Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.265
Peer-review started: October 28, 2016
First decision: November 21, 2016
Revised: November 26, 2016
Accepted: December 8, 2016
Article in press: December 8, 2016
Published online: January 14, 2017
Processing time: 76 Days and 13.1 Hours
To evaluate the impact of glycemic control and nutritional status after total pancreatectomy (TP) on complications, tumor recurrence and overall survival.
Retrospective records of 52 patients with pancreatic tumors who underwent TP were collected from 2007 to 2015. A series of clinical parameters collected before and after surgery, and during the follow-up were evaluated. The associations of glycemic control and nutritional status with complications, tumor recurrence and long-term survival were determined. Risk factors for postoperative glycemic control and nutritional status were identified.
High early postoperative fasting blood glucose (FBG) levels (OR = 4.074, 95%CI: 1.188-13.965, P = 0.025) and low early postoperative prealbumin levels (OR = 3.816, 95%CI: 1.110-13.122, P = 0.034) were significantly associated with complications after TP. Postoperative HbA1c levels over 7% (HR = 2.655, 95%CI: 1.299-5.425, P = 0.007) were identified as one of the independent risk factors for tumor recurrence. Patients with postoperative HbA1c levels over 7% had much poorer overall survival than those with HbA1c levels less than 7% (9.3 mo vs 27.6 mo, HR = 3.212, 95%CI: 1.147-8.999, P = 0.026). Patients with long-term diabetes mellitus (HR = 15.019, 95%CI: 1.278-176.211, P = 0.031) and alcohol history (B = 1.985, SE = 0.860, P = 0.025) tended to have poor glycemic control and lower body mass index levels after TP, respectively.
At least 3 mo are required after TP to adapt to diabetes and recover nutritional status. Glycemic control appears to have more influence over nutritional status on long-term outcomes after TP. Improvement in glycemic control and nutritional status after TP is important to prevent early complications and tumor recurrence, and improve survival.
Core tip: Considering that total pancreatectomy (TP) deprives patients of endocrine and exocrine pancreatic function, the decision of TP in the setting of pancreatic tumors continues to be a challenge. A series of postoperative clinical parameters ensure the instantaneity of objective reflection of metabolism, and analyses of their association with outcomes may have more clinical value, compared with the preoperative ones. It is concluded that postoperative glycemic control and nutritional status have an impact on clinical outcomes after TP. Improvement in postoperative management is important to prevent early complications and tumor recurrence and, more importantly, improve survival.
- Citation: Shi HJ, Jin C, Fu DL. Impact of postoperative glycemic control and nutritional status on clinical outcomes after total pancreatectomy. World J Gastroenterol 2017; 23(2): 265-274
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/265.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.265
Pancreatic surgery has taken a dominant position among the multimodal therapies of pancreatic tumors owing to standardization of care, multidisciplinary approach and centralization at high-volume centers[1]. Total pancreatectomy (TP), as a resection for the entire gland, seems to have a more profound and lasting impact on patients, compared with pancreaticoduodenectomy (PD) and distal pancreatectomy (DP). With improved surgical techniques and acceptable mortality, interest in TP has been renewed by alternative indications, in addition to invasive pancreatic cancer[2] and end-stage chronic pancreatitis[3]. The increasing recognition of intraductal papillary mucinous neoplasms (IPMN) that main duct tape is associated with malignant transformation along the entire pancreas gland, makes TP considered as a treatment for this diffuse lesion[4]. The presence of a microscopically positive surgical resection (R1) margin requires extended resection up to TP. In the case of life-threatening complications after partial pancreatectomy, emergency TP can be utilized to control debridement and sepsis[5]. The procedure can also be performed as a feasible strategy for tumor recurrence in the remnant, or a more aggressive management for hereditary pancreatic cancer in high-risk patients with genetic abnormalities[6] or multifocal diseases such as islet cell neoplasms or renal cell metastasis[7].
According to a surveillance epidemiology and end results (SEER) database review, the receipt of TP has increased over the years, as 9.3% of patients with pancreatic ductal adenocarcinoma underwent TP in 1998 compared with 14.3% in 2004[8]. Complete absence of pancreatic hormones, including insulin, glucagon and other islet regulation peptides, leads to tough management of brittle diabetes and dangerous episodes of hypoglycemia[9]. Lack of pancreatic digestive enzymes contributes to malabsorption, with symptoms of severe diarrhea and steatorrhea[10]. It has been hypothesized that glycemic control and nutritional status at the apancreatic state may have a potential impact on postoperative complications, tumor recurrence and long-term survival, but this has not yet been studied. Considering the complex postoperative medical management, the decision of TP in the setting of pancreatic tumors continues to be a challenge for many surgeons and patients.
The aim of the study presented herein was to clarify whether glycemic control and nutritional status are associated with complications, tumor recurrence and long-term survival by conducting a retrospective analysis of patients undergoing TP. In addition, risk factors for postoperative glycemic control and nutritional status were evaluated for further improvement of reduced pancreatic exocrine and endocrine insufficiency.
A total of 52 patients who underwent elective TP between 2007 and 2015 at Huashan Hospital, Fudan University. At our institute, elective TP was indicated for pancreatic ductal adenocarcinoma, cystadenocarcinoma, invasive IPMN, acinar adenocarcinoma, adenosquamous carcinoma and renal cell carcinoma metastasis (Table 1). Benign IPMN was not included because it is not a regular indication for TP in our nation and completion pancreatectomy is more acceptable for our patients once tumor recurrence occurs. Completion pancreatectomy was excluded as well. All data collected were consented by these patients and approved by the Ethical Committee and Institutional Review Board.
| Variable | No. of patients |
| Mean age ± SD (yr) | 60.3 ± 9.0 |
| Sex (male) | 32 |
| Pathology | |
| Adenocarcinoma | 43 |
| Cystadenocarcinoma | 2 |
| Invasive IPMN | 4 |
| Acinar adenocarcinoma | 1 |
| Adenosquamous carcinoma | 1 |
| Renal cell carcinoma metastasis | 1 |
| Tumor location | |
| Head | 28 |
| Corpus | 15 |
| Tail | 3 |
| > 1 location | 6 |
| Median primary tumor size (IQR) (cm) | 4.7 (3.0-6.0) |
| Venous invasion | 26 |
| Lymph node invasion | 18 |
| Splenectomy | 11 |
| Operative time (IQR) (min) | 472.5 (421.3-548.8) |
| Operative arrangement | |
| Planned | 20 |
| Unplanned | 32 |
| Postoperative complication1 | |
| Gastroplegia | 4 |
| Gastrointestinal or abdominal hemorrhage bleeding | 1 |
| Pneumonia | 14 |
| Abdominal infection | 9 |
| Hepatic failure | 1 |
| Respiratory failure | 1 |
| Length of postoperative hospital stay (IQR) (d) | 12.0 (10.0-17.8) |
| Neoadjuvant treatment | 14 |
| Adjuvant treatment | 52 |
| Median recurrence-free time (IQR) (mo) | 4.7 (2.7-13.6) |
| Median survival time (IQR) (mo) | 20.9 (10.2-51.0) |
| 1-year survival (%) | 63.4 |
| 3-year survival (%) | 24.2 |
| 5-year survival (%) | 12.5 |
Standard preoperative evaluation consisted of a baseline history, physical examination and routine laboratory tests. A preliminary diagnosis was made by contrast-enhanced multidetector computed tomography, completed by magnetic resonance imaging or endoscopic ultrasound with/without fine needle aspiration when appropriate. Preoperative biliary drainage, endoscopic retrograde biliary drainage or percutaneous transhepatic cholangial drainage was indicated for jaundice. Tumors were excluded from curative resection upon the presence of distant metastases, which were identified by preoperative imaging or intraoperative exploration.
All total pancreatectomies were performed by one surgical team. TP was utilized for invasive tumors located in the pancreatic corpus to avoid dissemination of tumor cells. Extension of a pancreatectomy was anticipated for positive frozen section of the pancreatic margin (R1). Twenty-six patients underwent portal/superior mesenteric vein resection and artificial blood vessel replacement. Extended resection of adjacent organs was performed when inevitable for locally advanced tumors. TP for suspected carcinoma was followed by a standard radical lymphadenectomy. The spleen was usually preserved when a tumor was deemed localized far from the structure. Pylorus-preserving TP was not performed in these cases.
Postoperative complications and biochemical data during all 3 d after surgery were recorded, and the means of these values were evaluated. Abdominal infection was confirmed by positive germiculture of peritoneal fluid from peritoneal drainage catheter. In the 3 d after surgery, patients received continuous intravenous insulin infusion by trace syringe pump while blood glucose levels were under dynamic monitoring. Subsequently, patients were referred to a consultant endocrinologist for subcutaneous insulin regimens. Exocrine insufficiency was managed by oral pancreatic enzymes (18600 U/capsule) during meals. Patients were aware of the need to adapt the number of pancreatic enzyme capsules to their fat intake.
All data were collected and recorded prospectively at each point of the routine follow-up. Patients were recommended to be evaluated by laboratory tests (and imaging) every month in the first year after TP and every 3 mo afterwards. The variables for evaluating the glycemic control included fasting blood glucose (FBG) and HbA1c, while those for evaluating the nutritional status included body mass index (BMI), serum total protein, albumin and prealbumin. Prognostic nutritional index [PNI; albumin (g/L) + 5 × total lymphocyte count (× 109/L)] was calculated and applied as an immunonutritional indice as well[11]. The goal of glycemic control was set at both less than 155 mg/dL of FBG and less than 7% of HbA1c, which was different from that of type 1 or type 2 diabetes mellitus to avoid hypoglycemia. The condition above the threshold was judged as poorly-controlled diabetes after TP. The judgement is based on the glucose level exceeding the threshold for at least 2 d. The point of the judgement was the fasting state in the morning. Recurrence-free survival was defined as the interval between surgery and tumor recurrence; if recurrence was not diagnosed, the recurrence-free survival period ended on the date of death or the last follow-up. Radiographic findings consistent with recurrent disease were considered adequate proof of recurrence. Overall survival was defined as the interval from the time of initial histological diagnosis to the date of death or last follow-up.
Summary statistics were reported using mean or median values where appropriate. Student’s t test or analysis of variance was used for mean comparison of continuous variables distributed normally, whereas Mann-Whitney U test or Kruskal-Wallis H test was used to compare skewed continuous variables. Risk factors for complications, postoperative glycemic control and changes in BMI levels were estimated by logistic and linear regression analyses. Prognostic factors for recurrence-free and overall survival were estimated by Cox proportional hazards models. The Kaplan-Meier method was used to analyze recurrence-free and overall survivals, and differences in survival were examined using the log-rank test. A two-sided P value of < 0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed utilizing SPSS statistics 20 (IBM corporation, Armonk, NY, United States).
Table 1 shows the clinicopathologic characteristics of 52 patients undergoing TP. More than half of the patients were male (n = 32) and the mean age was 60.3 years. Planned TP was performed in 20 patients, while the surgical plan was intraoperatively changed in 32 patients because of tumor invasiveness or R1 margin. The vast majority of malignant tumors were pancreatic ductal adenocarcinoma (n = 43). Half of the pancreatic tumors had venous invasion (n = 26), but about one-third had lymph node invasion (n = 18). All of the patients with malignant tumors received adjuvant chemotherapy. No patients died of hypoglycemic events or diabetes complications. The median recurrence-free and overall survivals were 4.7 mo and 20.9 mo, respectively.
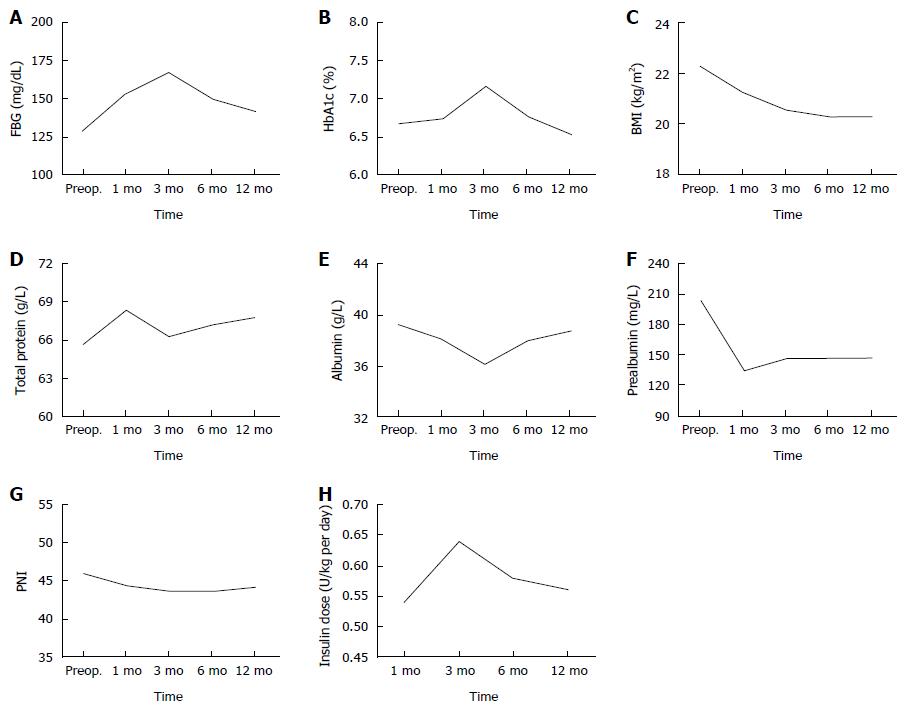
FBG and HbA1c levels were used to assess glycemic control during the follow-up (Table 2, Figure 1). FBG levels increased at 3 mo but returned to the preoperative levels at up to 12 mo (P = 0.078). HbA1c levels increased by the 3-mo follow-up but improved up to the preoperative levels by 12 mo (P = 0.330). BMI, serum total protein, albumin, prealbumin and PNI levels were used to measure nutritional status over the 12 mo of the follow-up (Table 2, Figure 1). BMI levels after TP decreased continuously by 12 mo (P = 0.001). Serum total protein levels were the highest postoperatively at 1 mo, and increased slightly up to 12 mo (P = 0.369), after a declination at 3 mo. Serum albumin levels decreased to the lowest levels at 3 mo, and recovered to the preoperative levels (P = 0.933). Serum prealbumin declined rapidly by 1 mo, and was maintained at a lower level up to 12 mo (P = 0.001). Insulin dose, which patients undergoing TP required, at 1, 3, 6 and 12 mo was 0.54 ± 0.12, 0.64 ± 0.11, 0.58 ± 0.13 and 0.56 ± 0.12, respectively. Therefore, there were significant differences between the preoperative and postoperative 12-mo BMI and serum prealbumin levels, respectively.
| Preop | Postop1 | Postop 1 mo | Postop 3 mo | Postop 6 mo | Postop 12 mo | P value2 | |
| FBG (mg/dL) | 130 ± 60 | 154 ± 39 | 153 ± 84 | 167 ± 94 | 149 ± 68 | 142 ± 79 | 0.078 |
| HbA1c (%) | 6.7 ± 1.5 | / | 6.7 ± 0.8 | 7.2 ± 1.0 | 6.8 ± 0.8 | 6.5 ± 0.8 | 0.330 |
| BMI (kg/m2) | 22.3 ± 3.2 | / | 21.2 ± 3.0 | 20.6 ± 2.8 | 20.3 ± 3.0 | 19.6 ± 2.5 | 0.001 |
| Total protein (g/L) | 65.7 ± 9.2 | 60.4 ± 6.6 | 68.4 ± 5.8 | 66.4 ± 4.9 | 67.2 ± 8.5 | 67.8 ± 6.3 | 0.369 |
| Albumin (g/L) | 39.3 ± 4.0 | 38.0 ± 4.2 | 38.2 ± 4.0 | 36.2 ± 4.4 | 38.0 ± 7.7 | 38.8 ± 4.6 | 0.933 |
| Prealbumin (mg/L) | 203.9 ± 54.3 | 171.6 ± 40.8 | 135.3 ± 34.6 | 147.9 ± 42.0 | 145.9 ± 48.8 | 148.2 ± 54.6 | 0.001 |
| PNI | 46.0 ± 3.4 | 47.8 ± 6.3 | 44.6 ± 4.0 | 43.8 ± 5.9 | 42.7 ± 5.9 | 47.3 ± 4.8 | 0.244 |
To determine which factors are associated with complications after TP, a univariate analysis was performed for preliminary screening, followed by a stepwise logistic regression analysis. Twenty-three patients had postoperative complications, including gastroplegia, gastrointestinal or abdominal hemorrhage, pneumonia, abdominal infection and hepatic or respiratory failure (Table 1). Five patients had two complications simultaneously, and one patient had three complications simultaneously. Mean values calculated from biochemical data in the first 3 d after surgery (early postoperative data) were evaluated in the analysis (Figure 2). In univariate analysis, postoperative complications occurred in 16 (69.6%) of 23 patients with higher early postoperative FBG levels compared with 10 (34.5%) of 29 patients with early postoperative FBG levels less than 155 mg/dL (P = 0.014). Patients with early postoperative prealbumin levels less than 185 mg/L had a significantly higher incidence of complications than those with higher level (14 of 23, 60.9% vs 8 of 29, 27.6%, P = 0.018) (Supplement 1). In multivariate analysis, high early postoperative FBG levels (OR = 4.074, 95%CI: 1.188-13.965, P = 0.025) and low early postoperative prealbumin levels (OR = 3.816, 95%CI: 1.110-13.122, P = 0.034) were significantly associated with complications after TP.
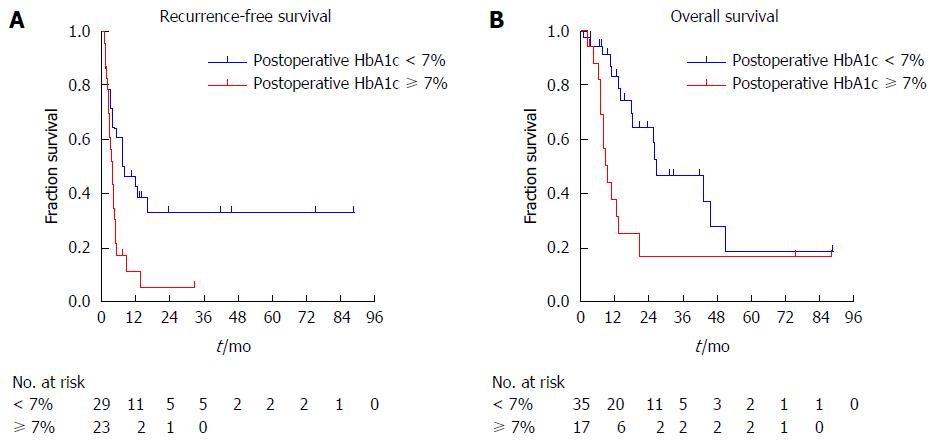
The results of the Cox regression hazard model for risk factors for tumor recurrence are shown in Table 3. The latest biochemical data during the follow-up before tumor recurrence (postoperative data) were evaluated in the analysis (Figure 2). Univariate analysis revealed that patients with preoperative FBG levels over 110 mg/dL (P = 0.045), venous invasion by tumor (P = 0.015), postoperative HbA1c levels over 7% (P = 0.010) or postoperative PNI levels less than 45 (P = 0.044) tended to have a diminished recurrence-free survival, respectively (Supplement 2). Multivariate analysis demonstrated that preoperative FBG (HR = 2.330, 95%CI: 1.204-4.510, P = 0.012), venous invasion (HR = 2.975, 95%CI: 1.502-5.892, P = 0.002) and postoperative HbA1c (HR = 2.655, 95%CI: 1.299-5.425, P = 0.007) were still retained as significant and independent risk factors for tumor recurrence. Patients with postoperative HbA1c levels over 7% had deceased recurrence-free survival compared to those with postoperative HbA1c levels less than 7% (4.2 mo vs 8.2 mo, P = 0.008) (Figure 3A).
| Variable | Multivariate analysis | ||
| Odds ratio/hazard ratio | 95%CI | P value | |
| Complications | |||
| Postop FBG1 | |||
| < 155 mg/dL | |||
| ≥ 155 mg/dL | 4.074 | 1.188-13.965 | 0.025 |
| Postop prealbumin1 | |||
| > 185 mg/L | |||
| ≤ 185 mg/L | 3.816 | 1.110-13.122 | 0.034 |
| Recurrence-free survival | |||
| Preop FBG | |||
| ≤ 110 mg/dL | |||
| > 110 mg/dL | 2.330 | 1.204-4.510 | 0.012 |
| Venous invasion | |||
| No | |||
| Yes | 2.975 | 1.502-5.892 | 0.002 |
| Postop HbA1c1 | |||
| < 7% | |||
| ≥ 7% | 2.655 | 1.299-5.425 | 0.007 |
| Postop PNI1 | |||
| > 45 | |||
| ≤ 45 | 1.760 | 0.897-3.455 | 0.100 |
| Overall survival | |||
| Diabetes mellitus | |||
| No | |||
| Short-term | 0.943 | 0.300-2.965 | 0.920 |
| Long-term | 2.305 | 0.560-9.483 | 0.247 |
| Preop FBG | |||
| ≤ 110 mg/dL | |||
| > 110 mg/dL | 1.112 | 0.357-3.462 | 0.854 |
| Postop HbA1c2 | |||
| < 7% | |||
| ≥ 7% | 3.212 | 1.147-8.999 | 0.026 |
| Postop total protein2 | |||
| > 55 g/L | |||
| ≤ 55 g/L | 1.152 | 0.304-4.364 | 0.835 |
| Postop albumin2 | |||
| > 35 g/L | |||
| ≤ 35 g/L | 2.894 | 0.551-15.195 | 0.209 |
| Postop prealbumin2 | |||
| > 185 mg/L | |||
| ≤ 185 mg/L | 3.961 | 0.976-16.068 | 0.054 |
| Postop PNI2 | |||
| > 45 | |||
| ≤ 45 | 1.143 | 0.242-5.408 | 0.866 |
Table 3 illustrates prognostic factors for patients undergoing TP using the Cox regression analysis. The latest biochemical data during the follow-up before patient death (postoperative data) were evaluated in the analysis (Figure 2). Preoperative long-term diabetes mellitus (P = 0.004), high preoperative FBG (P = 0.016) and postoperative HbA1c (P = 0.011) levels, low postoperative serum total protein (P = 0.028), albumin (P = 0.014), prealbumin (P = 0.009) and PNI (P = 0.021) levels were related to adverse prognosis (Supplement 3). However, multivariate analysis showed that patients with postoperative HbA1c levels over 7% had much poorer overall survival than those with less than 7% (9.3 mo vs 27.6 mo, HR = 3.212, 95%CI: 1.147-8.999, P = 0.026). Patients with postoperative HbA1c levels over 7% had much shorter overall survival compared to those with postoperative HbA1c levels less than 7% (9.3 mo vs 26.7 mo, P = 0.008) (Figure 3B).
Considering that apancreatic diabetic status played an important role in complications, tumor recurrence and overall survival, preoperative factors for the latest post-TP glycemic control during the follow-up were analyzed by logistic regression (Table 4). In univariate analysis, the probability of poorly-controlled glycemic control was significantly increased when patients presented with long-term diabetes mellitus (P = 0.009) and preoperative HbA1c (P = 0.043) (Supplement 4). Multivariate analysis showed long-term diabetes mellitus (HR = 15.019, 95%CI: 1.278-176.211, P = 0.031) as the only factor associated with glycemic control after pancreatectomy.
Linear regression was used to analyze association with changes in BMI (Table 4). The postoperative BMI was the latest value obtained during follow-up. Preoperative factors in univariate analysis were age (P = 0.038), pathology of pancreatic ductal carcinoma (P = 0.035) and alcohol history (P = 0.027) (Supplement 5). Multivariate analysis revealed alcohol history as an independent factor affecting BMI (B = 1.985, SE = 0.860, P = 0.025). Patients with alcohol history tended to have lower BMI levels postoperatively, suggesting a poorer overall nutritional status.
As the frequency of TP has increased over decades[12], meticulous and comprehensive management has expanded from preparation of surgery to postoperative rehabilitation. Except for tumor recurrence, metabolic problems of endocrine and exocrine insufficiency caused by the apancreatic state are a matter of continuous concern. In the current study, we found that high early postoperative FBG and low early serum prealbumin were significantly associated with complications after TP, respectively. Patients with postoperative HbA1c levels over 7% had much poorer recurrence-free and overall survival than those with HbA1c levels less than 7%. In addition, we figured out long-term diabetes mellitus and alcohol history as risk factors for glycemic control and changes in BMI after TP, respectively.
Of note, the majority of previous studies focused on preoperative clinical parameters to evaluate short-term and long-term outcomes after surgery. However, given that the pancreas plays a central role in glycemic control and nutritional status, pancreatic surgery involving resection of pancreatic parenchyma, TP in particular, breaks preoperative homeostasis and obliges the body to adapt to another new balance of glycemic and nutritional status. Thus, a series of postoperative clinical parameters ensure the instantaneity of objective reflection of metabolism, and analyses of their association with complications, recurrence and survival may have more clinical value, compared with the preoperative ones. In our study, we found that glycemic control tended to improve after an inflection point at 3 mo, and nutritional status tended to level off after reaching a nadir at 3 mo. These findings suggest that at least 3 mo are required for patients after TP to adapt to brittle diabetes and recover nutritional status, with the help of tight glycemic management and additional nutritional support.
It is known that hyperglycemia has an adverse impact on leukocyte function, including granulocyte adherence, impaired phagocytosis, delayed chemotaxis and depressed bactericidal capacity[13]. A degree of hyperglycemia as low as 200 mg/dL has been demonstrated to impair phagocytic function[14]. In hepato-biliary-pancreatic surgery, the surgical site infection rate was 20% among patients with serum glucose levels less than 200 mg/dL, which was significantly better than the rates of 52% among patients with serum glucose levels over 200 mg/dL[15]. The rates of infectious and noninfectious complications and mortality increased parallel to the degree of hyperglycemia[16]. Our study indicated that a higher early postoperative FBG was associated with a higher morbidity rate (69.6%) compared with a rate of 34.5% in patients with early postoperative FBG less than 155 mg/dL. It is inferred that early postoperative glycemic control predicts nosocomial infection rate, and postoperative infections improve with good glycemic control. In addition, we demonstrated that patients with postoperatively low serum prealbumin levels had a high prevalence of complications. As the frequency of postoperative pulmonary complications was also seen much more often in patients undergoing total gastrectomy, the impairment of the nutritional status was considered to be most responsible for the increased risk of postoperative pulmonary complications and abscess formation[17]. Previous studies in the literature have proved a significant association between prealbumin, rather than transferrin and albumin, and infectious complications or related death[18]. Given physiological changes after total pancreatectomy and approximately 2-d half-life of prealbumin[19], postoperative serum prealbumin is a more sensitive indicator than serum albumin, and suitable for evaluation of complications.
A retrospective analysis of TP revealed patient sex and tumor stage as factors influencing long-term survival[20]. However, stratification of metabolic factors has not been unequivocally established to further benefit patients undergoing TP. Cheon et al[21] and Fan et al[22] unanimously confirmed that preoperative HbA1c levels over 7.0% were associated with inferior overall survival of patients with pancreatic cancer, and that antidiabetic treatment, metformin in particular, might improve the long-term outcomes. We determined that postoperative HbA1c predicted recurrence and survival of patients undergoing TP. The relationship between postoperative HbA1c and recurrence and survival of patients undergoing TP may be attributed to the fact that pancreatic cancers depend heavily on glucose for growth. While the normal pancreas metabolizes glucose through oxidative phosphorylation, pancreatic cancer cells prefer aerobic glycolysis for glycometabolism, which is known as the Warburg effect[23]. This pattern generates less energy but more metabolites for biosynthetic functions to sustain and accelerate cell proliferation, thus conferring a survival advantage[24]. To compensate for insufficient energy and meeting the increasing requirement for biosynthesis, pancreatic cancer cells exhibit high efficiency of glucose uptake[25]. A dose-response meta-analysis confirmed the relations between blood glucose and risk of pancreatic cancer[26]. Although insulin is a mitogenic and growth-promoting hormone[27], we did not find any evidence that higher insulin administration prompted an early recurrence of disease. Therefore, it is concluded that hyperglycemia after TP, identified by HbA1c, may promote tumor recurrence by enhancing tumor cell proliferation and, thus, adversely impacting survival. On the other hand, no nutritional parameters were found to be associated with survival or recurrence after TP in multivariate analyses in our study. However, Geng et al[11] confirmed the role of PNI in prediction of survival in advanced pancreatic cancer. Moreover, Vashi et al[28] concluded that improvement in nutritional status during cancer treatment decreased the risk of mortality independent of previous treatment history. Therefore, it is important to maintain the postoperative nutritional status of patients with malignant tumors to allow repeated and aggressive chemotherapy for prevention of recurrence and to prolong survival.
Since the significant metabolic derangements of the apancreatic state may not immediately emerge in the postoperative inpatient recovery stage, a strict follow-up protocol should be available to supervise glycemic and nutritional index at internals during the postoperative period. These include FBG, HbA1c, weight measurement, serum protein levels, daily caloric intake and the number of stools per day. The majority of patients do not have underlying endocrine or exocrine insufficiency before surgery and have difficulty adjusting to complex diabetic and nutritional changes after TP. We demonstrated that patients with long-term diabetes mellitus tended to have poor glycemic control after TP, and elderly patients might be subject to poor nutritional status. It is possible that insulin resistance is less severe in patients with a shorter duration of diabetes mellitus than in patients with a longer duration, as observed in gastrectomy[29]. On the other hand, functions of nutrient absorption and regulation are likely to diminish with previous alcohol history. Chronic alcohol may cause direct toxicity to the liver and digestive tract, consulting either maldigestion and malabsorption or impaired utilization of nutrients[30]. Together with “empty” calories provided by alcohol, functional impairments of the liver and digestive tract reduce basic nutritional reserve for the body and, thus, place a burden on the nutritional recovery after TP.
The current study has several limitations. The major limitation was the retrospective nature of the analysis, which precludes inferring direct causation. Despite a time span of 9 years, only a relatively small sample size of patients was identified as the ones undergoing TP. The selected patients with different types of pancreatic tumors increased the heterogeneity of subjects. Also, we could not use the stool elastase and record steatorrhea and diarrhea during the follow-up to assess exocrine insufficiency and determine whether the quantity of enzymes administered was accurate. Although no hypoglycemia-related deaths were observed, hypoglycemia events after discharge were not included in the analyses to determine their influence on quality of life and survival. Another limitation was that target organ complications induced by hyperglycemia might have an impact on survival, although no diabetes-related deaths were recorded.
In conclusion, the apancreatic state after TP leads to diabetes and malabsorption dependent on insulin and enzyme replacement throughout one’s life. At least 3 mo are required for patients undergoing TP to adapt to diabetes and recover nutritional status, with the help of tight glycemic management and additional nutritional support. Postoperative glycemic control and nutritional status have an impact on clinical outcomes after TP, while glycemic control appears to have more influence over nutritional status on long-term outcomes in patients undergoing TP. Improvement in glycemic control and nutritional status after TP is important to prevent early complications and tumor recurrence and, more importantly, to improve survival. Improved postoperative management should include auto-islet cell transplantation and advances in artificial pancreas, which allow much tighter control of blood glucose, and addition of acid-suppressing agents to pancreatin enteric-coated capsules and surgical preservation of the pylorus, which will improve nutrient digestion and absorption. With new concepts and strategies, TP will no longer be a challenge to surgeons and patients.
Total pancreatectomy (TP) seems to have a more profound and lasting impact on patients due to an apancreatic state after TP. According to a review of the surveillance epidemiology and end results database, the receipt of TP has increased over the years. Considering the complex postoperative medical management, the decision of TP in the setting of pancreatic tumors continues to be a challenge for many surgeons and patients.
The majority of previous studies have focused on preoperative clinical parameters to evaluate short-term and long-term outcomes after surgery. Complete absence of pancreatic hormones leads to tough management of brittle diabetes and dangerous episodes of hypoglycemia. Lack of pancreatic digestive enzymes contributes to malabsorption with symptoms of severe diarrhea and steatorrhea. It has been hypothesized that glycemic control and nutritional status of the apancreatic state may have a potential impact on postoperative complications, tumor recurrence and long-term survival, but this has not yet been studied.
A series of postoperative clinical parameters ensure the instantaneity of objective reflection of metabolism, and analyses of their association with outcomes may have more clinical value, compared with the preoperative ones. In this study, high early postoperative fasting blood glucose levels and low early postoperative prealbumin levels were significantly associated with complications after TP. Patients with postoperative HbA1c levels over 7% had much poorer recurrence-free and overall survivals than those with HbA1c levels less than 7%. Therefore, postoperative glycemic control and nutritional status have an impact on clinical outcomes after TP.
Improvement in glycemic control and nutritional status after TP is important to prevent early complications and tumor recurrence and, more importantly, to improve survival. With new concepts and strategies, TP will no longer be a challenge to surgeons and patients.
Total pancreatectomy has been used to treat both benign and malignant disease of the pancreas, but its use has been limited by concerns about management of the apancreatic state with its concomitant total endocrine and exocrine insufficiency. Improvements in postoperative management include auto-islet cell transplantation, advances in artificial pancreas, addition of acid-suppressing agents to pancreatin enteric-coated capsules and surgical preservation of the pylorus.
This study showed that good glycemic control may improve the outcomes after surgery. This is very impressive study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Aosasa S, Barbier L, Buchler MW, Han HS S- Editor: Qi Y L- Editor: Filipodia E- Editor: Liu WX
| 1. | Karachristos A, Esnaola NF. Surgical management of pancreatic neoplasms: what’s new? Curr Gastroenterol Rep. 2014;16:397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 2. | Johnston WC, Hoen HM, Cassera MA, Newell PH, Hammill CW, Hansen PD, Wolf RF. Total pancreatectomy for pancreatic ductal adenocarcinoma: review of the National Cancer Data Base. HPB (Oxford). 2016;18:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Braganza JM, Lee SH, McCloy RF, McMahon MJ. Chronic pancreatitis. Lancet. 2011;377:1184-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 343] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | Griffin JF, Poruk KE, Wolfgang CL. Is It Time to Expand the Role of Total Pancreatectomy for IPMN? Dig Surg. 2016;33:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | de Castro SM, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Incidence and management of pancreatic leakage after pancreatoduodenectomy. Br J Surg. 2005;92:1117-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Brentnall TA. Management strategies for patients with hereditary pancreatic cancer. Curr Treat Options Oncol. 2005;6:437-445. [PubMed] |
| 7. | Wente MN, Kleeff J, Esposito I, Hartel M, Müller MW, Fröhlich BE, Büchler MW, Friess H. Renal cancer cell metastasis into the pancreas: a single-center experience and overview of the literature. Pancreas. 2005;30:218-222. [PubMed] |
| 8. | Nathan H, Wolfgang CL, Edil BH, Choti MA, Herman JM, Schulick RD, Cameron JL, Pawlik TM. Peri-operative mortality and long-term survival after total pancreatectomy for pancreatic adenocarcinoma: a population-based perspective. J Surg Oncol. 2009;99:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Dresler CM, Fortner JG, McDermott K, Bajorunas DR. Metabolic consequences of (regional) total pancreatectomy. Ann Surg. 1991;214:131-140. [PubMed] |
| 10. | Lindkvist B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J Gastroenterol. 2013;19:7258-7266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 135] [Cited by in RCA: 149] [Article Influence: 12.4] [Reference Citation Analysis (6)] |
| 11. | Geng Y, Qi Q, Sun M, Chen H, Wang P, Chen Z. Prognostic nutritional index predicts survival and correlates with systemic inflammatory response in advanced pancreatic cancer. Eur J Surg Oncol. 2015;41:1508-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Almond M, Roberts KJ, Hodson J, Sutcliffe R, Marudanayagam R, Isaac J, Muiesan P, Mirza D. Changing indications for a total pancreatectomy: perspectives over a quarter of a century. HPB (Oxford). 2015;17:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Hanazaki K, Maeda H, Okabayashi T. Relationship between perioperative glycemic control and postoperative infections. World J Gastroenterol. 2009;15:4122-4125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 70] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | MacRury SM, Gemmell CG, Paterson KR, MacCuish AC. Changes in phagocytic function with glycaemic control in diabetic patients. J Clin Pathol. 1989;42:1143-1147. [PubMed] |
| 15. | Ambiru S, Kato A, Kimura F, Shimizu H, Yoshidome H, Otsuka M, Miyazaki M. Poor postoperative blood glucose control increases surgical site infections after surgery for hepato-biliary-pancreatic cancer: a prospective study in a high-volume institute in Japan. J Hosp Infect. 2008;68:230-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Kiran RP, Turina M, Hammel J, Fazio V. The clinical significance of an elevated postoperative glucose value in nondiabetic patients after colorectal surgery: evidence for the need for tight glucose control? Ann Surg. 2013;258:599-604; discussion 604-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Yamanaka H, Nishi M, Kanemaki T, Hosoda N, Hioki K, Yamamoto M. Preoperative nutritional assessment to predict postoperative complication in gastric cancer patients. JPEN J Parenter Enteral Nutr. 1989;13:286-291. [PubMed] |
| 18. | Tempel Z, Grandhi R, Maserati M, Panczykowski D, Ochoa J, Russavage J, Okonkwo D. Prealbumin as a serum biomarker of impaired perioperative nutritional status and risk for surgical site infection after spine surgery. J Neurol Surg A Cent Eur Neurosurg. 2015;76:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Ingenbleek Y, Young V. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr. 1994;14:495-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 274] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 20. | Andrén-Sandberg A, Ihse I. Factors influencing survival after total pancreatectomy in patients with pancreatic cancer. Ann Surg. 1983;198:605-610. [PubMed] |
| 21. | Cheon YK, Koo JK, Lee YS, Lee TY, Shim CS. Elevated hemoglobin A1c levels are associated with worse survival in advanced pancreatic cancer patients with diabetes. Gut Liver. 2014;8:205-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Fan KY, Dholakia AS, Wild AT, Su Z, Hacker-Prietz A, Kumar R, Hodgin M, Hsu CC, Le DT, De Jesus-Acosta A. Baseline hemoglobin-A1c impacts clinical outcomes in patients with pancreatic cancer. J Natl Compr Canc Netw. 2014;12:50-57. [PubMed] |
| 23. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11809] [Article Influence: 738.1] [Reference Citation Analysis (0)] |
| 24. | Sah RP, Nagpal SJ, Mukhopadhyay D, Chari ST. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 2013;10:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 25. | Regel I, Kong B, Raulefs S, Erkan M, Michalski CW, Hartel M, Kleeff J. Energy metabolism and proliferation in pancreatic carcinogenesis. Langenbecks Arch Surg. 2012;397:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Xu JJ, Wang ZL, Xu D, Zhang LL, Zhang XB. Tailoring deposition and morphology of discharge products towards high-rate and long-life lithium-oxygen batteries. Nat Commun. 2013;4:2438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 242] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 27. | Call R, Grimsley M, Cadwallader L, Cialone L, Hill M, Hreish V, King ST, Riche DM. Insulin--carcinogen or mitogen? Preclinical and clinical evidence from prostate, breast, pancreatic, and colorectal cancer research. Postgrad Med. 2010;122:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Vashi P, Popiel B, Lammersfeld C, Gupta D. Outcomes of systematic nutritional assessment and medical nutrition therapy in pancreatic cancer. Pancreas. 2015;44:750-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | An JY, Kim YM, Yun MA, Jeon BH, Noh SH. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J Gastroenterol. 2013;19:9410-9417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |