Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.373
Peer-review started: July 25, 2016
First decision: August 22, 2016
Revised: September 6, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: January 14, 2017
Processing time: 174 Days and 7.8 Hours
Posterior reversible encephalopathy syndrome (PRES) is a neuro-radiologic diagnosis that has become more widely recognized and reported over the past few decades. As such, there are a number of known risk factors that contribute to the development of this syndrome, including volatile blood pressures, renal failure, cytotoxic drugs, autoimmune disorders, pre-eclampsia, and eclampsia. This report documents the first reported case of PRES in a patient with severe alcoholic hepatitis with hepatic encephalopathy and delves into a molecular pathophysiology of the syndrome.
Core tip: Posterior reversible encephalopathy syndrome (PRES) has been described in a number of settings, but not in the setting of severe alcoholic hepatitis, as is presented in this case report. There are clear molecular relationships between ammonia, which is detoxified to glutamine in the brain, causing astrocytic swelling, cerebral edema, and vasogenic edema. This vasogenic edema is a pivotal component of PRES and accounts for one of the major hypotheses of the syndrome. Thus, though a clear connection between hyperammonemia and PRES has never been documented, there is a theoretical relationship.
- Citation: John ES, Sedhom R, Dalal I, Sharma R. Posterior reversible encephalopathy syndrome in alcoholic hepatitis: Hepatic encephalopathy a common theme. World J Gastroenterol 2017; 23(2): 373-376
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/373.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.373
Posterior reversible encephalopathy syndrome (PRES) is a disorder characterized by various acute neurological symptoms and has been increasingly recognized over the past two decades due to advances in brain imaging. It is identified radiographically by subcortical vasogenic brain edema. PRES has been documented in patients with renal failure, labile blood pressure, cytotoxic drugs, autoimmune disorders, pre-eclampsia and eclampsia[1]. There has been one documented case of PRES in a patient with cirrhosis who presented with gastrointestinal bleeding, hypotension and hepatic encephalopathy[2]. We present the first reported case of PRES in the setting of severe alcoholic hepatitis with hepatic encephalopathy and the absence of the known predisposing factors described to date.
A 40-year-old female was readmitted to the hospital with a seizure following a 3-wk admission for hepatic encephalopathy due to severe alcoholic hepatitis. The patient returned to the hospital in less than 24 h of discharge following a witnessed tonic-clonic seizure at home. She had no prior history of seizures. She did not consume alcohol or non-prescription drugs between discharge and readmission. She reported compliance with prescribed medications at home.
During the preceding hospitalization, the patient presented with altered mental status, fever, jaundice, tender hepatomegaly, and a white blood cell count of 14.1 thousand/μL. Altered mental status was gauged by the West Haven Criteria, by which the patient had grade 3 hepatic encephalopathy. Her discriminant function was 99. Hepatic dysfunction was characterized by albumin of 3.0 g/dL, international normalized ratio (INR) of 2.36, ammonia of 300 mcg/dL, and bilirubin of 30.3 mg/dL. Her aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels were 241 IU/L and 62 IU/L, respectively. Body mass index was 16.5. Clinical and radiographic features were suggestive of chronic liver disease, including encephalopathy, ascites, asterixis, spider angiomata and esophageal varices without active gastrointestinal bleeding. Liver biopsy and histology were not obtained as the results would not affect management. Her serum ascites albumin gradient was 3.8 gm/dL and confirmed portal hypertension. Despite appropriate therapy with lactulose and rifaximin, the patient remained grade 3 hepatic encephalopathy. Thus, a magnetic resonance imaging (MRI) examination was performed. Although it was a limited study due to patient movement, bilateral temporal parietal restriction was described, raising concern for PRES. There was no evidence of seizure activity on 60-min electroencephalography (EEG) at the time. Despite mild intermittent headaches, she remained stable without focal neurologic deficits, and was discharged home on the recommended steroid taper for alcoholic hepatitis, ciprofloxacin for spontaneous bacterial peritonitis prophylaxis, fluconazole for candidal esophagitis found on upper endoscopy, nadolol for grade 1 esophageal nonbleeding varices, lactulose and rifaximin for hepatic encephalopathy, and spironolactone and furosemide for ascites.
The patient was readmitted in less than 24 h following a witnessed tonic-clonic seizure. She was intubated for airway protection and rapidly extubated within 24 h. Her admission vital signs included a temperature of 97.2 F, pulse of 95 beats/min, respiratory rate of 8 breaths/min and a blood pressure of 114/78 mmHg. Off sedation, there were no focal neurologic findings. Labs were significant for hemoglobin of 10.0 g/dL, INR of 1.79, prothrombin time of 19.4 s, creatinine of 0.3 mg/dL, bicarbonate of 15.5 mmol/L, anion gap of 21 mEq/L, total bilirubin of 10.8 mg/dL, direct bilirubin of 6.5 mg/dL, alkaline phosphatase of 133 IU/L, ALT of 54 IU/L, and AST of 112 IU/L, all relatively unchanged from her discharge labs. Urine drug screen was negative and alcohol level was undetectable.
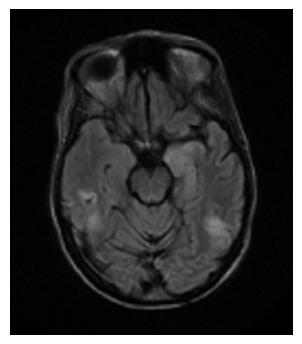
A repeat MRI was performed while the patient was post-ictal and grade 3 HE, which showed a high signal intensity in the subcortical, and periventricular white matter of the bilateral temporal and parietal lobes on the fluid attenuated inversion recovery (FLAIR) sequence of MRI was consistent with PRES (Figure 1). The MRI was unchanged from the previous MRI, though it was better quality because there was less artifact from patient movement. EEG showed high focal epileptogenic potential in this same temporal-parietal area. Subsequent neurologic exam revealed right visual field deficits, and psychomotor retardation with subjective complaint of headaches, but no asterixis or other focal deficits. She was started on lacosamide for further seizure prevention and continued on lactulose and rifaximin. Fluconazole was switched to itraconazole to prevent the lowering of her seizure threshold. Prior to discharge, her neurologic deficits and headaches had completely resolved.
PRES, first described in 1996 in the New England Journal of Medicine, is a clinic neuro-radiological diagnosis. While the pathogenesis of PRES is not fully understood, two prevailing hypotheses have been proposed, but neither has been fully validated thus far. The more popular theory purports that severe and rapidly developing hypertension can devastate auto-regulation, resulting in hyperperfusion with endothelial injury/vasogenic edema[3]. The posterior brain is more affected by hyperperfusion, as minimal sympathetic innervation exists in the posterior fossa. The original hypothesis conversely suggests that hypoperfusion causes vasoconstriction, resulting in brain ischemia and consequent vasogenic edema[4].
The increased intrahepatic resistance from cirrhosis causes portal hypertension, and is worsened by hepatic and non-hepatic endothelial dysfunction[4], a component found in the prevalent theory of PRES pathophysiology. Specifically, hypoactive endothelial cells decrease nitric oxide production that consequently initiates portal hypertension. This, in turn, results in endothelial dysfunction in the splanchnic and systemic circulation (extrahepatic). There is a subsequent superfluous amount of vasodilators, resulting in vasodilation that further contributes to exacerbating the portal hypertension[4].
The pathophysiology of hepatic encephalopathy is not completely understood, but ammonia has been recognized as a pivotal player in the process. There are two forms of ammonia - ammonium (NH4+) and ammonia (NH3) - the latter of which is more predominant in alkalotic states and described in approximately 70% of patients with decompensated liver disease[5]. As the brain has no inherent cycle of urea metabolism, ammonia reaching the astrocytes is detoxified by glutamine synthetase in the presence of glutamate to form glutamine. Glutamate is a major transmitter involved in neuro-excitation in 80% of synapses. Glutamine over-production promotes swelling of astrocytes, which results in cerebral edema, resulting in intracranial pressure[6]. In fact, using magnetic resonance diffusion tensor imaging, an increase in interstitial brain water in patients with cirrhosis and hepatic encephalopathy has been shown. In this study, the higher grades of hepatic encephalopathy corresponded to an increased brain water content. Similarly, treating hyperammonemia resulted in decreased brain water content[7]. Lastly, hyperammonemia has been implicated in the dysregulation of cerebral blood flow and consequent cerebral vasodilation causing vasogenic edema[8,9]. It is the presence of additional vasogenic edema that has also been implicated in the specific pathogenesis of PRES. As evidenced, portal hypertension and hepatic encephalopathy result in changes that are concurrent with the changes found in PRES. However, there is a dearth of literature regarding PRES and hepatic encephalopathy in the setting of portal hypertension irrespective of the underlying etiology.
Clinical manifestations of PRES have been variable. Most frequently it presents as various degrees of encephalopathy or seizures. It can also manifest as headaches, visual disturbances, other focal neurological deficits, or status epileptics[10]. Symptoms are usually acute or subacute. As stated earlier, symptoms are most often seen in the setting of renal failure, labile blood pressures, autoimmune disorders, preeclampsia/eclampsia or cytotoxic or immunosuppressive drugs such as calcineurin inhibitors, cyclosporine, and cisplatin, but not steroids[10]. The patient was on steroids, but there has been no defined relationship in the literature between the use of steroids and PRES. There have been sparse case reports of PRES occurring in patients with acute hepatic failure[11], and one in a cirrhotic patient[1] with hepatic encephalopathy. Our patient was hemodynamically stable and did not present with any of these known risk factors. Her ammonia level was significantly elevated despite lactulose and rifaximin therapy. The hyperammonemia could have been the triggering factor for developing PRES, while the fluconazole used to treat esophageal candidiasis likely lowered her seizure threshold, resulting in the witnessed tonic-clonic seizure.
Radiologic confirmation is an important component of diagnosis. Neuroradiologic images of PRES show characteristic white-gray matter edema predominantly involving the posterior region of the brain and best seen with brain MRI. Our patient had bilateral temporal and parietal lobe findings, as seen in Figure 1, which are two common areas that are affected in PRES. It can also frequently be seen in the parieto-occipital region, watershed regions, and frontal lobes.
Treatment of PRES has not been well studied; symptoms usually resolve once the underlying cause has been treated. Patients with seizures, such as our patient, should be placed on anti-epileptic medications. Once the underlying disorder has been treated, prognosis is usually favorable. Our case poses an interesting challenge because it introduces another potential etiology of PRES, which has not been studied in the past. Further research is needed to better understand the pathophysiology of PRES, a neurologic entity that can occur in many clinical conditions. Other possible etiologies, including exaggerated immune response or cytokine release may enhance systemic endothelial activation. This case adds to the literature of potential etiologies leading to PRES, an extremely rare clinical and radiologic diagnosis.
In conclusion, this is the first reported case of acute alcoholic hepatitis with hepatic encephalopathy developing PRES in the absence of known risk factors such as hypotension, ischemia, and blood pressure fluctuations.
The patient is a known alcoholic, who had presented with altered mental status.
On physical exam, the patient had tender hepatomegaly, jaundice, asterixis, and altered mental status consistent with grade 3 hepatic encephalopathy; on her readmission, she presented with a post-ictal state after having a witnessed seizure.
The seizures were attributed to posterior reversible encephalopathy syndrome (PRES), but other things considered included alcohol withdrawal, hepatic encephalopathy, and drug abuse.
The patient was initially admitted with fever, jaundice, tender hepatomegaly, leukocytosis, hyperammonemia, and a history of heavy alcohol abuse. Upon her re-admission when she presented with seizures; she was re-admitted with similar labs.
The imaging was the crux of the case, as magnetic resonance imaging revealed PRES, though the patient did not have any of the typical risk factors for developing PRES.
There were no pathological diagnoses made in this case.
The patient was treated with lactulose and rifaximin on her first admission; during the re-admission, she was treated with lacosamide, and continued with the treatment of hepatic encephalopathy, including lactulose and rifaximin.
There is one other case of PRES in a patient with cirrhosis who presented with gastrointestinal bleeding, hypotension and hepatic encephalopathy, however the case presented in this report is unique in that there is no associated volatile blood pressure, which is well documented as a cause of PRES.
PRES: Posterior reversible encephalopathy syndrome is a radiographic condition that has been well documented in patients with renal failure, labile blood pressure, cytotoxic drugs, autoimmune disorders, pre-eclampsia and eclampsia.
It is crucial to recognize the relationship between hyperammonemia and hepatic encephalopathy and PRES as a side effect as avoiding medications that can further lower the seizure threshold.
The strengths of this article include the novel proposed pathophysiologic relationship between hyperammonemia and PRES, an association that has not been explored in the past. The weakness of this article is that there was no liver biopsy to prove hepatic impairment, though this can be clinically determined.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: McMillin MA, Yadav SK S- Editor: Yu J L- Editor: Filipodia E- Editor: Liu WX
| 1. | Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015;14:914-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 716] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 2. | Chawla R, Smith D, Marik PE. Near fatal posterior reversible encephalopathy syndrome complicating chronic liver failure and treated by induced hypothermia and dialysis: a case report. J Med Case Rep. 2009;3:6623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Bartynski WS, Boardman JF. Catheter angiography, MR angiography, and MR perfusion in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 199] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Iwakiri Y, Groszmann RJ. Vascular endothelial dysfunction in cirrhosis. J Hepatol. 2007;46:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Butterworth RF. Pathogenesis of hepatic encephalopathy in cirrhosis: the concept of synergism revisited. Metab Brain Dis. 2015; Nov 2; Epub ahead of print. [PubMed] |
| 6. | Lemberg A, Fernández MA. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann Hepatol. 2009;8:95-102. [PubMed] |
| 7. | Kale RA, Gupta RK, Saraswat VA, Hasan KM, Trivedi R, Mishra AM, Ranjan P, Pandey CM, Narayana PA. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 8. | Larsen FS, Adel Hansen B, Pott F, Ejlersen E, Secher NH, Paulson OB, Knudsen GM. Dissociated cerebral vasoparalysis in acute liver failure. A hypothesis of gradual cerebral hyperaemia. J Hepatol. 1996;25:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 60] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Blei AT, Larsen FS. Pathophysiology of cerebral edema in fulminant hepatic failure. J Hepatol. 1999;31:771-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Servillo G, Bifulco F, De Robertis E, Piazza O, Striano P, Tortora F, Striano S, Tufano R. Posterior reversible encephalopathy syndrome in intensive care medicine. Intensive Care Med. 2007;33:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Mettananda S, Fernando AD, Ginige N. Posterior reversible encephalopathy syndrome in a survivor of valproate-induced acute liver failure: a case report. J Med Case Rep. 2013;7:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |