Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.297
Peer-review started: October 7, 2016
First decision: November 9, 2016
Revised: November 22, 2016
Accepted: December 8, 2016
Article in press: December 8, 2016
Published online: January 14, 2017
Processing time: 100 Days and 5.2 Hours
To assess the effect of long-term oral nucleos(t)ide analogues (NUCs) therapy on liver volume change in patients with suppress hepatitis B virus (HBV)-related liver cirrhosis.
We reviewed the data of naïve patients with HBV-related liver cirrhosis, who had taken oral NUCs therapy, between 2003 and 2007 at Chonbuk University Hospital. We analyzed two consecutive sets of abdominal computerized tomography scans-one at the time of treatment initiation and another at the second-year follow-up. Liver volume was calculated by 3-dimensional liver extraction volumetry program.
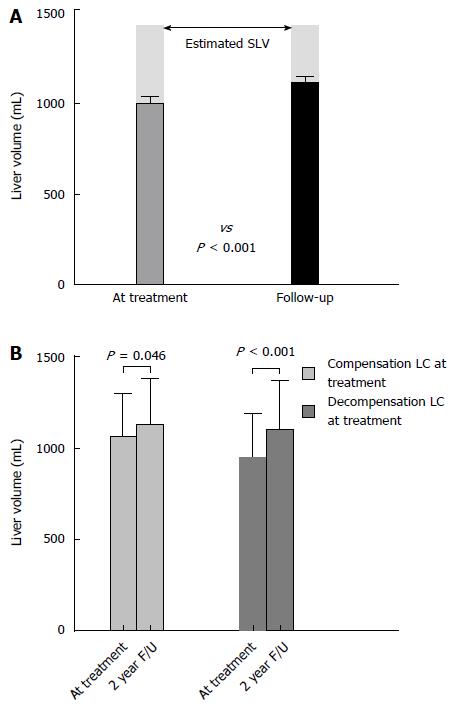
A total of 55 patients (34 males) were included. There was 114.3 mL ± 167.8 mL (12.9% ± 17.9%) of increase in liver volume during the two years of NUCs therapy (993.8 mL ± 242.8 mL at baseline vs 1108.1 mL ± 263.3 mL at two-year follow-up, P < 0.001). The ratio of the measured baseline liver volume to the estimated standard liver volume was improved from 70.8% to 78.0%. An increase in liver volume was shown not only in patients with compensated cirrhosis (P = 0.046) but also in those with decompensated cirrhosis (P < 0.001). Significant factors for volume increases were Child-Turcotte-Pugh grade and model for end-stage liver disease score improvement without virological breakthrough. In multiple linear regression analysis, delta albumin and delta alanine aminotransferase levels showed a significant association with the increase in liver volume (P = 0.002 and 0.005, respectively).
Long-term oral NUCs therapy in patients with HBV-related liver cirrhosis lead to significant increase in liver volume assessed with 3-dimensional liver extraction volumetry program.
Core tip: Liver volume change may represent the hepatic functional and regenerative capacity. We inspected the effect of oral nucleos(t)ide analogues (NUCs) on liver volume in patients with hepatitis B virus-related cirrhosis. The result showed the increase in liver volume during two years of NUCs therapy and the volume increase was shown both in compensated and decompensated cirrhosis. The increase in liver volume was well correlated with several markers representing hepatic functional status. More volume increase may be attained regarding aggressive damage at baseline and marked improvement during the treatment period. Our data support the reversal of fibrosis and enhancement of liver regeneration of damaged liver in patients with liver cirrhosis after the treatment with NUCs.
- Citation: Lee CH, Kim IH, Moon JC, Seo SY, Kim SH, Kim SW, Lee SO, Lee ST, Kim DG, Yang JD, Yu HC. 3-Dimensional liver volume assessment in patients with hepatitis B virus-related liver cirrhosis during long-term oral nucleos(t)ide analogues therapy. World J Gastroenterol 2017; 23(2): 297-305
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.297
Chronic hepatitis B virus (HBV) infection remains a major global public health problem with the potential to cause liver cirrhosis, hepatic decompensation, hepatocellular carcinoma (HCC), and liver-related mortality. According to studies from Taiwan regarding the Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer (REVEAL), serum HBV DNA levels were independent predictors for progression of chronic HBV infection[1,2]. Fortunately, oral nucleos(t)ide analogues (NUCs), including lamivudine (LAM), telbivudine (LdT), adefovir (ADV), entecavir (ETV), and tenofovir (TDF), have been approved to suppress HBV replication with potent reduction of serum HBV DNA levels and improvement of hepatic necroinflammation and fibrosis[3,4]. Furthermore, a long-term treatment with oral NUCs has been known to significantly reduce the rates of disease progression and development of HCC in patients with HBV-related cirrhosis[5,6].
Precise measurement of liver volume has been made possible due to the availability of rapid volumetric scanning and reconstruction techniques of modified discrete cosine transform (MDCT). Imaging-based volumetry has been increasingly utilized in current clinical practice to obtain accurate measurements of liver volume. This modality is particularly useful prior to major hepatic resection and living donor liver transplantation, where the size of the remnant liver and liver graft, respectively, affects procedural success and postoperative mortality and morbidity. Liver cirrhosis is a progressive condition accompanied by morphologic changes of the liver and deterioration of hepatic function. Clinically, Child-Turcotte-Pugh (CTP) classification and model for end-stage liver disease (MELD) score are mainly used in assessing the severity of liver cirrhosis. Previous studies demonstrated that changes in liver volume were positively correlated with CTP classification and could be used as a severity marker for liver cirrhosis[7-9]. However, there was no study that evaluated the changes of liver volume in cirrhotic patients undergoing oral NUCs therapy. In this study, we aimed to assess the effect of long-term oral NUCs therapy on the change of liver volume in patients with HBV-related liver cirrhosis using a 3-dimensional (D) virtual liver extraction measurement program.
This retrospective study included naïve HBV-related liver cirrhosis patients, who had received oral NUCs therapy for two years between January 2003 and December 2012 at Chonbuk National University Hospital. Patients were eligible if they had taken two abdominal computerized tomography (CT) scans-one at the time of treatment initiation and another at the second-year follow up. HBV-related cirrhosis was defined as a detectable serum hepatitis B surface antigen (HBsAg) level for more than 6 mo with clinical diagnosis of liver cirrhosis. Liver cirrhosis was clinically diagnosed based on compatible radiological findings of cirrhosis with one of the following three biochemical findings: platelet count of less than 100000/mm3, serum albumin level of less than 3.5 g/dL, and prothrombin time international normalized ratio of 1.3 or more[10]. Radiological findings compatible with liver cirrhosis included morphologic changes (nodularity of liver surface, atrophy of the right lobe, hypertrophy of the left and caudate lobe, expansion of the periportal space, and intrahepatic nodule), ascites, and presence of portal hypertension (presence of collateral vessels or splenomegaly)[10]. Based on the CTP score, patients with cirrhosis were categorized as compensated (CTP class A) or decompensated (CTP class B or C). Patients with the following were excluded from the study: history of previous NUCs treatment, space occupying lesions within the liver, hepatic vascular thrombosis, bile duct dilatation, and alcoholism. Additional exclusion criteria included co-infection with human immunodeficiency virus, toxic hepatitis, and congestive heart disease. During the study period, 192 patients with HBV-related liver cirrhosis were treated with oral NUCs for at least 24 mo. After excluding 137 of patients without consecutive CT images at baseline and 2-year follow-up data, a total of 55 naïve patients were included for final analysis. TDF was not available in Korea during the study period, and oral NUCs therapy was initiated on one of the NUCs, including LAM (100 mg), LdT (600 mg), and ETV (0.5 mg). ADV (10 mg) and ETV (1.0 mg) were used as a rescue drug for patients with treatment failure, defined as the emergence of virological breakthrough with or without genotypic resistance after the initial NUCs therapy. The strategies of oral NUCs therapy-treatment initiation, monitoring, and management of treatment failure-were conducted in accordance with the clinical practice guideline for chronic hepatitis B (CHB)[4].
Sociodemographic data, biochemical tests, including complete blood count (leukocytes, hemoglobin, platelets), liver function tests (aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, gamma glutamyl transpeptidase, alkaline phosphatase, and bilirubin), prothrombin time, renal tests (blood urea nitrogen, creatinine), alpha-fetoprotein, HBeAg, anti-HBe, and HBV DNA level, type of initial oral NUCs were collected at baseline. During the follow-up period, each visitation data included oral NUCs therapy-related outcomes (serum ALT, HBeAg, anti-HBe, serum HBV DNA, viral breakthrough, resistance, changing antivirals), any cirrhosis-related complications, HCC development, and death. Serum ALT was measured with an enzymatic assay and defined as normal if it was less than 40 IU/L. Serum HBsAg, anti-HBs, HBeAg, and anti-HBe were detected by an electrochemiluminescence immunoassay (Roche Diagnostics, Mannheim, Germany). Serum HBV DNA was quantified by a real-time polymerase chain reaction (PCR) assay using the COBAS Taq-Man HBV quantitative test (Roche Molecular Systems Inc., Branchburg, NJ, United States), which had a lower limit of quantification (20 IU/mL). Genotypic analysis of HBV DNA polymerase was performed in patients showing virological breakthrough during therapy using a matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS)-based genotyping assay, which was previously known as the Restriction Fragment Mass Polymorphism (RFMP) assay[11].
Liver volume was calculated from two consecutive sets of abdominal CT scans-one at the time of treatment initiation (baseline) and another at the second-year follow-up. We used CT scans that had been taken within three months at each time point. The Liver extraction software was Dr. Liver version 04.2013 (POSTECH Inc., Pohang, South Korea), which extracts liver information from abdominal CT images at the prephase, arterial phase, portal phase, and delayed phase[12,13]. We calculated the volume of liver parenchyma and compared the changes of liver volume between the baseline and follow-up. The standard liver volume (SLV) was estimated via an equation proposed by Yu et al[14] and the estimated SLV of enrolled patients was 1436.0 mL ± 170.2 mL. This study was conducted in compliance with the World Medical Association Declaration of Helsinki and was approved by the Ethics Committee at our institution.
A descriptive analysis of liver volume change during the treatment period was performed using a paired t-test for qualitative variables. Factors associated with volume change were analyzed by univariate and multiple linear regression analyses. A P value of less than 0.05 was considered statistically significant, and all reported P values were two sided. Statistical software package SPSS version 18.0 (SPSS Inc., Chicago, Ill) was used for analysis.
The baseline characteristics of patients are shown in Table 1. The mean age was 53.2 ± 9.3, and there were 34 males and 21 females. The mean body mass index was 24.0 ± 2.5 (ranged from 16.5 to 29.0), and 27 patients exceeded the upper normal range by more than 22.9. There were 23 patients who showed elevated ALT levels by two times or more. HBeAg negativity was shown in 21 patients, and only one patient showed HBV DNA titer level in undetectable range at baseline. The AFP level was elevated in 28 patients. The mean CTP score was 7.8 ± 2.3, and decompensated liver cirrhosis (LC) patients accounted for about 60% among the study population. The mean MELD score was 9.0 ± 4.9, and the estimated 90-d mortality rate was 0.05.
| Clinical features | Values1 |
| Total number | 55 |
| Age (yr) | 53.2 ± 9.3 |
| Sex , male: female | 34:21 |
| Body mass index (kg/m2) | 24.0 ± 2.5 |
| Diabetes mellitus | 11 (20.0) |
| Laboratory results | |
| WBC (× 103/μL) | 4507.6 ± 1703.3 |
| Hemoglobin (g/dL) | 13.2 ± 1.8 |
| Platelet (× 103/μL) | 91.9 ± 51.9 |
| Prothrombin time (INR) | 1.4 ± 0.3 |
| Total bilirubin (mg/dL) | 2.1 ± 2.0 |
| AST (IU/L) | 115.7 ± 118.2 |
| ALT (IU/L) | 109.8 ± 151.8 |
| ALP | 218.5 ± 89.0 |
| GGT | 100.8 ± 94.5 |
| Albumin (g/dL) | 3.5 ± 0.6 |
| Creatinine | 0.8 ± 0.2 |
| AFP | 142.4 ± 299.4 |
| HBeAg positive | 34 (61.8) |
| HBV DNA (log IU/mL) | 5.8 ± 1.2 |
| CTP score | 7.8 ± 2.3 |
| CTP grade (A/B/C) | 21 (38.2)/21 (38.2)/13 (23.6) |
| MELD score | 9.0 ± 4.9 |
After the 2-year treatment period, two patients showed elevated ALT level by two times or more, and HBeAg seroconversion was observed in 28 (50.9%) patients. HBV DNA undetectability was shown in 37 (67.3%) patients, and the mean HBV DNA level of overall patients was 2.0 ± 2.3. The mean CTP score was 5.9 ± 1.1 and the mean MELD score was 5.9 ± 2.5 at the second-year follow-up.
The overall changes of liver volume between the treatment initiation and second-year follow-up are shown in Figure 1. The liver volume at baseline and at second-year follow-up was 993.8 mL ± 242.8 mL and 1108.1 mL ± 263.3 mL, respectively. There was 114.3 mL ± 167.8 mL (12.9% ± 17.9%) of volume increase during the two-year treatment period. The measured liver volume from baseline to second-year follow-up showed an improvement; from 70.8% to 78.0% of estimated SLV (Figure 1A). Fourteen patients showed a volume decrease at the follow-up compared with that at baseline. Among them, 7 patients had experienced virological breakthrough and HCC had developed in 4 patients.
When divided into two groups-compensated liver cirrhosis (CTP class A) group and decompensated liver cirrhosis (CTP class B and C) group, the baseline liver volume was lower in the decompensated cirrhosis group. The volume changes between the two groups are shown in Figure 1B. Liver volume increase was shown not only in patients with compensated cirrhosis but also in those with decompensated cirrhosis. In patients with compensated liver cirrhosis, the baseline liver volume was 1063.7 mL ± 238.9 mL and the follow-up liver volume was 1127.9 mL ± 250.8 mL (P = 0.046). In patients with decompensated liver cirrhosis, the baseline liver volume was 950.0 mL ± 238.4 mL and the follow-up liver volume was 1095.9 mL ± 273.7 mL (P < 0.001).
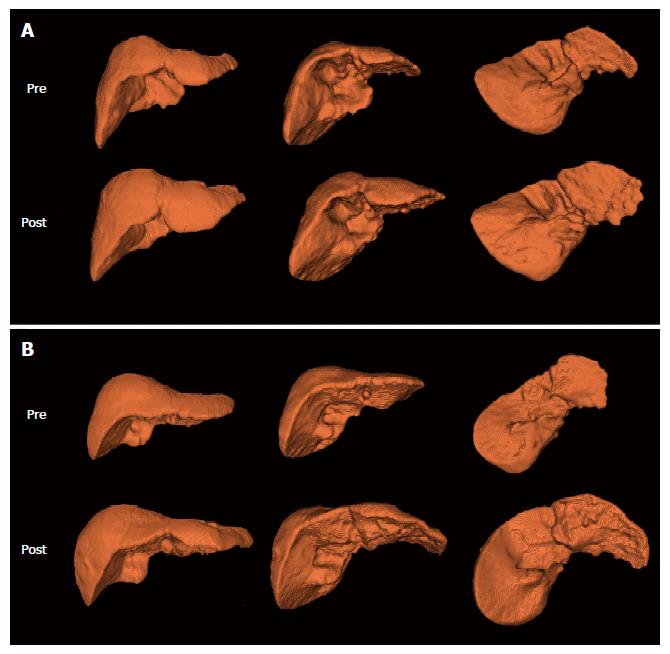
Representative 3-D images are shown in Figure 2. A representative case in the compensated liver cirrhosis group was a 71-year-old woman who showed an increase in liver volume by 321 mL (35.0%) after treatment (918 mL at baseline and 1239 mL at two years of follow-up) (Figure 2A). Her baseline AST, ALT, and total bilirubin levels were 67 IU/L, 31 IU/L, and 1.95 mg/dL, respectively, and her DNA titer was 8.25 × 106. IU/mL. At the follow-up, her AST, ALT, and total bilirubin levels were normalized, and her HBV DNA titer was within the undetectable range. A representative case in the decompensated liver cirrhosis group was a 48-year-old man, show showed an increase in liver volume by 555 mL (69.1%) during the two years of treatment period (803 mL of baseline liver volume and 1358 mL of follow-up liver volume) (Figure 2B). His baseline serum AST, ALT, and total bilirubin levels were 723 IU/L, 824 IU/L, and 5.7 mg/dL, respectively, and his HBV DNA titer was 1.62 × 106 IU/mL. After treatment, serum AST, ALT, and total bilirubin levels were normalized, and his HBV DNA titer was within the undetectable range.
Patients were divided into two groups in accordance with several independent variables, and the changes of liver volume were compared between the two groups. The results are shown in Table 2. The liver volume changes regarding baseline sex, initial NUCs, and presence of decompensation were not significantly different. Thirty-one patients showed an improvement in their CTP grade during the treatment period, revealing a significantly greater liver volume increase than those without CTP grade improvement (168.9 mL ± 164.8 mL vs 43.7 mL ± 146.6 mL, P < 0.005). The group with MELD score showed a decrease by more than 5, which also represented a significantly greater volume increase (P = 0.013). Virological breakthrough was developed in 13 patients; they showed a greater increase in liver volume than those without virological breakthrough (26.9 mL ± 153.3 mL vs 141.3 mL ± 164.5 mL, P = 0.030). Other variables, such as HCC development, virological response, virological resistance, and history of stop medication, tended to show reliable differences, but without statistical significance.
| Variables | No | Pre-Volume | Post-Volume | Volume change | Volume change (%) | P value |
| Male | 34 | 1089.6 ± 222.6 | 1210.9 ± 245.0 | 121.3 ± 189.5 | 12.6 ± 19.4 | 0.695 |
| Female | 21 | 838.8 ± 191.3 | 941.6 ± 202.6 | 102.8 ± 128.7 | 13.4 ± 15.5 | |
| Initial NUCs: LAM or LdT | 32 | 1022.2 ± 230.6 | 1125.1 ± 235.7 | 102.9 ± 155.2 | 11.4 ± 14.5 | 0.559 |
| Initial NUCs: ETV | 23 | 954.4 ± 258.8 | 1084.5 ± 301.4 | 130.0 ± 186.4 | 12.9 ± 17.9 | |
| Compensated LC | 21 | 1063.8 ± 238.9 | 1127.9 ± 250.8 | 64.1 ± 137.7 | 6.6 ± 12.5 | 0.081 |
| Decompensated LC | 34 | 950.6 ± 238.4 | 1095.9 ± 273.6 | 145.2 ± 178.9 | 16.7 ± 19.7 | |
| CTP grade improvement | 31 | 944.4 ± 224.4 | 1113.3 ± 271.6 | 168.9 ± 164.8 | 18.9 ± 18.8 | 0.005 |
| No CTP grade improvement | 24 | 1057.7 ± 255.3 | 1101.4 ± 257.7 | 43.7 ± 146.6 | 5.1 ± 13.2 | |
| MELD score decrease > 5 | 17 | 1012.9 ± 172.6 | 1209.9 ± 214.2 | 197.0 ± 199.2 | 21.1 ± 22.0 | 0.013 |
| No MELD score decrease > 5 | 38 | 985.3 ± 270.0 | 1062.6 ± 272.8 | 77.2 ± 139.3 | 9.2 ± 14.5 | |
| HCC development | 6 | 998.3 ± 202.8 | 990.0 ± 196.8 | -9.2 ± 124.3 | -0.5 ± 11.3 | 0.056 |
| No HCC development | 49 | 993.3 ± 249.1 | 1122.7 ± 268.2 | 129.4 ± 167.2 | 14.5 ± 17.9 | |
| Virological response | 38 | 974.1 ± 230.2 | 1111.1 ± 270.3 | 137.0 ± 162.8 | 15.1 ± 18.6 | 0.134 |
| No virological response | 17 | 1038.1 ± 271.0 | 1101.5 ± 254.8 | 63.4 ± 172.7 | 8.0 ± 15.5 | |
| Virological breakthrough | 13 | 1064.6 ± 255.3 | 1091.5 ± 168.7 | 26.9 ± 153.3 | 4.9 ± 14.5 | 0.030 |
| No virological breakthrough | 42 | 971.9 ± 237.7 | 1113.2 ± 287.8 | 141.3 ± 164.5 | 15.4 ± 18.2 | |
| Virological resistance | 8 | 992.0 ± 206.0 | 1059.3 ± 132.5 | 67.3 ± 129.7 | 8.9 ± 14.2 | 0.134 |
| No virological resistance | 41 | 969.0 ± 236.5 | 1125.0 ± 286.7 | 156.0 ± 154.1 | 16.8 ± 17.3 | |
| History of stop medication | 2 | 1369.5 ± 44.5 | 1257.0 ± 141.4 | -112.5 ± 96.9 | -8.3 ± 7.3 | 0.051 |
| Continuation of medication | 53 | 979.7 ± 235.7 | 1102.5 ± 265.9 | 122.8 ± 164.4 | 13.7 ± 17.7 |
A linear regression analysis was performed to investigate the association between liver volume change and variables. The results from a univariate linear regression analysis revealed that the baseline AST, ALT, total bilirubin, AFP, and CTP and MELD scores were significantly independent variables associated with liver volume change (Table 3). At the follow-up, prothrombin time, total bilirubin, and albumin levels were associated with liver volume change. In addition, delta AST, ALT, prothrombin time, total bilirubin, albumin, serum HBV DNA level, CTP score, and MELD score showed a significant association with liver volume change via a univariate linear regression analysis.
| Variables | R2 | β | Standard error | P value |
| Age | 0.002 | -0.766 | 2.473 | 0.758 |
| Body mass index | 0.000 | -0.419 | 10.708 | 0.969 |
| At treatment (baseline) | ||||
| WBC | 0.027 | 0.016 | 0.013 | 0.228 |
| Hemoglobin | 0.013 | 10.858 | 12.864 | 0.402 |
| Platelet | 0.000 | 0.050 | 0.445 | 0.912 |
| AST | 0.162 | 0.571 | 0.179 | 0.002 |
| ALT | 0.191 | 0.483 | 0.137 | 0.001 |
| Prothrombin time, INR | 0.061 | 157.794 | 85.278 | 0.070 |
| Total bilirubin | 0.101 | 26.334 | 10.797 | 0.018 |
| Albumin | 0.036 | -53.958 | 38.081 | 0.162 |
| Creatinine | 0.000 | -12.157 | 153.255 | 0.937 |
| AFP | 0.122 | 0.196 | 0.072 | 0.009 |
| Serum HBV DNA | 0.014 | 16.265 | 18.788 | 0.391 |
| CTP score | 0.070 | 18.954 | 9.470 | 0.050 |
| MELD score | 0.081 | 9.650 | 4.466 | 0.035 |
| Follow-up | ||||
| ALT | 0.013 | -0.936 | 1.135 | 0.414 |
| Prothrombin time, INR | 0.097 | -415.040 | 173.546 | 0.020 |
| Total bilirubin | 0.096 | -79.343 | 33.496 | 0.022 |
| Albumin | 0.165 | 147.769 | 45.702 | 0.002 |
| Creatinine | 0.015 | 106.249 | 116.561 | 0.366 |
| Serum HBV DNA | 0.035 | -13.407 | 9.720 | 0.174 |
| CTP score | 0.068 | -39.884 | 20.313 | 0.055 |
| MELD score | 0.009 | -6.209 | 9.098 | 0.498 |
| Delta (Follow-up - Baseline) | ||||
| ALT | 0.210 | -0.513 | 0.137 | < 0.001 |
| Prothrombin time, INR | 0.158 | -254.936 | 80.880 | 0.003 |
| Total bilirubin | 0.158 | -31.355 | 9.943 | 0.003 |
| Albumin | 0.237 | 132.268 | 32.604 | < 0.001 |
| Creatinine | 0.024 | 151.708 | 134.270 | 0.264 |
| Serum HBV DNA improvement (log) | 0.084 | 12.505 | 5.681 | 0.032 |
| CTP score | 0.236 | -49.313 | 12.183 | < 0.001 |
| MELD score | 0.126 | -12.873 | 4.655 | 0.008 |
| Duration of virological response | 0.000 | -0.016 | 0.176 | 0.930 |
According to the results of a multiple linear regression analysis, delta albumin (y = 105.006x + 31.820, P = 0.002) and delta ALT level (y = -0.388x + 0.131, P = 0.005) showed a significant association with the increase in liver volume (R2 = 0.347) (Table 4).
| R2 | β | Standard error | P value | |
| Delta albumin | 0.347 | 105.006 | 31.820 | 0.002 |
| Delta ALT | -0.388 | 0.131 | 0.005 |
Liver cirrhosis is a condition induced by a multifactorial chronic liver injury[15]. Liver cirrhosis induces various complications of portal hypertension and decompensation, such as variceal bleeding, ascites, spontaneous bacterial peritonitis, and encephalopathy, which may result in poor survival outcome[16]. Recently, there have been reports that showed not only the prevention of such complications, but also the regression of fibrosis and cirrhosis through a long-term treatment with oral NUCs[17,18]. Moreover, a sustained reduction of HBV replication has also been known to lower the risk of HCC in HBV-related cirrhosis[19].
Liver is one of the major regenerative organs. Liver regeneration is an important mechanism for maintaining liver function, and it is a well-orchestrated phenomenon, which is initiated when liver functional capacity is diminished[20]. After partial hepatectomy or portal vein embolization, the regeneration process is accelerated as a compensatory mechanism in normal livers. In cirrhotic patients, however, liver volume is commonly decreased with morphological change due to repeated hepatic damage. In addition, the regeneration process in cirrhosis patients after partial hepatectomy may be incomplete[8,21]. Liver volume increase in cirrhotic patients can be induced by reduced liver damage and enough restoration of functional capacity. By measuring liver volume change, we can suggest the regenerative capacity and the role of regeneration. Moreover, liver volume increase in patients with LC shows the potential for the reversal of fibrosis. Although liver biopsy is the gold standard for the assessment of hepatic fibrosis, and despite the introduction of many other measuring tools with functional capacity, liver volumetry can be a non-invasive and effective tool in clinical practice.
We analyzed the liver volume change during a two-year treatment period in patients with HBV-related liver cirrhosis. The two-year duration period was deemed appropriate based on previous studies showing that the liver regeneration process is usually completed within 3 to 6 mo after partial hepatectomy[20]. Unlike a hepatectomy state, treatment with NUCs is a restorative process of the damaged liver, and the effect of antiviral therapy may be represented slowly and minimally during the treatment period. Therefore, we set a prolonged period of observation time sufficient enough to observe the changes in liver volume post NUCs treatment. We hypothesized that taking NUCs would maintain a baseline status of liver volume, because it prevents hepatic damage and stops the fibrotic process.
In our study, the serum levels of AST and ALT at baseline were elevated with high HBV DNA levels, and two thirds of enrolled patients were in a decompensated status. The liver volume of liver cirrhosis patients was shown to decrease compared with the estimated standard liver volume (SLV) calculated by patients’ height and weight at baseline. The baseline liver volume of decompensated cirrhotic patients was lower than that of compensated cirrhotic patients. However, after treatment with NUCs, liver functional profiles were improved and the liver volume increased approximately up to the standard liver volume. Interestingly, liver volume increased in patients with compensated liver cirrhosis, as well as decompensated liver cirrhosis. Liver fibrosis or cirrhosis was considered irreversible in the past, but advancements in research in the field has shown that regression of liver fibrosis is possible[22]. Our study may support such reversal of cirrhotic liver as liver volume increases even in patients with decompensated LC after NUCs therapy. Administration of NUCs in both compensated and decompensated cirrhotic patients seems to be beneficial for maintaining the regeneration capacity and reversal of liver fibrosis.
Improvement of liver function can be represented by normalized AST, ALT, and bilirubin levels, elevated albumin level, and improved CTP and MELD scores. A prolonged sustained virological response without virological breakthrough or resistance is an important parameter correlated with the clearance of virus. We analyzed the liver volume change depending on these variables. In this study, there was a significantly greater increase of liver volume in patients with improvements in CTP and MELD scores by more than 5, when compared with others. Because these two parameters are well-known markers for the severity of liver cirrhosis, we suggest that improved clinical stage may induce more liver volume increase. Conversely, virologic breakthrough cases showed liver volume decrease. This suggests that continuous administration of NUCs in sustained virologic response is important in increasing liver volume. Other liver functional parameters also showed similar tendencies, but failed to have statistically significant differences. This result indicates that well-treated clinical states without virological breakthrough are important parameters for the regeneration capacity and reversal liver fibrosis.
Finally, we underwent a regression analysis to define the associated factors with liver volume change. Delta (differences between baseline and follow-up) scores were also analyzed because the alteration of parameters during the treatment period can be important in liver regeneration. Our data showed that several markers representing hepatic functional status are well correlated with liver volume change. Among them, in a multiple linear regression analysis, delta albumin and delta ALT scores were significantly correlated with liver volume change in this study. These markers can be useful for predicting liver volume change, liver regeneration capacity, and reversal of liver fibrosis. Because high ALT level at baseline and low ALT level cause high delta ALT score, more volume increase may be attained regarding aggressive damage at baseline and marked improvement during the treatment period. Delta albumin also represents improved synthetic liver function; thus, marked liver function improvement seems to cause marked volume increase. This result may show that aggressive damage at baseline and marked improvement during the treatment period may induce a greater liver volume increase, which may reflect a high rate of hepatic fibrosis reversal.
Our study has several limitations to consider. First, because we chose the duration of treatment for patients, we do not know the real-time liver volume change after NUCs treatment. Second, we did not divide the groups according to the medication type of NUCs. Moreover, we did not analyze segmental liver volume change. With regard to the 3-dimentional liver images, although the liver volume in decompensated liver cirrhosis patients tended to have a greater increase in the left side than in compensated liver cirrhosis patients, we were unable to measure the exact segmental volume change due to the limitation of this program. Previous studies showed that liver volume decreased in the right and left lobes and increased in the caudate lobe so that the analysis of segmental liver volume change may be helpful. Lastly, we did not analyze the associated factors, such as survival or complication rates, to predict the outcomes of patients. Despite these limitations, this study is, to the best of our knowledge, the first study measuring liver volume change after NUCs treatment in cirrhotic patients and supports the importance of antiviral therapy in CHB with liver cirrhosis patients.
In conclusion, a long-term oral NUCs therapy in patients with HBV-related liver cirrhosis significantly increased liver volume, and the volume increase was shown both in those with compensated and decompensated cirrhosis. The increase in liver volume was well correlated with several markers representing hepatic functional status, and significant variables associated with the increase in liver volume were delta albumin and delta ALT levels. Our data supports the reversal of fibrosis and enhancement of liver regeneration of damaged liver in patients with liver cirrhosis after the treatment with NUCs.
Long-term oral nucleos(t)ide analogues (NUCs) are known to suppress hepatitis B virus (HBV) replication effectively with an improvement of hepatic fibrosis.
Reversal of fibrosis and liver regeneration in patients with liver cirrhosis are important issues in the research field. Because liver volume change may represent the hepatic functional and regenerative capacity, assessing liver volume changes after long-term oral NUCs therapy in patients with HBV-relative liver cirrhosis could be highly remarkable in the clinical field.
Previous studies demonstrated that changes in liver volume were positively correlated with Child-Turcotte-Pugh classification and could be used as a severity marker for liver cirrhosis. However, there was no study that evaluated the changes of liver volume in cirrhotic patients undergoing oral NUCs therapy. The authors first analyzed the effect of long-term oral NUCs therapy on the change of liver volume in patients with HBV-related liver cirrhosis using a 3-dimensional (D) virtual liver extraction measurement program.
The result showed the increase in liver volume during two years of NUCs therapy and the volume increase was shown both in compensated and decompensated cirrhosis. Significant variables associated with the increase in liver volume were delta albumin and delta ALT levels. More volume increase may be attained regarding aggressive damage at baseline and marked improvement during the treatment period. This data support the reversal of fibrosis and enhancement of liver regeneration of damaged liver in patients with liver cirrhosis after the treatment with NUCs.
The authors found that NUCs therapy in these patients with HBV-related cirrhosis significantly increases liver volume and improves liver functions as measurements of ALT/AST show. This is the first study which evaluates liver volume in cirrhotic patients. This work is highly significant for the field of liver cirrhosis and treatments of patients. The study uses a special technique/program which is called a 3 dimensional virtual liver extraction measurement program. The results of the study are convincing. In summary, the manuscript provides a significant contribution to the understanding of liver cirrhosis and treatments by NUCs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cao WK, Timchenko N S- Editor: Qi Y L- Editor: A E- Editor: Liu WX
| 1. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1164] [Cited by in RCA: 1174] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 2. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2309] [Cited by in RCA: 2363] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2400] [Article Influence: 184.6] [Reference Citation Analysis (0)] |
| 4. | Korean Association for the Study of the Liver. KASL Clinical Practice Guidelines: Management of chronic hepatitis B. Clin Mol Hepatol. 2012;18:109-162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1739] [Cited by in RCA: 1740] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 6. | Hosaka T, Suzuki F, Kobayashi M, Seko Y, Kawamura Y, Sezaki H, Akuta N, Suzuki Y, Saitoh S, Arase Y. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology. 2013;58:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 540] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 7. | Saygili OB, Tarhan NC, Yildirim T, Serin E, Ozer B, Agildere AM. Value of computed tomography and magnetic resonance imaging for assessing severity of liver cirrhosis secondary to viral hepatitis. Eur J Radiol. 2005;54:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Zhou XP, Lu T, Wei YG, Chen XZ. Liver volume variation in patients with virus-induced cirrhosis: findings on MDCT. AJR Am J Roentgenol. 2007;189:W153-W159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Tong C, Xu X, Liu C, Zhang T, Qu K. Assessment of liver volume variation to evaluate liver function. Front Med. 2012;6:421-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH, Kim YS, Lee JW, Kim DJ, Cho SW. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol. 2012;18:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Han KH, Hong SP, Choi SH, Shin SK, Cho SW, Ahn SH, Hahn JS, Kim SO. Comparison of multiplex restriction fragment mass polymorphism and sequencing analyses for detecting entecavir resistance in chronic hepatitis B. Antivir Ther. 2011;16:77-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Yang X, Lee W, Choi Y, You H. Development of A User-Centered Virtual Liver Surgery Planning System. Proceedings of the Human Factors and Ergonomics Society Annual Meeting. 2012;56:772-776. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Lee CH, Kim SH, Kim IH, Kim SW, Lee ST, Kim DG, Yang JD, Yu HC, Cho BH, Lee SO. Endoscopic stenting in bile duct cancer increases liver volume. Gastrointest Endosc. 2014;80:447-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Yu HC, You H, Lee H, Jin ZW, Moon JI, Cho BH. Estimation of standard liver volume for liver transplantation in the Korean population. Liver Transpl. 2004;10:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Brown JJ, Naylor MJ, Yagan N. Imaging of hepatic cirrhosis. Radiology. 1997;202:1-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | de Jongh FE, Janssen HL, de Man RA, Hop WC, Schalm SW, van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology. 1992;103:1630-1635. [PubMed] |
| 17. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 18. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1228] [Cited by in RCA: 1365] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 19. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [PubMed] |
| 20. | Khan AZ, Mudan SS. Liver regeneration: mechanisms, mysteries and more. ANZ J Surg. 2007;77:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Yamanaka N, Okamoto E, Kawamura E, Kato T, Oriyama T, Fujimoto J, Furukawa K, Tanaka T, Tomoda F, Tanaka W. Dynamics of normal and injured human liver regeneration after hepatectomy as assessed on the basis of computed tomography and liver function. Hepatology. 1993;18:79-85. [PubMed] |
| 22. | Ismail MH, Pinzani M. Reversal of liver fibrosis. Saudi J Gastroenterol. 2009;15:72-79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |