatocellular carcinoma: two case reports and a literature review. Hepatol Res. 2004;29:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Nouso K, Uematsu S, Shiraga K, Okamoto R, Harada R, Takayama S, Kawai W, Kimura S, Ueki T, Okano N. Regression of hepatocellular carcinoma during vitamin K administration. World J Gastroenterol. 2005;11:6722-6724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 52. | Evdokimova VN, Liu Y, Potter DM, Butterfield LH. AFP-specific CD4+ helper T-cell responses in healthy donors and HCC patients. J Immunother. 2007;30:425-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Wada Y, Nakashima O, Kutami R, Yamamoto O, Kojiro M. Clinicopathological study on hepatocellular carcinoma with lymphocytic infiltration. Hepatology. 1998;27:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 301] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 54. | Abiru S, Kato Y, Hamasaki K, Nakao K, Nakata K, Eguchi K. Spontaneous regression of hepatocellular carcinoma associated with elevated levels of interleukin 18. Am J Gastroenterol. 2002;97:774-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Ohba K, Omagari K, Nakamura T, Ikuno N, Saeki S, Matsuo I, Kinoshita H, Masuda J, Hazama H, Sakamoto I. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575-577. [PubMed] |
| 56. | Ohtani H, Yamazaki O, Matsuyama M, Horii K, Shimizu S, Oka H, Nebiki H, Kioka K, Kurai O, Kawasaki Y. Spontaneous regression of hepatocellular carcinoma: report of a case. Surg Today. 2005;35:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Maio M. Melanoma as a model tumour for immuno-oncology. Ann Oncol. 2012;23 Suppl 8:viii10-viii14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 58. | Scott M, Lawrance J, Dennis M. Regression of a renal cell carcinoma following allogeneic peripheral blood stem cell transplant for acute myeloid leukaemia: evidence of a graft-versus-tumour effect without significant graft-versus-host disease. Br J Haematol. 2012;159:1. [PubMed] |