Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.242
Peer-review started: June 28, 2016
First decision: September 6, 2016
Revised: October 4, 2016
Accepted: November 13, 2016
Article in press: November 13, 2016
Published online: January 14, 2017
Processing time: 201 Days and 3.1 Hours
To investigate the protective effect of a recombinant adeno-associated virus carrying thymosin β4 (AAV-Tβ4) on murine colitis via intracolonic administration.
AAV-Tβ4 was prepared and intracolonically used to mediate the secretory expression of Tβ4 in mouse colons. Dextran sulfate sodium (DSS) was applied to induce the murine ulcerative colitis, and 2,4,6-trinitrobenzene sulfonic acid (TNBS) was used to establish a mouse colitis model resembling Crohn’s disease. The disease severity and colon injuries were observed and graded to reveal the effects of AAV-Tβ4 on colitis. The activities of myeloperoxidase (MPO) and superoxide dismutase (SOD) and the content of malondialdehyde (MDA) were determined using biochemical assays. Colonic levels of tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-10 were measured using ELISA, and mucosal epithelial cell apoptosis and proliferation were detected by TUNEL assay and immunochemistry, respectively.
Recombinant AAVs efficiently delivered LacZ and Tβ4 into the colonic tissues of the mice, and AAV-Tβ4 led to a strong expression of Tβ4 in mouse colons. In both the DSS and TNBS colitis models, AAV-Tβ4-treated mice displayed distinctly attenuated colon injuries and reduced apoptosis rate of colonic mucosal epithelia. AAV-Tβ4 significantly reduced inflammatory cell infiltrations and relieved oxidative stress in the inflamed colons of the mice, as evidenced by decreases in MPO activity and MDA content and increases in SOD activity. AAV-Tβ4 also modulated colonic TNF-α, IL-1β and IL-10 levels and suppressed the compensatory proliferation of colonic epithelial cells in DSS- and TNBS-treated mice.
Tβ4 exerts a protective effect on murine colitis, indicating that AAV-Tβ4 could potentially be developed into a promising agent for the therapy of inflammatory bowel diseases.
Core tip: We confirmed that intracolonically administered recombinant adeno-associated virus (AAVs) efficiently mediated the ectopic expression of LacZ and thymosin β4 (Tβ4) in mouse colonic mucosa. The current study first indicated that AAV-Tβ4 could improve murine colitis induced either by dextran sulfate sodium or 2,4,6-trinitrobenzene sulfonic acid by suppressing inflammatory cell infiltration, alleviating oxidative stress and epithelial apoptosis, and modulating the production of inflammatory mediators in the inflamed colon. Furthermore, locally overexpressed Tβ4 could attenuate the proliferation of colonic mucosal epithelia. In summary, these results suggest a protective role of Tβ4 in inflammatory bowel diseases (IBD) and indicate that AAV-Tβ4 has therapeutic potential for IBD patients.
- Citation: Zheng XY, Lv YF, Li S, Li Q, Zhang QN, Zhang XT, Hao ZM. Recombinant adeno-associated virus carrying thymosin β4 suppresses experimental colitis in mice. World J Gastroenterol 2017; 23(2): 242-255
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/242.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.242
Thymosin β4 (Tβ4) consists of 43 amino acids and is a highly conserved protein belonging to the beta-thymosins family. This small molecule spreads in nearly all cells and exists in various body fluids, such as tears, saliva, blood, plasma and wound fluid[1,2]. Following translation, Tβ4 undergoes complex modifications, such as transglutamination, phosphorylation, N acetylation, and proteolysis[3]. Extracellular Tβ4, released via either active secretion or cell lysis[4], can be rapidly internalized via an unknown mechanism and acts as a paracrine growth factor or cytokine[5,6]. The biological actions of Tβ4 are partially mediated via the activation of integrin-linked kinase[7], upregulation of Bcl-2 production and inhibition of caspase activity[8]. Systemic administration of Tβ4 decreases lethality and inflammatory responses in sepsis, prevents myocardial rupture and improves cardiac function after myocardial infarction, rehabilitates neural function following stroke and encephalomyelitis, and accelerates fracture healing[1,9-13]. Topical use of Tβ4 improves corneal injury, promotes skin wound healing, and accelerates hair growth[1,14-16]. These data suggest a great therapeutic potential for Tβ4 in wound healing and inflammatory diseases.
Tβ4 is expressed in human intestines where it modulates the intestinal immune system[17,18]. Moreover, Tβ4 has been considered to be an applicable treatment of gastrointestinal disorders[19]. Increased serum Tβ4 levels have been demonstrated in patients with inflammatory bowel diseases (IBDs)[20]. However, the role and function of Tβ4 in IBDs have not been elucidated. Given the anti-inflammatory, anti-apoptotic, and pro-tissue regeneration properties of Tβ4 that have been previously reported, Tβ4 is supposed to be a beneficial factor for IBDs. To verify this speculation, we constructed a recombinant adeno-associated virus (AAV) to achieve persistent secretory expression of Tβ4 in mouse colons and assessed its effects in two murine colitis models.
Self-complementary recombinant adeno-associated virus (subtype 2) were constructed by applying an AAV Helper-Free System (Cell Biolabs, Inc., San Diego, CA, United States). For secretory expression of Tβ4, the N-terminal 80 amino acid signal peptide of human neurotrophin-4 preproprotein was fused to the N-terminus of human Tβ4 (GenBank NM_021109.3). The coding DNA of the fusion protein was synthesized with the addition of EcoRI and BamHI restriction-enzyme sites (AuGCT DNA-SYN Biotechnology Inc., Beijing, China) and inserted into pscAAV-MCS to yield the pscAAV-NT-4-Tβ4 plasmid. Recombinant AAV containing Tβ4 (AAV-Tβ4) was generated via co-transfection of pscAAV-NT-4-Tβ4, pHelper and pAAV-RC5 into AAV-293 cells using polyethylenimine (PEI). Similarly, a recombinant AAV carrying LacZ (AAV-LacZ) was constructed as a control virus. Seventy-two hours after transfection, the cells were collected for viral particle isolation, purification and quantitative analysis.
TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA, United States) was employed to determine the rAAVs titters and the abundance of the rAAVs in the colon. The primers against the cytomegalovirus promoter region were as follows: 5’-AGACTTGGAAATCCCCGTGAGT-3’ (forward) and 5’-CGTATTAGTCATCGCTATTACCATGGT-3’ (reverse). The sequence of the probe was 5’-6FAM-AACCGCTATCCACGCCCATTGATG-TAMRA-3’. The collected data were analyzed by the standard curve method.
Male BALB/c mice, 6 wk of age, were purchased from the Experimental Animal Centre of Xi’an Jiaotong University and housed under pathogen-free conditions at 22 ± 2 °C with a 12:12 h light-dark cycle. All animal experiments were approved by the Institutional Animal Ethics Committee of Xi’an Jiaotong University.
To verify the transduction efficiency of the recombinant AAV (rAAV) in mouse colons, 12 mice were divided into the following 3 equal groups: phosphate-buffered saline (PBS), AAV-LacZ and AAV-Tβ4. After a 12-h fast, the mice were given an enema of PBS or rAAVs accordingly. Under anesthesia with chloral hydrate[21], a soft catheter was inserted into the mouse anus at a depth of 4 centimeters, and 0.2 mL of PBS, AAV-LacZ [4 × 1010 viral genome (vg) or AAV-Tβ4 (4 × 1010 vg] was instilled into the mouse colon via this catheter. The mouse was then held in an upside-down position for 1 min to allow the enema to distribute throughout the colon. After recovery from the anesthesia, water and food were provided. Two weeks later, mice of all of the groups were sacrificed for their colons. The colon was longitudinally opened, washed with cold normal saline, and cut into three parts as follows: the first was for DNA extraction and sequent qPCR assays, the second was cryosectioned for X-Gal staining as described previously[22], and the third was homogenized in radioimmunoprecipitation assay lysis buffer for Western blotting.
The following two models were studied: dextran sulfate sodium (DSS) colitis to simulate human ulcerative colitis (UC) and 2,4,6-trinitrobenzene sulfonic acid (TNBS) colitis to model Crohn’s disease (CD).
DSS-induced colitis: The mice were divided into a normal control (NC) group, a PBS/DSS group, an AAV-LacZ/DSS group and an AAV-Tβ4/DSS group (10 mice per group). Prior to inducing DSS colitis, 0.2 mL of AAV-LacZ (4 × 1010 vg) and AAV-Tβ4 (4 × 1010 vg) were intracolonically (ic) dosed for the mice of the AAV-LacZ/DSS and AAV-Tβ4/DSS groups, respectively. Meanwhile, the NC and PBS/DSS mice received an enema of PBS. One week later, mice from the three DSS groups were given 50 g/L DSS (MW: 5000, Sigma-Aldrich, St. Louis, MO, United States) as drinking water for 7 d to establish the colitis model, while the mice in the NC group received DSS-free drinking water.
TNBS-induced colitis: The mice, 10 per group, were randomly assigned to the ethanol control (EC), PBS/TNBS, AAV-LacZ/TNBS and AAV-Tβ4/TNBS groups. Mice of the three TNBS groups were given a 0.2 mL enema of TNBS (Sigma-Aldrich, St. Louis, MO, United States, dissolved in 500 mL/L ethanol) once per week for 6 wk. TNBS was dosed at 50 mg/kg for the first 2 wk, 100 mg/kg for the next 2 wk and 150 mg/kg for the last 2 wk. Meanwhile, mice of the EC group received an equal volume of enema with 500 mL/L ethanol. AAVs were ic administered at a dose of 4 × 1010 vg in 0.2 mL, 3 d after the first TNBS instillation, while mice in the other two groups were given PBS instead. At the end of the modeling, mice of all of the groups were sacrificed and colonic tissues were isolated.
The health status of all the mice was observed during the modeling. The disease activity index (DAI) was estimated daily (DSS-induced colitis) or weekly (TNBS-induced colitis) in accordance with a well-established scoring system[23].
Macroscopic damage of the colon was carefully observed under an anatomic microscope (Nikon, Tokyo, Japan) and graded according to a previously used scoring system[23]. Small colon segments were fixed, embedded and sectioned for hematoxylin and eosin staining and immunohistochemistry. Histological alterations were graded by observing the H&E stained sections according to a previously established criteria[24].
Genomic DNA (gDNA) was isolated from the colonic tissues using a DNeasy Kit (Qiagen, Valencia, CA, United States). For the qPCR analysis, 1 μg of the total gDNA was used in each PCR assay. Small pieces of colonic tissue were weighed and mechanically homogenized in normal saline on an ice-bath to obtain 100 mg/L colonic homogenates for biochemical detections and ELISA.
Briefly, proteins were extracted from the colonic tissue samples, applied to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Tris-glycine system for β-actin; Tris-Tricine system for Tβ4) and transferred onto polyvinylidene fluoride membranes (Bio-Rad, Hercules, CA, United States). Western blots were performed with specific antibodies targeting β-actin (Sigma-Aldrich, St. Louis, MO, United States) and Tβ4 (Abcam, San Francisco, CA, United States).
Myeloperoxidase (MPO) activity, malondialdehyde (MDA) content and superoxide dismutase (SOD) activity in the colonic homogenates were assessed by applying the test kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s protocols. The results of the MPO activity, MDA content and SOD activity were, respectively, represented as units per gram of tissue (U/g), nmol per mg protein (nmol/mgprot) and U/mgprot.
The supernatants of the colonic homogenates were collected to determine the tumor necrosis factor-α (TNF-α), interleukin (IL)-1β and IL-10 levels. Three ELISA kits (TNF-α, R&D Systems, Minneapolis, MN, United States; IL-1β and IL-10, eBioscience, San Diego, CA, United States) were used in accordance with the manufacturers’ instructions. The results were expressed as pictogram per mg protein (pg/mgprot).
Immunohistochemistry was performed with primary antibodies against PCNA and Tβ4, according to a previously reported method[22].
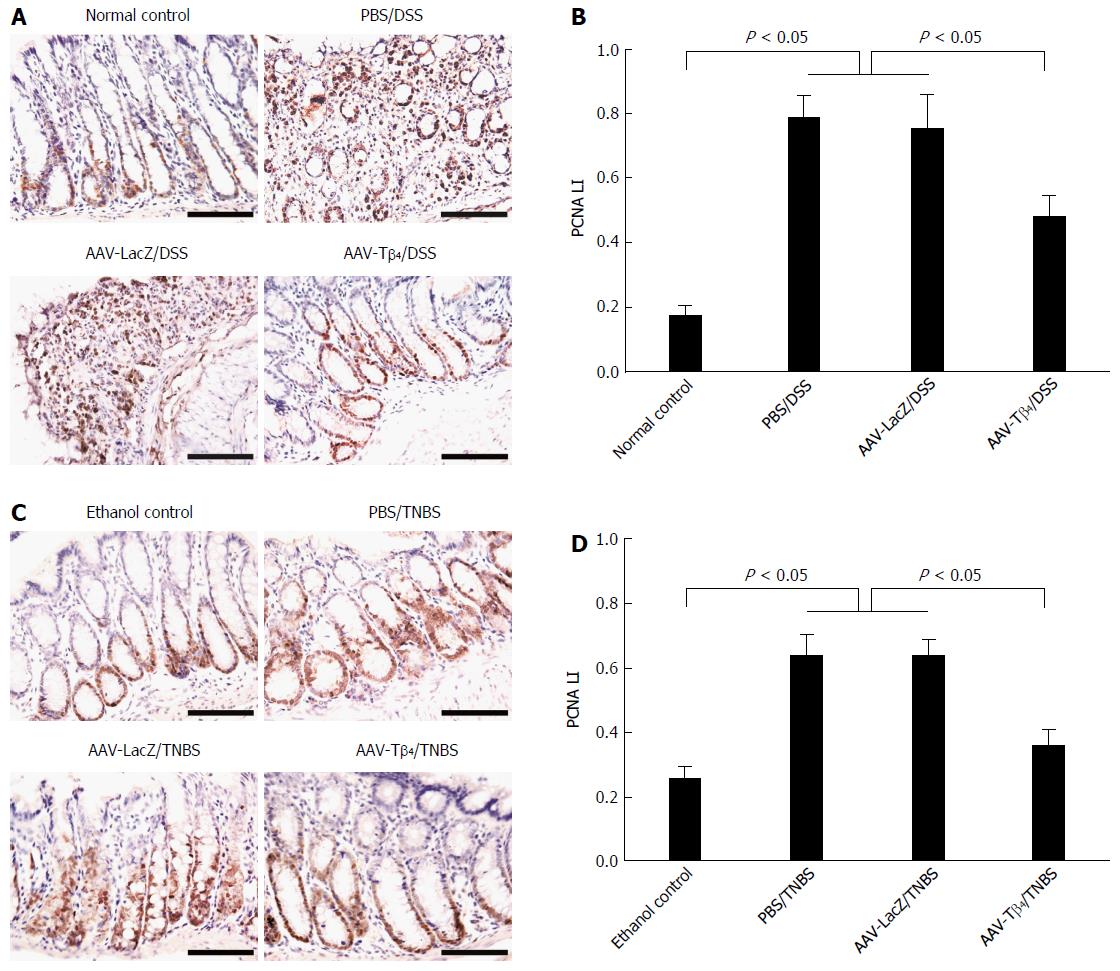
The proliferation of mucosal epithelial cells was estimated by PCNA immunostaining of the colonic sections. PCNA labeling index (PCNA LI) was determined by calculating the ratio of the number of PCNA-positive cells to the total number of counted cells in 10 randomly chosen fields at × 400 magnification.
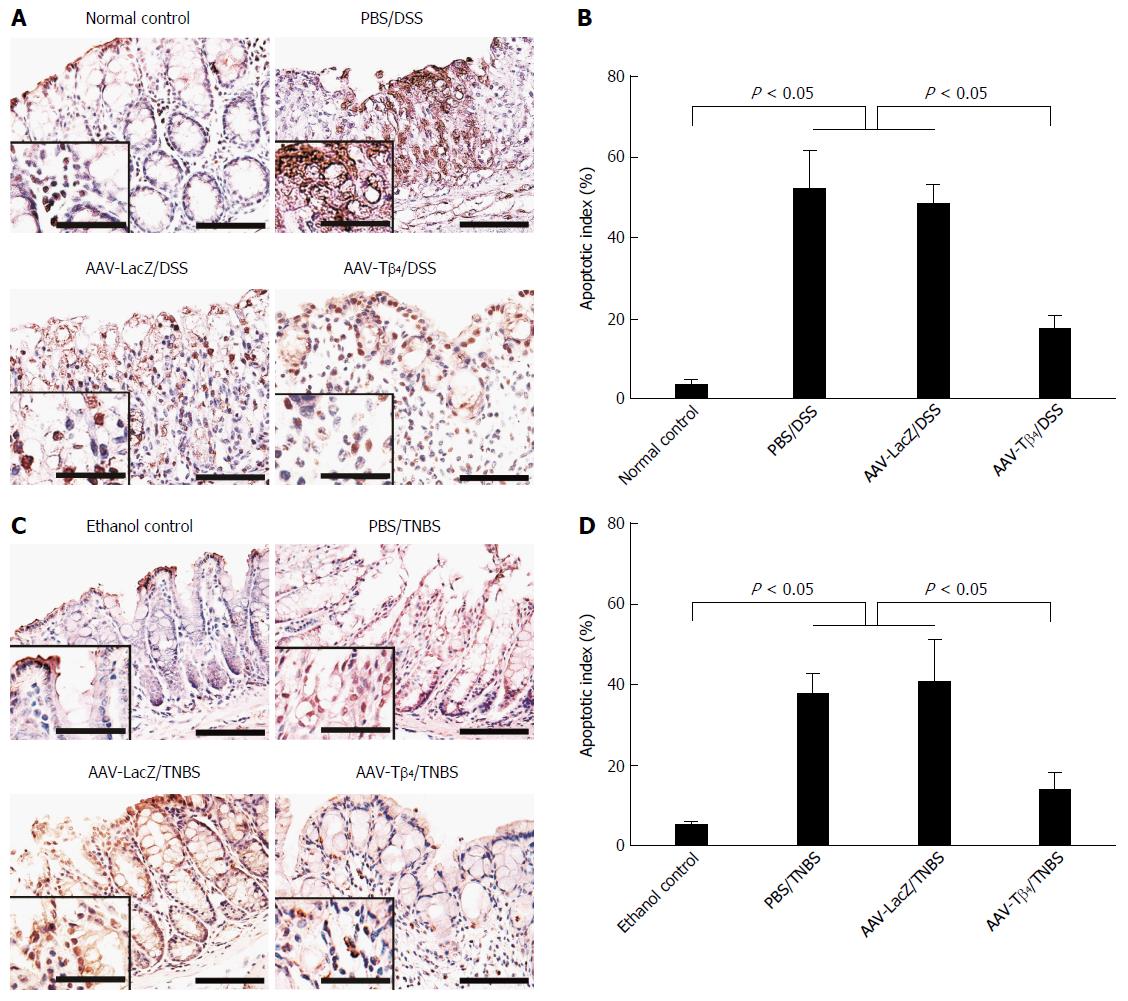
Epithelial cell apoptosis in colonic mucosa was assessed by applying the DeadEnd Colorimetric TUNEL System (Promega, Madison, WI, United States) based on the manufacturer’s protocol. One thousand mucosal epithelial cells from 10 randomly selected fields (magnification × 400) were counted in each colonic section. The percentage of TUNEL-labelled epithelia was used to present the apoptotic index (AI).
The Kruskal-Wallis test was applied to analyze the nonparametric data, and the one-way analysis of variance followed by Tukey’s post hoc test was adopted for the quantitative data. P < 0.05 was considered to represent statistical significance.
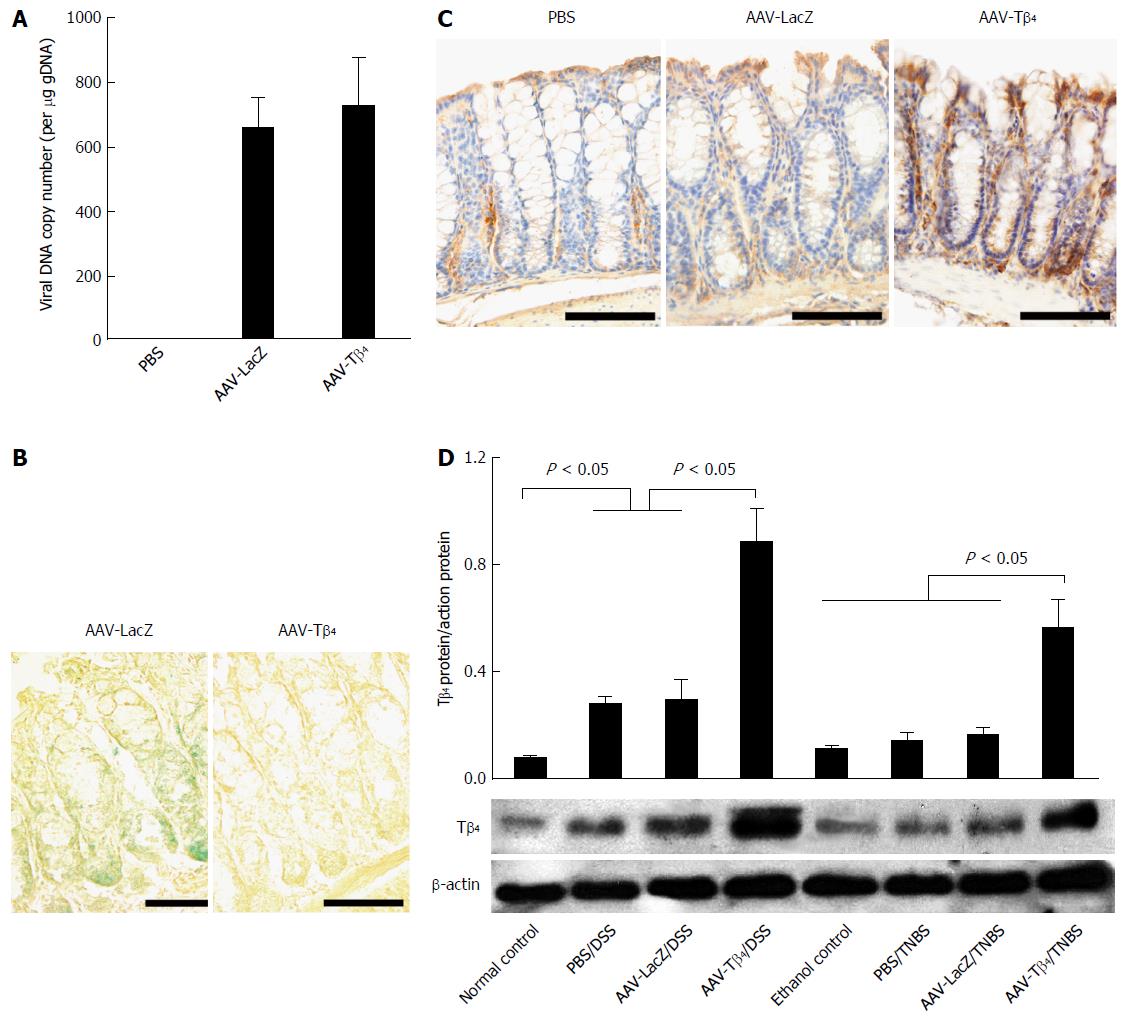
To verify the transduction efficiency of ic AAV, we first used real-time PCR to determine the abundance of vector DNA in the mouse colon. As shown in Figure 1A, real-time PCR revealed the presence of AAV vector DNA in the mouse colon after the administration of rAAVs. Next, we performed X-gal staining and Tβ4 immunostaining to determine the AAV-mediated expression of foreign genes. X-gal staining localized the ectopic β-galactosidase expression in the colonic epithelium and lamina propria of the AAV-LacZ-treated mice (Figure 1B). Immunohistochemistry showed an increased expression of Tβ4 in the AAV-Tβ4-treated mice compared to the mice administered with PBS or AAV-LacZ (Figure 1C). Moreover, we measured the colonic expression of Tβ4 using Western blot in the two sets of colitic mice. The results showed that the colonic expression of Tβ4 was notably upregulated after DSS treatment while slightly increased after enemas of TNBS. Intracolonic AAV-Tβ4 led to a drastically increased colonic expression of Tβ4 in both colitis models (Figure 1D). These findings provide further evidence that rAAV by ic could efficiently deliver foreign genes into the mouse colonic tissue, and AAV-Tβ4 could mediate the secretory expression of Tβ4.
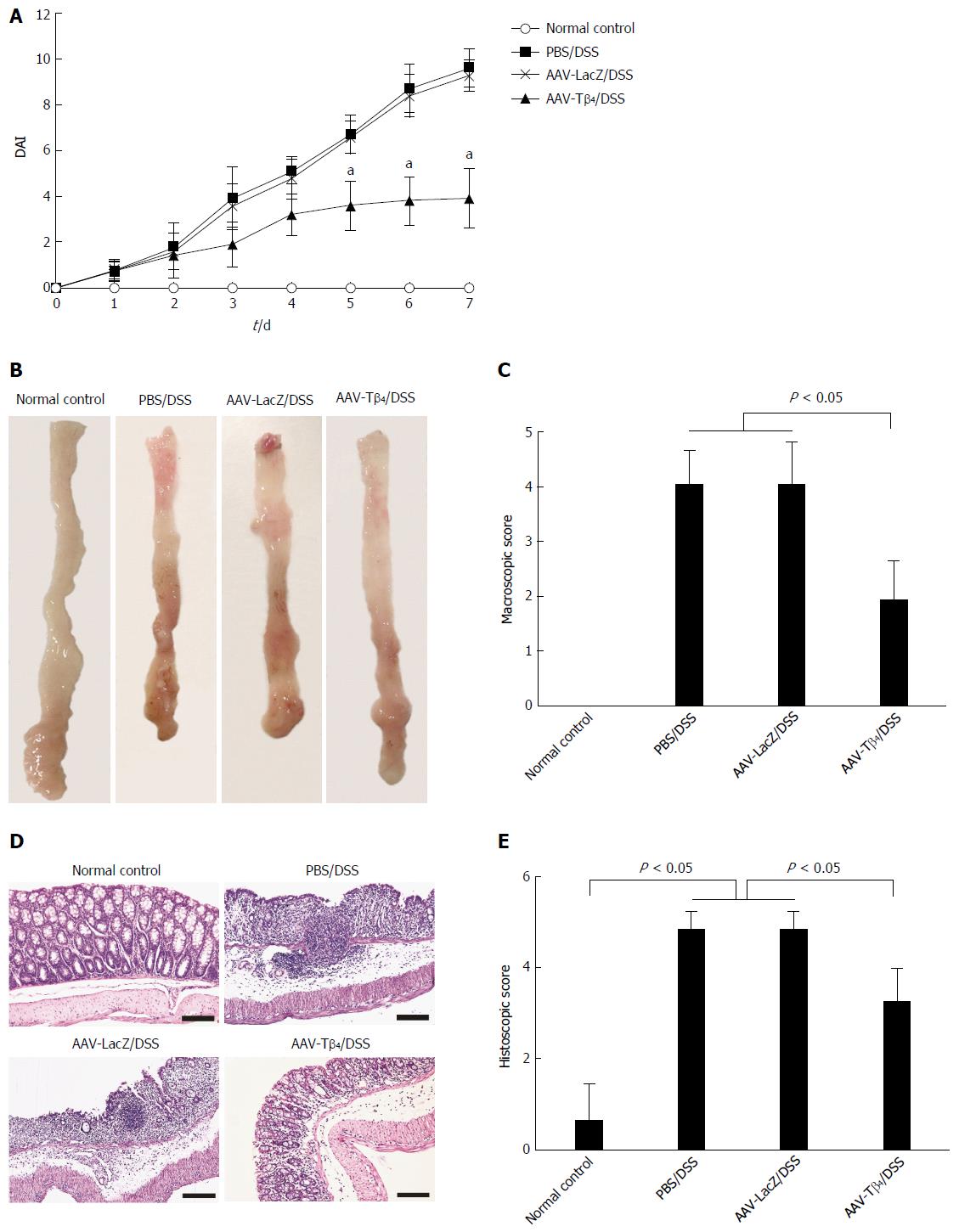
Mice in the PBS/DSS and AAV-LacZ/DSS groups suffered distinct weight loss, diarrhea and hematochezia, with higher DAI scores than normal mice. AAV-Tβ4 administration ameliorated DSS-induced colitic manifestations and DAI scores (Figure 2A). Macroscopic examination revealed that DSS treatment led to extensive colonic damages characterized by hyperemia, edema and ulceration (Figure 2B), while pre-treatment with AAV-Tβ4 significantly attenuated the colonic damages, presenting low macroscopic scores (Figure 2B and C). Similarly, DSS-induced microscopic lesions, such as loss of goblet cells and crypts, epithelia necrosis, inflammatory cell infiltrations and crypt abscesses, were also markedly alleviated by AAV-Tβ4 treatment (Figure 2D and E).
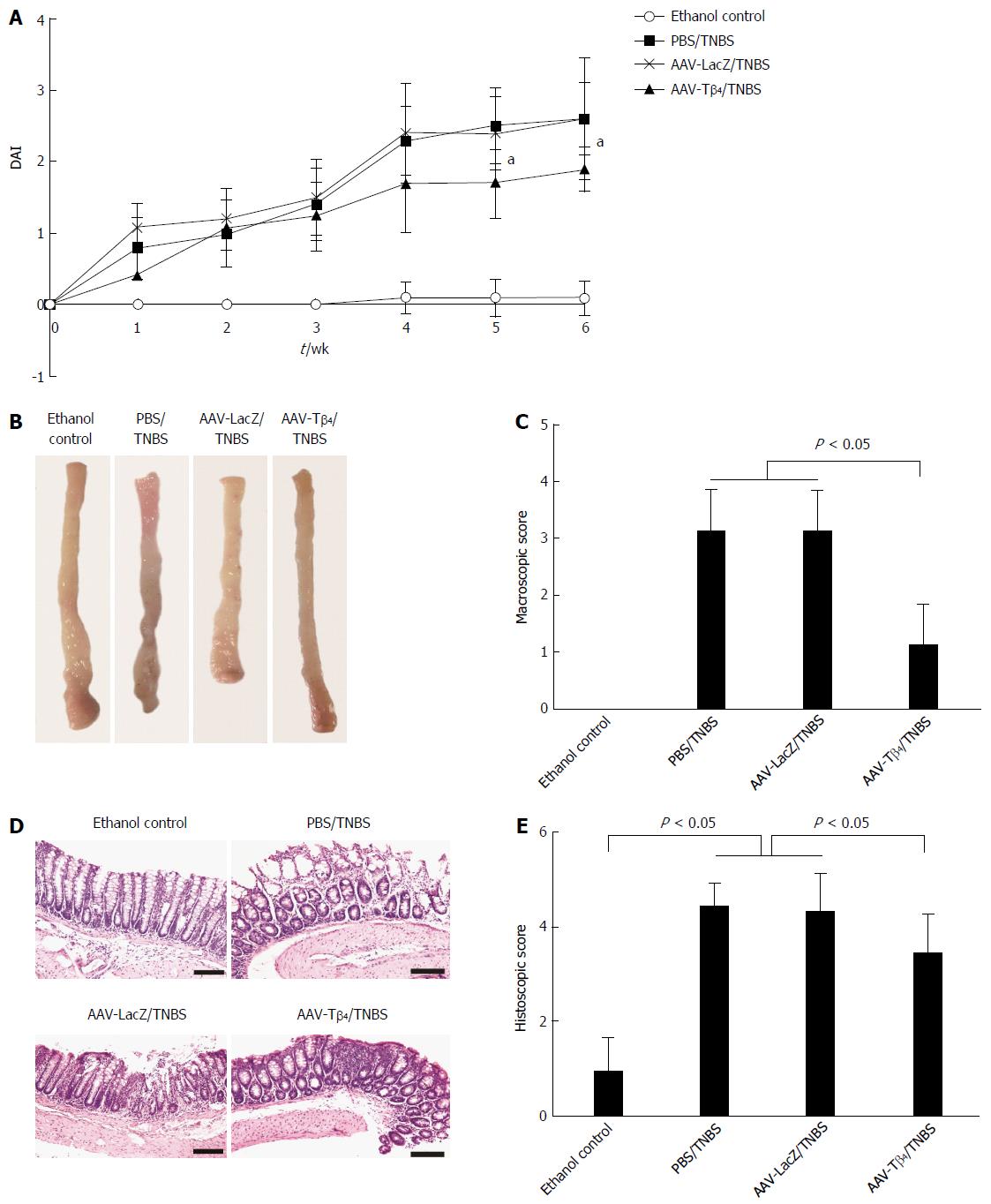
Repeated TNBS enemas led to similar but milder symptoms as those caused by the DSS treatment. Mice in the PBS/TNBS and AAV-LacZ/TNBS groups presented notably higher DAI scores compared with the EC mice. The AAV-Tβ4 treatment alleviated mouse suffering, as indicated by the significantly lower DAI scores after the fifth week of modeling (Figure 3A). Similar to the findings in the DSS colitis model, AAV-Tβ4 administration markedly alleviated TNBS-induced mucosal damage both macroscopically (Figure 3B and C) and microscopically (Figure 3D and E).
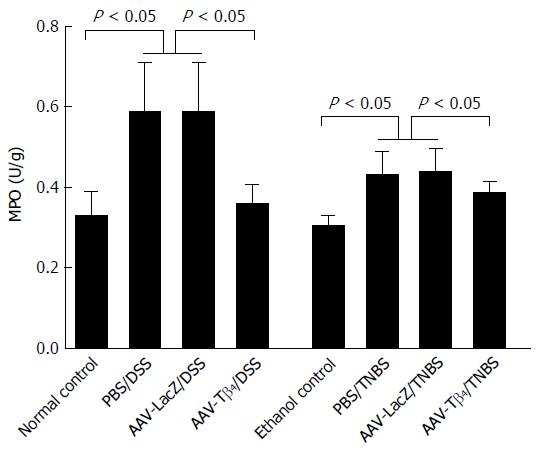
Colonic MPO activity, a common index for neutrophil and macrophage infiltrations, was increased in both colitis models compared to their respective control groups. The significantly decreased colonic MPO activities in the colitic mice administered with AAV-Tβ4 indicate the efficacy of Tβ4 in attenuating inflammatory cell infiltration (Figure 4).
Apoptosis of the colonic mucosal epithelia disrupts mucosal integrity and, consequently, affects the barrier function of the colonic mucosa. TUNEL staining was performed to assess the effect of Tβ4 on colonic epithelial apoptosis in the two colitis models. As shown in Figure 5, only a few apoptotic cells were distributed on the surface of the normal colonic mucosa. Both DSS and TNBS treatments remarkably led to the apoptosis of the mucosal epithelial cells, while AAV-Tβ4 administration significantly attenuated these changes. The AI of the AAV-Tβ4 groups was markedly lower than that of the PBS or AAV-LacZ groups.
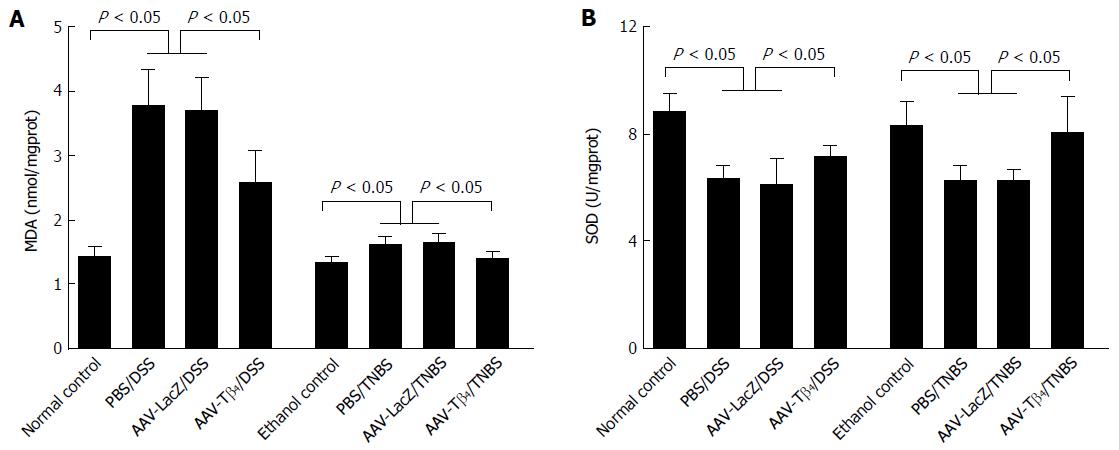
Figure 6 shows that MDA content was distinctly increased after DSS treatment, while it was mildly increased in the TNBS model. AAV-Tβ4 markedly decreased the colonic MDA levels in both DSS- and TNBS-treated mice. DSS and TNBS treatments decreased the colonic SOD activities similarly, while AAV-Tβ4 reversed these changes. These results demonstrate the efficacy of Tβ4 in the alleviation of oxidative stress in colitis.
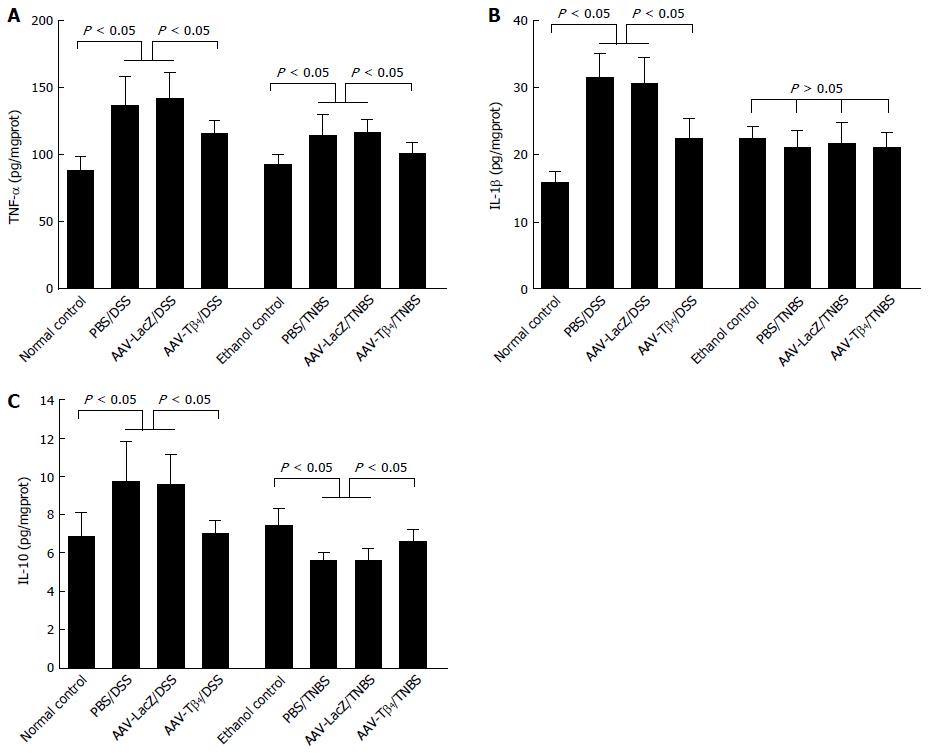
Colonic productions of inflammatory cytokines were determined to make a preliminary exploration of the underlying mechanisms of the anti-inflammatory effects of Tβ4. As shown in Figure 7, DSS treatment resulted in distinct increases in colonic TNF-α, IL-1β and IL-10 levels, while ectopically expressed Tβ4 significantly inhibited these increases. Repeated TNBS enemas increased colonic TNF-α levels and decreased IL-10 levels, while AAV-Tβ4 administration attenuated these alterations (Figure 7A and C). Unlike the findings in the DSS model, neither TNBS treatment nor ic AAV-Tβ4 significantly altered colonic IL-1β levels (Figure 7B, P > 0.05). Collectively, these data indicate that Tβ4 could modulate the production of inflammatory cytokines in inflamed colons.
PCNA immunostaining demonstrated that both DSS and TNBS caused notable increases in the proportions of PCNA-positive cells in the colonic mucosa, while ectopic Tβ4 mediated by AAV significantly suppressed these increases (Figure 8), indicating that Tβ4 alleviated the acceleration of cell proliferation in the inflamed colons.
Although increased levels of serum Tβ4 were reported in IBD patients[20], the role of Tβ4 in the development of IBDs is still elusive. In this study, the colonic expression of Tβ4 was increased after colitis induction, especially in the DSS model, and the increase was positively related to the colitis severity. These findings suggest that differential expressions of Tβ4 might be helpful to predict and assess the severity of IBDs. The present study also showed that AAV-mediated overexpression of Tβ4 in mouse colons alleviated both DSS- and TNBS-induced colitis. Collectively, these data suggest a dose-dependent protective effect of Tβ4 in inflamed colons. The increased production of local Tβ4 in mice with severe colitis reflected an adaptive response of these mice to colonic damages, while the increased expression of endogenous Tβ4 might not be sufficient to withstand the colonic injury and present the protective effect. Only a dramatic increase in the local Tβ4 content as in the present study achieved by AAV delivery, can distinctly attenuate the colitic manifestations in mice. Furthermore, our subsequent results indicated that the protective effect of Tβ4 may involve suppressing oxidant damage and inflammatory cell infiltrations, regulating colonic inflammatory cytokine levels, preventing mucosal epithelia from apoptosis and regulating cell regeneration in the inflamed colonic tissues.
Inflammation is the core component of IBDs, in which neutrophils and macrophages play crucial roles. MPO is mainly produced by macrophages and neutrophils, and its activity is usually considered as an indicator of mucosal inflammation in IBDs. Previous studies by others demonstrated that Tβ4 treatment reduced the infiltration of MPO-positive neutrophils into the infarct zone after myocardial infarction[25], and reduced the MPO activity in bleomycin-intoxicated lungs in mice[26]. In this study, MPO activity was distinctly decreased after AAV-Tβ4 administration in both DSS- and TNBS-treated mice, enhancing our understanding of the inflammation-inhibitory efficacy of Tβ4.
TNF-α is the most potent pro-inflammatory cytokine involved in IBDs, which was verified by the successful application of TNF-α antibodies for the treatment of IBDs[27-29]. Consistent with the previous study in which systemic administration of Tβ4 decreased the TNF-α level in mice with sepsis[9], our results show that AAV-Tβ4 markedly alleviated the upregulated expression of TNF-α in the injured colonic tissues, suggesting that suppressing TNF-α expression is an essential mechanism for the Tβ4-mediated alleviation of colitis.
IL-1β is an important mediator of inflammation, and its level is often increased during the development of UC. However, reports regarding alterations of IL-1β levels in CD are controversial[28,30-32]. Distinct upregulation of IL-1β was found in the colons of DSS-treated mice, while the IL-1β content was hardly changed in the TNBS model. Tβ4 reversed the DSS-induced increase in colonic IL-1β expression, which suggests that the suppression of IL-1β expression contributes to the protective effect of Tβ4 on colitis.
IL-10, a potent anti-inflammation cytokine, is considered a negative regulator in IBDs[33,34]. However, the colonic IL-10 expression profile in patients with IBD is controversial. Most reports indicate that mucosal levels of IL-10 are increased in UC[27,35], but normal[28], decreased[36,37] and elevated[38,39] mucosal IL-10 levels are reported for CD. Recent studies have demonstrated that polymorphisms of the IL-10 gene are correlated with the development of UC and CD[40]. In our study, AAV-Tβ4 attenuated the DSS-induced increase and TNBS-induced decrease in colonic IL-10 levels. It is known that TNBS colitis exhibits enhanced Th1-mediated inflammatory response, while DSS colitis is characterized by a predominant Th2 response (increase in IL-10). A previous study reported that Tβ4 had inhibitory effects on human colonic lamina propria lymphocytes[18]. Thus, we speculate that the disparate effects of Tβ4 on IL-10 production might be mediated via its suppression on the differently enhanced responses of immune cells in the two models. Overall, our results suggest that restoring the balance of cytokines might be one of the underlying mechanisms of Tβ4 in attenuating colitis.
Oxidant/antioxidant imbalance plays an essential role in the pathogenesis of IBDs. The present study demonstrates that AAV-Tβ4 administration decreased MDA production and increased SOD activity in the inflamed colons, which illustrates the efficacy of Tβ4 in scavenging ROS and boosting anti-oxidative defense during colitis. ROS mainly originate from inflammatory cells. Therefore, the mechanism of Tβ4 in the prevention of oxidant damage in colonic tissue should be mainly attributable to the alleviation of the infiltration of inflammatory cells. In addition, previous studies have revealed that Tβ4 reduced ROS levels in corneal epithelial cells, cardiac fibroblasts and cardiomyocytes via the upregulation of the expression of antioxidant enzyme SOD and catalase[41-43]. A direct enhancement of SOD expression by Tβ4 might also contribute to the protection of oxidative damage.
Intestinal epithelial apoptosis is a representative indicator of mucosal damage in IBDs. In the present study, the attenuation of colonic cell apoptosis by Tβ4 should be mainly due to its inhibitory effects on inflammation and oxidative reaction, which protect mucosal epithelia against death-inducing factors. Moreover, there is substantial evidence that Tβ4 suppresses apoptosis of corneal epithelia, cardiomyocytes, neurons, normal intestinal epithelia, and colorectal cancer cells by regulating several intracellular signaling events such as Akt activation, c-Jun phosphorylation, Bcl-2 phosphorylation, and decreased caspase-3 activity[8,44-48]. Thus, a direct anti-apoptotic activity of Tβ4 might also participate in this protective effect. As apoptosis is a dynamic pathophysiologic process with considerable complexity, further investigation is needed to clarify the underlying mechanism(s) of Tβ4 for inhibiting colonic epithelial apoptosis.
Tβ4 can promote the regeneration of the cornea, skin, heart, and intestine epithelial cells[1,46,47]; nevertheless, PCNA immunostaining in the current study revealed that Tβ4 attenuated the accelerated proliferation of colonic mucosal cells following DSS or TNBS treatment. One possible explanation may be that the attenuation of mucosal damage by Tβ4 reduces the compensatory regeneration secondary to tissue damage, which surpasses the proliferation-promoting effect mediated by Tβ4.
Because IBD is a chronic and relapsing disorder mainly involving the intestines, local and persistent expression of therapeutic molecules should be a suitable approach to IBD treatment. AAV is characterized by a wide range of hosts, long-term expression, low immunogenicity and lack of toxicity. Repeated application of AAV is also feasible. Therefore, AAV emerges as a promising vector in gene therapy for chronic diseases, and it has been widely utilized in clinical trials to treat a variety of diseases[49-51]. In the present study, we constructed AAV-Tβ4 and confirmed its efficacy in transducing mouse colonic cells and mediating high expression of secretory Tβ4.
In conclusion, our study verifies that Tβ4 is a protective molecule in murine colitis and suggests that ic AAV-Tβ4 could potentially be developed into a promising therapeutic approach for IBDs. To further clarify the importance of endogenous Tβ4 in the maintenance of intestinal homeostasis and in the development of IBDs, tissue-specific ablation of Tβ4 in mouse colons or neutralization of extracellular Tβ4 by immunizing animals with a Tβ4-carrier protein conjugates should be carried out. Besides, the “treatment” effect of AAV-Tβ4 on chronic colitis such as IL-10 knock-out mouse model is also under our consideration.
Although increased serum thymosin β4 (Tβ4) level has been reported in inflammatory bowel diseases (IBDs) patients, its role in the development of IBDs is still unclear. Preclinical and clinical studies have confirmed that Tβ4 can promote wound healing and attenuate inflammation, suggesting a possible beneficial role of Tβ4 in colitis. Recombinant adeno-associated virus (AAV), when intracolonically (ic) administered, can efficiently infect the colonic mucosa and therefore it is a suitable vector to mediate ectopic expression of therapeutic genes for both research and therapeutic applications in IBDs.
This study is novel in demonstrating that Tβ4 is a protective factor in colitis by relieving oxidant damage, attenuating epithelial apoptosis, reducing inflammatory cell infiltrations, and modulating tumor necrosis factor-α, interleukin (IL)-1β and IL-10 levels in the inflamed colonic mucosa of mice.
To achieve persistent, secretory overexpression of Tβ4 in mouse colons, the authors constructed a recombinant AAV carrying a fusion gene composed of the N-terminal 80 amino acid signal peptide of human neurotrophin-4 preproprotein fused to the N-terminus of the coding region of Tβ4 and employed it via ic administration. Moreover, the authors tested the effects of ic AAV-Tβ4 in both the dextran sulfate sodium colitis (ulcerative colitis-like) and TNBS (Crohn’s disease-like) colitis models.
The results of the present study suggest that intracolonic AAV-Tβ4 is likely to be developed as an efficient and convenient therapeutic agent for IBDs.
Tβ4, a conserved 43-amino acid protein, is present in nearly all cells and in various body fluids. Despite the lack of a signal peptide, Tβ4 can be released to extracellular spaces by either secretion, cell lysis or necrosis, while extracellular or exogenous Tβ4 can be rapidly internalized by presently unknown mechanisms. Tβ4 possesses many physiological activities, including promotion of cell migration, adhesion, angiogenesis, cell survival, stem cell maturation and down-regulation of inflammatory cytokines. There is evidence that the biological actions of Tβ4 are mediated in part by activating integrin-linked kinase, increasing bcl-2 expression and decreasing caspase activation.
This is a well-designed study. The role of Tβ4 in colitis is novel and the data of the present study indicate that AAV-Tβ4 may be explored as a promising therapeutic agent for IBDs.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gupta S S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Crockford D, Turjman N, Allan C, Angel J. Thymosin beta4: structure, function, and biological properties supporting current and future clinical applications. Ann N Y Acad Sci. 2010;1194:179-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 2. | Jo JO, Kang YJ, Ock MS, Kleinman HK, Chang HK, Cha HJ. Thymosin β4 expression in human tissues and in tumors using tissue microarrays. Appl Immunohistochem Mol Morphol. 2011;19:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Hannappel E. Thymosin beta4 and its posttranslational modifications. Ann N Y Acad Sci. 2010;1194:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Mannherz HG, Hannappel E. The beta-thymosins: intracellular and extracellular activities of a versatile actin binding protein family. Cell Motil Cytoskeleton. 2009;66:839-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 96] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Ho JH, Chuang CH, Ho CY, Shih YR, Lee OK, Su Y. Internalization is essential for the antiapoptotic effects of exogenous thymosin beta-4 on human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:27-33. [PubMed] |
| 6. | Cierniewski CS, Sobierajska K, Selmi A, Kryczka J, Bednarek R. Thymosin β4 is rapidly internalized by cells and does not induce intracellular Ca2+ elevation. Ann N Y Acad Sci. 2012;1269:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 518] [Cited by in RCA: 518] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 8. | Sosne G, Siddiqi A, Kurpakus-Wheater M. Thymosin-beta4 inhibits corneal epithelial cell apoptosis after ethanol exposure in vitro. Invest Ophthalmol Vis Sci. 2004;45:1095-1100. [PubMed] |
| 9. | Badamchian M, Fagarasan MO, Danner RL, Suffredini AF, Damavandy H, Goldstein AL. Thymosin beta(4) reduces lethality and down-regulates inflammatory mediators in endotoxin-induced septic shock. Int Immunopharmacol. 2003;3:1225-1233. [PubMed] |
| 10. | Peng H, Xu J, Yang XP, Dai X, Peterson EL, Carretero OA, Rhaleb NE. Thymosin-β4 prevents cardiac rupture and improves cardiac function in mice with myocardial infarction. Am J Physiol Heart Circ Physiol. 2014;307:H741-H751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Zhang J, Zhang ZG, Morris D, Li Y, Roberts C, Elias SB, Chopp M. Neurological functional recovery after thymosin beta4 treatment in mice with experimental auto encephalomyelitis. Neuroscience. 2009;164:1887-1893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Morris DC, Cui Y, Cheung WL, Lu M, Zhang L, Zhang ZG, Chopp M. A dose-response study of thymosin β4 for the treatment of acute stroke. J Neurol Sci. 2014;345:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | Brady RD, Grills BL, Schuijers JA, Ward AR, Tonkin BA, Walsh NC, McDonald SJ. Thymosin β4 administration enhances fracture healing in mice. J Orthop Res. 2014;32:1277-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, Hazlett LD. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Exp Eye Res. 2002;74:293-299. [PubMed] |
| 15. | Philp D, Badamchian M, Scheremeta B, Nguyen M, Goldstein AL, Kleinman HK. Thymosin beta 4 and a synthetic peptide containing its actin-binding domain promote dermal wound repair in db/db diabetic mice and in aged mice. Wound Repair Regen. 2003;11:19-24. [PubMed] |
| 16. | Philp D, St-Surin S, Cha HJ, Moon HS, Kleinman HK, Elkin M. Thymosin beta 4 induces hair growth via stem cell migration and differentiation. Ann N Y Acad Sci. 2007;1112:95-103. [PubMed] |
| 17. | Nemolato S, Cabras T, Cau F, Fanari MU, Fanni D, Manconi B, Messana I, Castagnola M, Faa G. Different thymosin Beta 4 immunoreactivity in foetal and adult gastrointestinal tract. PLoS One. 2010;5:e9111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Elitsur Y, Mutchnick MG, Sakr WA, Luk GD. Thymosin alpha 1 and thymosin beta 4 modulate human colonic lamina propria lymphocyte function. Immunopharmacology. 1990;20:89-96. [PubMed] |
| 19. | Goldstein A, Finkelstein J; inventors; Regenerx Biopharmaceuticals, Inc. assignee. Treatment of infections and other disorders. United States patent US 20110144020 A1. 2011 June 16. . |
| 20. | Mutchnick MG, Lee HH, Hollander DI, Haynes GD, Chua DC. Defective in vitro gamma interferon production and elevated serum immunoreactive thymosin beta 4 levels in patients with inflammatory bowel disease. Clin Immunol Immunopathol. 1988;47:84-92. [PubMed] |
| 21. | Yang J, Liu XX, Fan H, Tang Q, Shou ZX, Zuo DM, Zou Z, Xu M, Chen QY, Peng Y. Extracellular Vesicles Derived from Bone Marrow Mesenchymal Stem Cells Protect against Experimental Colitis via Attenuating Colon Inflammation, Oxidative Stress and Apoptosis. PLoS One. 2015;10:e0140551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Hao Z, Yang X, Lv Y, Li S, Purbey BK, Su H. Intracolonically administered adeno-associated virus-bone morphogenetic protein-7 ameliorates dextran sulphate sodium-induced acute colitis in rats. J Gene Med. 2012;14:482-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Lv Y, Yang X, Huo Y, Tian H, Li S, Yin Y, Hao Z. Adenovirus-mediated hepatocarcinoma-intestine-pancreas/pancreatitis-associated protein suppresses dextran sulfate sodium-induced acute ulcerative colitis in rats. Inflamm Bowel Dis. 2012;18:1950-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Ohkawara T, Nishihira J, Takeda H, Hige S, Kato M, Sugiyama T, Iwanaga T, Nakamura H, Mizue Y, Asaka M. Amelioration of dextran sulfate sodium-induced colitis by anti-macrophage migration inhibitory factor antibody in mice. Gastroenterology. 2002;123:256-270. [PubMed] |
| 25. | Evans MA, Smart N, Dubé KN, Bollini S, Clark JE, Evans HG, Taams LS, Richardson R, Lévesque M, Martin P. Thymosin β4-sulfoxide attenuates inflammatory cell infiltration and promotes cardiac wound healing. Nat Commun. 2013;4:2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Conte E, Genovese T, Gili E, Esposito E, Iemmolo M, Fruciano M, Fagone E, Pistorio MP, Crimi N, Cuzzocrea S. Thymosin β4 protects C57BL/6 mice from bleomycin-induced damage in the lung. Eur J Clin Invest. 2013;43:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | Szkaradkiewicz A, Marciniak R, Chudzicka-Strugała I, Wasilewska A, Drews M, Majewski P, Karpiński T, Zwoździak B. Proinflammatory cytokines and IL-10 in inflammatory bowel disease and colorectal cancer patients. Arch Immunol Ther Exp (Warsz). 2009;57:291-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 107] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | León AJ, Gómez E, Garrote JA, Bernardo D, Barrera A, Marcos JL, Fernández-Salazar L, Velayos B, Blanco-Quirós A, Arranz E. High levels of proinflammatory cytokines, but not markers of tissue injury, in unaffected intestinal areas from patients with IBD. Mediators Inflamm. 2009;2009:580450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641-1657. [PubMed] |
| 30. | Andoh A, Ogawa A, Kitamura K, Inatomi O, Fujino S, Tsujikawa T, Sasaki M, Mitsuyama K, Fujiyama Y. Suppression of interleukin-1beta- and tumor necrosis factor-alpha-induced inflammatory responses by leukocytapheresis therapy in patients with ulcerative colitis. J Gastroenterol. 2004;39:1150-1157. [PubMed] |
| 31. | Yamamoto T, Umegae S, Kitagawa T, Matsumoto K. Postoperative change of mucosal inflammation at strictureplasty segment in Crohn’s disease: cytokine production and endoscopic and histologic findings. Dis Colon Rectum. 2005;48:749-757. [PubMed] |
| 32. | Ruffolo C, Scarpa M, Faggian D, Pozza A, Navaglia F, D’Incà R, Hoxha P, Romanato G, Polese L, Sturniolo GC. Cytokine network in rectal mucosa in perianal Crohn’s disease: relations with inflammatory parameters and need for surgery. Inflamm Bowel Dis. 2008;14:1406-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Rennick DM, Fort MM. Lessons from genetically engineered animal models. XII. IL-10-deficient (IL-10(-/-) mice and intestinal inflammation. Am J Physiol Gastrointest Liver Physiol. 2000;278:G829-G833. [PubMed] |
| 34. | Fedorak RN, Gangl A, Elson CO, Rutgeerts P, Schreiber S, Wild G, Hanauer SB, Kilian A, Cohard M, LeBeaut A. Recombinant human interleukin 10 in the treatment of patients with mild to moderately active Crohn’s disease. The Interleukin 10 Inflammatory Bowel Disease Cooperative Study Group. Gastroenterology. 2000;119:1473-1482. [PubMed] |
| 35. | Fonseca-Camarillo G, Furuzawa-Carballeda J, Llorente L, Yamamoto-Furusho JK. IL-10-- and IL-20--expressing epithelial and inflammatory cells are increased in patients with ulcerative colitis. J Clin Immunol. 2013;33:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Correa I, Veny M, Esteller M, Piqué JM, Yagüe J, Panés J, Salas A. Defective IL-10 production in severe phenotypes of Crohn’s disease. J Leukoc Biol. 2009;85:896-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 37. | Gologan S, Iacob R, Iancu D, Iacob S, Cotruta B, Vadan R, Catuneanu AM, Constantinescu I, Barbarii L, Gheorghe C. Inflammatory gene expression profiles in Crohn’s disease and ulcerative colitis: a comparative analysis using a reverse transcriptase multiplex ligation-dependent probe amplification protocol. J Crohns Colitis. 2013;7:622-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Wang AH, Lam WJ, Han DY, Ding Y, Hu R, Fraser AG, Ferguson LR, Morgan AR. The effect of IL-10 genetic variation and interleukin 10 serum levels on Crohn’s disease susceptibility in a New Zealand population. Hum Immunol. 2011;72:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Santaolalla R, Mañé J, Pedrosa E, Lorén V, Fernández-Bañares F, Mallolas J, Carrasco A, Salas A, Rosinach M, Forné M. Apoptosis resistance of mucosal lymphocytes and IL-10 deficiency in patients with steroid-refractory Crohn’s disease. Inflamm Bowel Dis. 2011;17:1490-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Andersen V, Ernst A, Christensen J, Østergaard M, Jacobsen BA, Tjønneland A, Krarup HB, Vogel U. The polymorphism rs3024505 proximal to IL-10 is associated with risk of ulcerative colitis and Crohns disease in a Danish case-control study. BMC Med Genet. 2010;11:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 41. | Ho JH, Tseng KC, Ma WH, Chen KH, Lee OK, Su Y. Thymosin beta-4 upregulates anti-oxidative enzymes and protects human cornea epithelial cells against oxidative damage. Br J Ophthalmol. 2008;92:992-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Kumar S, Gupta S. Thymosin beta 4 prevents oxidative stress by targeting antioxidant and anti-apoptotic genes in cardiac fibroblasts. PLoS One. 2011;6:e26912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 43. | Wei C, Kumar S, Kim IK, Gupta S. Thymosin beta 4 protects cardiomyocytes from oxidative stress by targeting anti-oxidative enzymes and anti-apoptotic genes. PLoS One. 2012;7:e42586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 44. | Srivastava D, Saxena A, Michael Dimaio J, Bock-Marquette I. Thymosin beta4 is cardioprotective after myocardial infarction. Ann N Y Acad Sci. 2007;1112:161-170. [PubMed] |
| 45. | Cheng P, Kuang F, Zhang H, Ju G, Wang J. Beneficial effects of thymosin β4 on spinal cord injury in the rat. Neuropharmacology. 2014;85:408-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Chao TC, Chan LC, Ju SY, Tang MC, Liu CY, Chen PM, Tzeng CH, Su Y. In vivo growth suppression of CT-26 mouse colorectal cancer cells by adenovirus-expressed small hairpin RNA specifically targeting thymosin beta-4 mRNA. Cancer Gene Ther. 2014;21:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 47. | Chao TC, Chen KJ, Tang MC, Chan LC, Chen PM, Tzeng CH, Su Y. Thymosin beta-4 knockdown in IEC-6 normal intestinal epithelial cells induces DNA re-replication via downregulating Emi1. J Cell Physiol. 2014;229:1639-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 48. | Moon EY, Song JH, Yang KH. Actin-sequestering protein, thymosin-beta-4 (TB4), inhibits caspase-3 activation in paclitaxel-induced tumor cell death. Oncol Res. 2007;16:507-516. [PubMed] |
| 49. | Vardas E, Kaleebu P, Bekker LG, Hoosen A, Chomba E, Johnson PR, Anklesaria P, Birungi J, Barin B, Boaz M. A phase 2 study to evaluate the safety and immunogenicity of a recombinant HIV type 1 vaccine based on adeno-associated virus. AIDS Res Hum Retroviruses. 2010;26:933-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Nathwani AC, Reiss UM, Tuddenham EG, Rosales C, Chowdary P, McIntosh J, Della Peruta M, Lheriteau E, Patel N, Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N Engl J Med. 2014;371:1994-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 897] [Cited by in RCA: 985] [Article Influence: 89.5] [Reference Citation Analysis (0)] |
| 51. | Bainbridge JW, Mehat MS, Sundaram V, Robbie SJ, Barker SE, Ripamonti C, Georgiadis A, Mowat FM, Beattie SG, Gardner PJ. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372:1887-1897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 565] [Article Influence: 56.5] [Reference Citation Analysis (0)] |