Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.216
Peer-review started: July 13, 2016
First decision: August 29, 2016
Revised: September 6, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: January 14, 2017
Processing time: 184 Days and 16.2 Hours
To assess the feasibility of SPECT-computed tomography (CT) in rats with trinitrobenzene sulfonic acid (TNBS)-induced acute colitis and confront it with model inflammatory characteristics.
Colitis was induced in Sprague-Dawley rats by intrarectal injection of TNBS (n = 10) while controls received vehicle (n = 10). SPECT-CT with intravenous injection of 10 MBq of 67Ga-Citrate was performed at day 2. SPECT-CT criteria were colon wall thickness and maximal wall signal intensity. Laboratory parameters were assessed: colon weight:length ratio, colon cyclooxygenase-2 expression by western blot and histological inflammatory score.
Colon weight/length ratio, colon COX-2 expression and histological inflammatory score were significantly higher in the TNBS group than in the control group (P = 0.0296, P < 0.0001, P = 0.0007 respectively). Pixel max tend to be higher in the TNBS group than in the control group but did not reach statistical significance (P = 0.0662). Maximal thickness is significantly increased in the TNBS group compared to the control group (P = 0.0016) while colon diameter is not (P = 0.1904). Maximal thickness and colon diameter were correlated to colon COX-2 expression (P = 0.0093, P = 0.009 respectively) while pixel max was not (P = 0.22). Maximal thickness was significantly increased when inflammation was histologically observed (P = 0.0043) while pixel max and colon diameter did not (P = 0.2452, P = 0.3541, respectively).
SPECT-CT is feasible and easily distinguished control from colitic rats.
Core tip: Transsectional imaging such as magnetic resonance imaging are emerging to evaluate colonic inflammation but they do not allowed inflammation quantification. Functional imaging by SPECT-computed tomography (CT) could be interesting under quantification. This present study validates the use of SPECT in colitis models. We confronted SPECT-CT parameters to lab parameters and we found that maximal thickness was correlated to inflammatory parameters such as colon COX-2 expression or histological inflammatory score. Evaluating animal models may be important an important tool for preclinical studies in inflammatory bowel diseases models. It also allows optimizing the technique prior to patient contact.
- Citation: Marion-Letellier R, Bohn P, Modzelewski R, Vera P, Aziz M, Guérin C, Savoye G, Savoye-Collet C. SPECT-computed tomography in rats with TNBS-induced colitis: A first step toward functional imaging. World J Gastroenterol 2017; 23(2): 216-223
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/216.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.216
Natural history of inflammatory bowel diseases (IBD) consists in repetition of flares leading to transmural inflammation that affects the gastrointestinal tract[1]. These long-term post-inflammatory consequences usually lead to fibrosis development and can be responsible of disabling symptoms. However, the management of inflammation by IBD therapy has markedly progressed over the past 20 years thanks to the availability of immunosuppressive and biologics (anti-TNF or anti-adhesion molecules)[2]. Biologics enable to achieve a better and more long-term response for IBD patients. Lesions improvement during biologic therapy makes possible to look beyond symptoms[3]. Consequently current therapeutic objective is not only to control disease activity but also to prevent progression of structural bowel damage[4].
Currently, endoscopy is the gold standard technique for assessing mucosal inflammation in IBD, as well as evaluating the treatment efficacy on mucosa[2], it does however represent a minor risk of severe complications[5]. Endoscopy can be incomplete[6] and is limited to mucosa and therefore cannot reflect parietal damage. Moreover, extra-luminal disease complications, such as fistulae or abscesses leading to surgery, cannot be detected by endoscopy, even though they are decisive in the natural course of CD. Consequently, trans-sectional imaging techniques including magnetic resonance imaging (MRI), ultrasound and computed tomography (CT) are emerging to evaluate small bowel and colonic inflammation in IBD[7-9].
The evaluation of treatment impact on inflammatory lesions also requires the development of innovative treatment evaluation methods at preclinical stage. Much of what we have learned regarding the pathophysiological mechanisms underlying IBD genesis and progress has come from experimental studies using animal models of intestinal inflammation[10]. The 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitis model is a reference acute colitis models with transmural inflammation close in some regards to those observed in CD[11,12]. We previously validate the use of MRI to assess inflammation and fibrosis in colitis models[13,14]. We now aim to evaluate the SPECT to assess inflammation. SPECT is a main technique in nuclear imaging to obtain functional images compared to anatomical MRI images.
This study aimed to validate the feasibility of SPECT in rats with TNBS-induced colitis and compare results with clinical, biological and histological characteristics of the model.
Animal care and experimentation complied with both French and European Community regulations (Official Journal of the European Community L358, 18/12/1986) and Rachel Marion-Letellier is authorized by the French Government to use this rat model (Authorization no. 76-106).
Sprague-Dawley male rats weighing 200-250 g were obtained from Janvier (Le Genest St Isle, France). They were randomized into a colitic group (10 rats) and a control noncolitic group (10 rats). Water and food were provided ad libitum until SPECT exam. The rats were weighed on the day of colitis induction. After the imaging session, they were killed by lethal anesthesia and their colon was removed. It was then cleaned with phosphate-buffered saline (PBS) to remove fecal residues, then measured and weighed to determine the colon weight:length ratio, which is an inflammatory marker. Pieces of colon were collected and then stored at -80 °C. One piece was fixed in formalin and stored at 4 °C.
Colitis was induced at day 0 by administration of TNBS (Sigma-Aldrich, Saint-Quentin Fallavier, France) as previously described[13,15,16]. The rats were then briefly anesthetized with an intraperitoneal injection of ketamine and chlorpromazine following 24-h food deprivation. Colitic rats received TNBS dissolved in 50% (v/v) ethanol (25 mg in a volume of 0.25 mL) by intrarectal injection through a cannula, whereas control rats received the vehicle (0.25 mL of 50% ethanol). Following instillation of the hapten, the rats were maintained in a head-down position for a few minutes to prevent leakage of the intracolon instillate.
SPECT-CT (Triumph®, Trifoil Imaging) with intravenous injection of 10 MBq of 67Ga-Citrate was performed at day 2: 67Ga gamma camera imaging with multi-pinholes collimators and 93 keV and 184 keV ± 10% photopeaks energy window.
Rats were injected via a tail vein with about 185 MBq of 67Ga, under continuous isoflurane anaesthesia (2.5% in O2, 1 L/min). At 1 h post-injection, SPECT scans were acquired with 64 projections over 360° (radius of rotation = 9.3 cm, 30 s/projection).
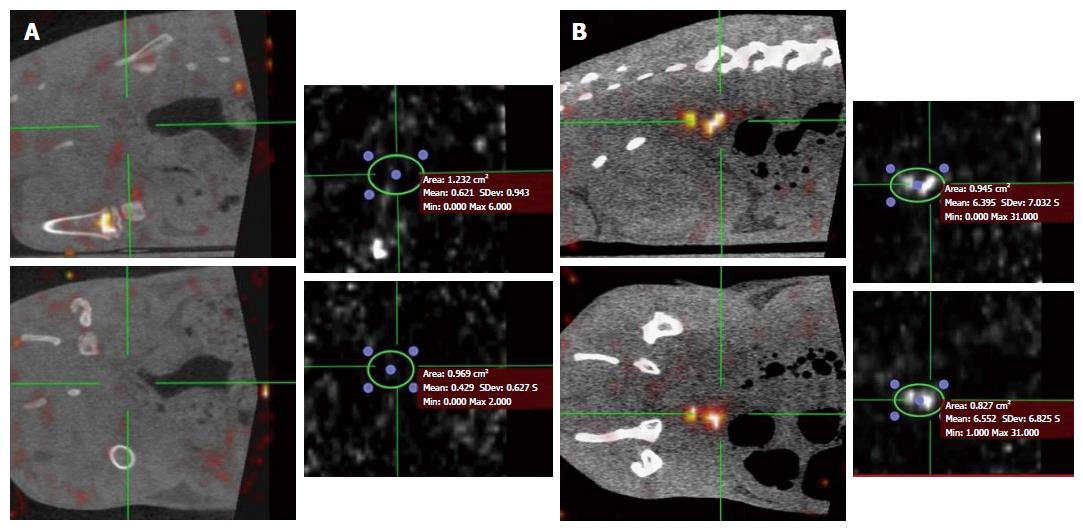
SPECT projections were reconstructed with OSEM reconstruction algorithm (5 iterations, 8 subsets). Each reconstructed matrix (80 × 80) was composed of 80 transverse images with cubic voxels of 2 mm3. SPECT acquisitions were followed by CT scans, acquired at 80 kV and 180 μA with 512 projections over 360° and a field of view (FOV) of 91 mm. Each reconstructed matrix (512 × 512) was composed of 512 transverse images with cubic voxels of 0.15 mm3. Reconstructed data from the SPECT and CT scans were visualized and co-registered with OsiriX 64 imaging software (Figure 1). SPECT-CT criteria were colon wall thickness and maximal wall signal intensity.
PBS and Protease inhibitor cocktail, were purchased from Sigma Aldrich-Company (Saint-Quentin Fallavier, France). The 4%-12% Bis-Tris gels, Invitrolon PVDF membranes, Seeblue multi-colored standard were obtained from Invitrogen (Cergy Pontoise, France). The goat polyclonal antibody anti COX-2 (sc-1747), and the secondary antibodies IgG1 horseradish conjugated were obtained from Santa Cruz biotechnology (Tebu, Le Perray-en-Yvelines, France).
Frozen colon samples were homogenized in PBS with 0.1% protease inhibitor cocktail (Sigma) and 1% phosphatase inhibitor cocktail (Sigma). Homogenates were centrifuged (12000 g, 15 min, 4 °C) and the supernatants were collected. Protein concentration was determined following Bradford’s colorimetric method. Aliquots of supernatants containing equal amounts of protein (25 μg) were separated on 4%-12% NuPAGE gel (Invitrogen) and then transferred to a nitrocellulose membrane (Hybond, GE Healthcare, United Kingdom). After blocking, membranes were incubated with COX-2 primary antibodies at the dilution of 1:500. After three washes, filter was then incubated with the secondary horseradish peroxidase linked anti-goat IgG. To check equal loading, the blots were analysed for β-actin expression. Immunodetection was performed using enhanced chemiluminiscence light-detecting kit (Amersham, Arlinghton Heights, IL, United States). Densitometric data were measured following normalisation to the control (house-keeping gene) Immunocomplexes were revealed by using the ECL detection system (GE Healthcare). Immunoblots were scanned with ImageScanner II (GE Healthcare) previously calibrated by using a greyscale marker (Kodak) and digitalized with Labscan 6.00 software (GE Healthcare).
After formalin fixation (40%), colon samples were embedded in paraffin wax blocks and 5 µm sections were stained with hematoxylin-eosin-safran. A pathologist (Moutaz Aziz) blind to treatment allocation scored these 5 μm sections. Epithelial necrosis, inflammatory cells infiltration and thickness of the mucosa were assessed using semi-quantitative scores which ranged from 0 to 3 for each variable (0, no inflammation; 1, very low level of inflammation; 2, moderate level of leukocyte infiltration; 3, high levels of leukocytes infiltration and vascular density, ulcerations) using the analysis software Leica QWin (Leica Microsystems, Bensheim, Germany).
Statistical comparisons were performed using GraphPadPrism 5. Data are expressed as mean ± SEM. Parametric quantitative data were analyzed with Student t-test and non-parametric quantitative data with Mann Whitney test. Qualitative data were analyzed with χ2 test and Fisher’s test for low number (n < 5). Correlation between parametric quantitative data was analyzed by simple linear regression and Pearson’s correlation. For correlation between non-parametric data, Spearman rank-order correlation was used and Spearman correlation coefficient r was calculated. Differences were considered significant at P < 0.05.
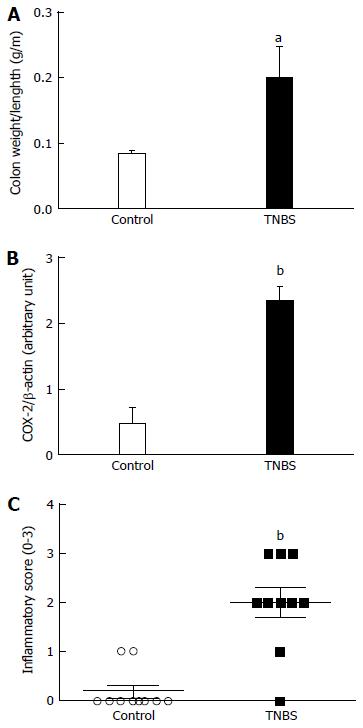
Colon weight/length ratio was significantly higher in the TNBS group (0.20 ± 0.05 g/m; Figure 2A) than in the control group (0.08 ± 0.00 g/m, P = 0.0296). Colon COX-2 expression was significantly increased in the TNBS group (2.33 ± 0.24; Figure 2B) than in the control group (0.46 ± 0.25, P < 0.0001). Histological inflammatory score was significantly higher in the TNBS group (2.00 ± 0.29) than in the control group (0.20 ± 0.13, P = 0.0007; Figure 2C).
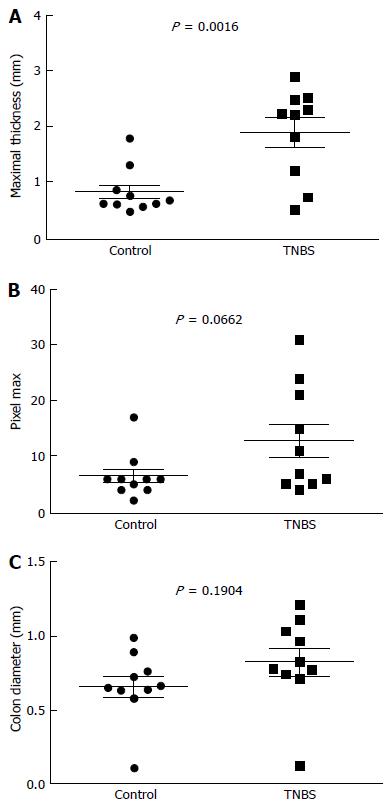
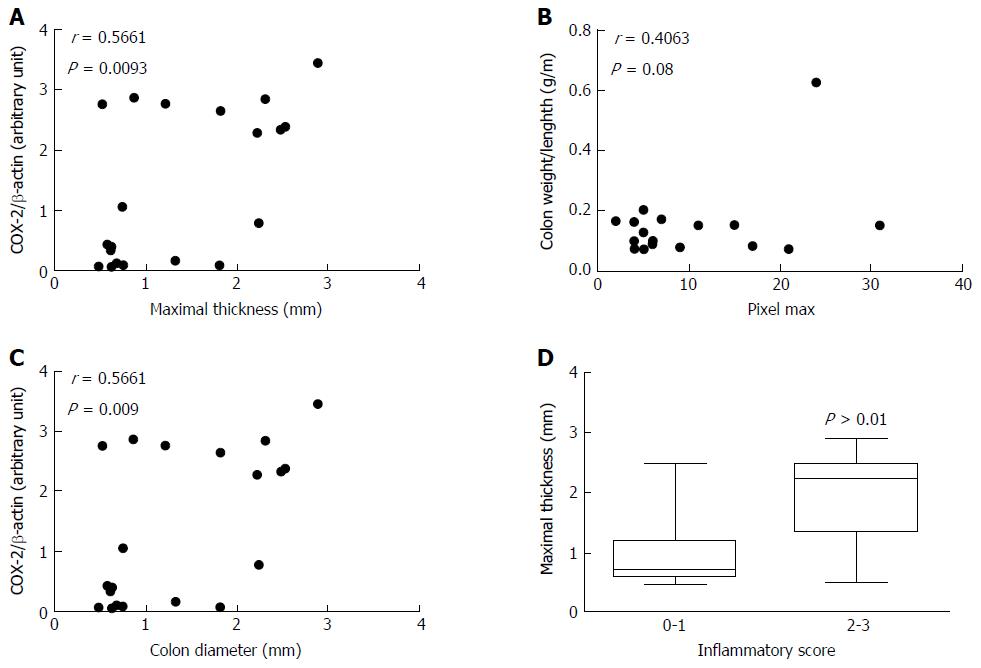
Maximal thickness is significantly increased in the TNBS group (1.9 ± 0.3 mm, Figure 3A) compared to the control group (0.8 ± 0.1 mm, P = 0.0016). Maximal thickness was correlated to colon COX-2 expression (r = 0.5661, P = 0.0093; Figure 4A) but not to colon weight/length ratio (r = 0.01626, P = 0.9458, data not shown).
Pixel max tend to be higher in the TNBS group (12.9 ± 3.0, Figure 3B) than in the control group (6.5 ± 1.3) but did not reach statistical significance (P = 0.0662). Pixel max was not correlated with colon COX-2 expression (r = 0.2188, P = 0.22, data not shown) but tends to be correlated with colon weight/length ratio (r = 0.4063, P = 0.08; Figure 4B).
Colon diameter is not significantly different among groups (P = 0.1904; Figure 3C).Colon diameter is significantly correlated with colon COX-2 expression (r = 0.5661, P = 0.009; Figure 4C) but not to colon weight/length ratio (r = 0.01960, P = 0.94, data not shown).
Maximal thickness was significantly increased when inflammation was histologically observed (inflammatory score > 1, P = 0.0043; Figure 4D) while pixel max and colon diameter did not (P = 0.2452, P = 0.3541 respectively, data not shown).
Our data show that SPECT is feasible in rats with TNBS-induced colitis and was able to detect colitic rats from controls. Three common criteria previously described in human and rodent studies were used: pixel max, bowel wall maximal thickness and colon wall diameter and bowel wall thickness was the most relevant in our study to distinguish inflammation. Similarly, we have previously shown that measurement of maximal bowel wall thickness was also relevant to detect inflammation by MRC in rats with TNBS-induced colitis[13].
Similarly to previous studies[13-15,17-20], we observed colon inflammation demonstrated by stiffened colon, histological inflammatory score or increased inflammatory markers. We also noted SPECT signs of inflammation such as an increased maximal thickness of colon. Nevertheless, some SPECT parameters such as pixel max or colon diameter were numerally different among groups but did not reach statistical significance. One limitation of our study was the small sample size with 10 rats per group. We also hypothesized that this absence of effect was also the result of the heterogeneity of TNBS-induced colitis model including patchy lesions[13]. The absence of association between SPECT parameters and COX-2 expression was also observed in one of our previous study. Indeed, we previously found that some MRI parameters such as maximal wall thickness were not correlated with COX-2 expression[13] in the same acute colitis model. We used COX-2 as an inflammatory marker because its lack of basal COX-2 expression in normal tissue. Nevertheless, COX-2 is involved in angiogenesis processes and chronic inflammation and be more relevant in chronic colitis assessment. Indeed, we have previously shown that MRC criteria were associated with COX-2 expression in rats with chronic TNBS-induced colitis[14]. Evaluation of correlation between SPECT criteria and colon COX-2 expression required further evaluation in rats with chronic colitis.
Despite the fact we found statistical differences among groups, some parameters did not reach statistical significance. For instance, pixel max was not significantly increased in the TNBS group and was not associated with clinical parameters such as colon COX-2 expression or colon weight/length ratio.
Few studies have investigated the use of SPECT in colitis models[21-24] but their purpose was to visualize a specific cells trafficking such as macrophages[24] or lymphocytes[21-23].
SPECT is a non-invasive scintigraphic technique. Despite its exposure to ionizing radiation, this tool enables to study longitudinal inflammatory processes and the potential follow-up of experimental treatment in colitis. It also allows in accordance with the animal ethics committee a drastic reduction of the number of animals used in a series of experiments. For example, SPECT is able to monitor chronic inflammatory diseases[25].
This pilot study validates the use of SPECT in colitis models. Future prospective is to use SPECT with specific tracers. As SPECT has a high sensitivity and a good tissue penetration depth, this technique is a good candidate for targeted molecular imaging. Indeed, use of radiotracers that are capable of targeting specific biological events associated with disease progression and therapeutic success are crucial for a better understanding of inflammatory processes.
Numerous radiotracers of angiogenesis from other organs may be used in IBD context. In rat with liver fibrosis, progression and recovery of the disease were monitored by using an integrin αvβ3 in a SPECT-CT approach[26]. This αvβ3 integrin is strongly expressed at an endothelial level in IBD patients[27]. By a similar approach, we may monitor the progression and the recovery of angiogenesis processes in IBD. As angiogenesis is a key process in IBD pathogenesis[27], targeted imaging of angiogenesis within the gastrointestinal tract has the potential for non-invasive assessment of the underlying molecular signal events associated with the angiogenic process and may be utilized to predict and evaluate clinical outcomes in the setting of IBD. Similarly, intestinal fibrosis is also a key issue in IBD. Indeed, chronic inflammation results in intestinal fibrosis which leads to strictures. Development of these fibrosis strictures can conduct to intestinal obstruction, a frequent CD complication. Mechanisms underlying fibrosis development in CD are poorly understood. In small animal studies, fibrosis processes from other organs can be monitored by a SPECT approach. This is the case of liver fibrosis in mice. Very recently, Zhang et al[28] develop a method of SPECT imaging to quantify and stage liver fibrosis in mice. They used a tracer targeting asialoglycoprotein receptor and they validated that expression of this tracer correlated with liver fibrosis progression[28].
SPECT is a validated and reproducible technique. In IBD patients, anatomical considerations are crucial in the diagnosis and novel anatomical imaging approaches have emerged. MRI is a reference imaging procedure because of three techniques: gadolinium-enhanced imaging, diffusion- weighted imaging and more recently magnetization transfer imaging[29]. These anatomical imaging techniques techniques enable to quantify and characterize inflammatory processes. These tools provide diagnostic elements concerning intestinal lesions in IBD. However, they did not highlight the molecular mechanisms behind the inflammatory processes. By contrast, SPECT is a molecular imaging technique leading to a better understanding of in vivo molecular signaling events and mays open doors for real functional imaging of the bowel. The advantages of SPECT technique is the availability of specific tracers leading to a better understanding of the disease or the monitoring of therapy efficacy. Novel imaging approach can be bimodal and images can be commonly acquired by a combination of SPECT and MRI to capitalize their respective properties.
In IBD patients, SPECT approach is already investigated. Endoscopy remains the gold standard for IBD screening but is an invasive technique with a potential risk for perforation. Aarntzen et al[30] developed a 99mTc-labeled CXCL-8 tracer to detect CXL8 receptors mediating chemotaxis of immune cells to the site of inflammation. These authors were able to detect active disease by this tracer in thirty IBD patients with good diagnostic accuracy and they also found a correlation between the accumulation of the tracer and the degree of neutrophil influx in the mucosa[30]. While endoscopy can be used to identify intestinal inflamed lesions into the bowel wall, SPECT imaging is a molecular imaging technique[31]. Contrary to anatomical imaging techniques such as endoscopy, SPECT provides molecular insights in order to identify potential therapeutic targets or to better understand molecular inflammatory processes leading to intestinal inflammation[31]. The possibility to use specific tracer enables to highlight the cellular and molecular changes that take place in vivo beyond the anatomical lesions.
SPECT is a relevant imaging technique that can target specific steps early in disease progression. It may provide important information on disease characteristics in IBD patient. This might be particularly relevant in efforts to stratify treatment of IBD.
A supplementary potential of the use of SPECT is to assess potential therapeutic effect of IBD treatment. For example, SPECT imaging can also be used to monitor the effect of a radiotherapeutics in a longitudinal study. Recently, Li et al[25] have monitored the efficacy of 131I-anti epidermal growth factor liposomes in a colorectal cancer mouse model. These authors were able to follow the behavior of the radiotherapeutic molecule such as the tumor uptake of the molecule in the same animal at different time points[25]. Anti-TNF therapy is common in IBD patients. In a recent study, infliximab was radiolabeled with 99mTc and its uptake was recorded in patients with pulmonary sarcoidosis[32]. The authors of this study were able by a SPECT-CT approach to study 99mTc-infliximab accumulation in the target tissue[32]. The authors validated the feasibility of this marker because its tracer accumulation correlates with cytokine concentration or lung function parameters. A similar approach may be used in IBD patients.
In conclusion, in vivo imaging is an important tool for preclinical studies in IBD models. It enables to studying the molecular and structural basis of IBD models by using specific tracers. In addition, the advances made in preclinical imaging have important implications for clinical practice. It allows to optimize the technique prior to patient contact and to develop novel probed with higher degree of specificity or safety.
Anatomical imaging such as magnetic resonance imaging (MRI) are emerging to evaluate colonic inflammation. These structural techniques do not allowed inflammation quantification. By contrast, functional imaging by SPECT-computed tomography (CT) could be interesting under quantification.
This study validates the use of SPECT in colitis models. Indeed, imaging parameters are associated to lab parameters. By using SPECT-CT, we are able to distinguish colitis rats from control rats.
SPECT is a molecular imaging technique leading to a better understanding of in vivo molecular signaling events and mays open doors for real functional imaging of the bowel. The advantages of SPECT technique is the availability of specific tracers leading to a better understanding of the disease or the monitoring of therapy efficacy. Novel imaging approach can be bimodal and images can be commonly acquired by a combination of SPECT and MRI to capitalize their respective properties.
Evaluating animal models may be important an important tool for preclinical studies in inflammatory bowel diseases models. It also allows optimizing the technique prior to patient contact.
SPE-CT: nuclear imaging technique that can monitor radiation emitted by tracers.
This is a study aimed to assess the feasibility of SPECT-CT in rats with trinitrobenzene sulfonic acid-induced acute colitis and confront it with model inflammatory characteristics.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: France
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Engin AB, Stein J S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Cosnes J. Measuring structural damage in Crohn’s disease. Gastroenterol Hepatol (N Y). 2013;9:103-104. [PubMed] |
| 2. | Baert F, Moortgat L, Van Assche G, Caenepeel P, Vergauwe P, De Vos M, Stokkers P, Hommes D, Rutgeerts P, Vermeire S. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology. 2010;138:463-468; quiz e410-461. |
| 3. | D’Haens GR, Fedorak R, Lémann M, Feagan BG, Kamm MA, Cosnes J, Rutgeerts PJ, Marteau P, Travis S, Schölmerich J. Endpoints for clinical trials evaluating disease modification and structural damage in adults with Crohn’s disease. Inflamm Bowel Dis. 2009;15:1599-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Pariente B, Cosnes J, Danese S, Sandborn WJ, Lewin M, Fletcher JG, Chowers Y, D’Haens G, Feagan BG, Hibi T. Development of the Crohn’s disease digestive damage score, the Lémann score. Inflamm Bowel Dis. 2011;17:1415-1422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 412] [Cited by in RCA: 463] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 5. | Ko CW, Dominitz JA. Complications of colonoscopy: magnitude and management. Gastrointest Endosc Clin N Am. 2010;20:659-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 6. | Gupta M, Holub JL, Eisen G. Do indication and demographics for colonoscopy affect completion? A large national database evaluation. Eur J Gastroenterol Hepatol. 2010;22:620-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Shoenut JP, Semelka RC, Magro CM, Silverman R, Yaffe CS, Micflikier AB. Comparison of magnetic resonance imaging and endoscopy in distinguishing the type and severity of inflammatory bowel disease. J Clin Gastroenterol. 1994;19:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937-1949. [PubMed] |
| 9. | Kettritz U, Isaacs K, Warshauer DM, Semelka RC. Crohn’s disease. Pilot study comparing MRI of the abdomen with clinical evaluation. J Clin Gastroenterol. 1995;21:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Jones-Hall YL, Grisham MB. Immunopathological characterization of selected mouse models of inflammatory bowel disease: Comparison to human disease. Pathophysiology. 2014;21:267-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 602] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 12. | Palmen MJ, Dieleman LA, van der Ende MB, Uyterlinde A, Peña AS, Meuwissen SG, van Rees EP. Non-lymphoid and lymphoid cells in acute, chronic and relapsing experimental colitis. Clin Exp Immunol. 1995;99:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Charpentier C, Marion-Letellier R, Savoye G, Nicol L, Mulder P, Aziz M, Vera P, Déchelotte P, Savoye-Collet C. Magnetic resonance colonography in rats with TNBS-induced colitis: a feasibility and validation study. Inflamm Bowel Dis. 2012;18:1940-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Melchior C, Loeuillard E, Marion-Letellier R, Nicol L, Mulder P, Guerin C, Bôle-Feysot C, Aziz M, Déchelotte P, Vera P. Magnetic resonance colonography for fibrosis assessment in rats with chronic colitis. PLoS One. 2014;9:e100921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Bertrand J, Tennoune N, Marion-Letellier R, Goichon A, Chan P, Mbodji K, Vaudry D, Déchelotte P, Coëffier M. Evaluation of ubiquitinated proteins by proteomics reveals the role of the ubiquitin proteasome system in the regulation of Grp75 and Grp78 chaperone proteins during intestinal inflammation. Proteomics. 2013;13:3284-3292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Ibrahim A, Mbodji K, Hassan A, Aziz M, Boukhettala N, Coëffier M, Savoye G, Déchelotte P, Marion-Letellier R. Anti-inflammatory and anti-angiogenic effect of long chain n-3 polyunsaturated fatty acids in intestinal microvascular endothelium. Clin Nutr. 2011;30:678-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Bertrand J, Marion-Letellier R, Azhar S, Chan P, Legrand R, Goichon A, Ghouzali I, Aziz M, Vaudry D, Savoye G. Glutamine enema regulates colonic ubiquitinated proteins but not proteasome activities during TNBS-induced colitis leading to increased mitochondrial activity. Proteomics. 2015;15:2198-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Hassan A, Ibrahim A, Mbodji K, Coëffier M, Ziegler F, Bounoure F, Chardigny JM, Skiba M, Savoye G, Déchelotte P. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. J Nutr. 2010;140:1714-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Ibrahim A, Aziz M, Hassan A, Mbodji K, Collasse E, Coëffier M, Bounoure F, Savoye G, Déchelotte P, Marion-Letellier R. Dietary α-linolenic acid-rich formula reduces adhesion molecules in rats with experimental colitis. Nutrition. 2012;28:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Mbodji K, Charpentier C, Guérin C, Querec C, Bole-Feysot C, Aziz M, Savoye G, Déchelotte P, Marion-Letellier R. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-κB in rats with TNBS-induced colitis. J Nutr Biochem. 2013;24:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Kanwar B, Gao DW, Hwang AB, Grenert JP, Williams SP, Franc B, McCune JM. In vivo imaging of mucosal CD4+ T cells using single photon emission computed tomography in a murine model of colitis. J Immunol Methods. 2008;329:21-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Bennink RJ, van Montfrans C, de Jonge WJ, de Bruin K, van Deventer SJ, te Velde AA. Imaging of intestinal lymphocyte homing by means of pinhole SPECT in a TNBS colitis mouse model. Nucl Med Biol. 2004;31:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | van Montfrans C, Bennink RJ, de Bruin K, de Jonge W, Verberne HJ, Ten Kate FJ, van Deventer SJ, Te Velde AA. In vivo evaluation of 111In-labeled T-lymphocyte homing in experimental colitis. J Nucl Med. 2004;45:1759-1765. [PubMed] |
| 24. | Wu Y, Briley-Saebo K, Xie J, Zhang R, Wang Z, He C, Tang CY, Tao X. Inflammatory bowel disease: MR- and SPECT/CT-based macrophage imaging for monitoring and evaluating disease activity in experimental mouse model--pilot study. Radiology. 2014;271:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Li W, Ji YH, Li CX, Liu ZY, Li N, Fang L, Chang J, Tan J. Evaluation of therapeutic effectiveness of (131)I-antiEGFR-BSA-PCL in a mouse model of colorectal cancer. World J Gastroenterol. 2016;22:3758-3768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Yu X, Wu Y, Liu H, Gao L, Sun X, Zhang C, Shi J, Zhao H, Jia B, Liu Z. Small-Animal SPECT/CT of the Progression and Recovery of Rat Liver Fibrosis by Using an Integrin αvβ3-targeting Radiotracer. Radiology. 2016;279:502-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 27. | Danese S, Sans M, de la Motte C, Graziani C, West G, Phillips MH, Pola R, Rutella S, Willis J, Gasbarrini A. Angiogenesis as a novel component of inflammatory bowel disease pathogenesis. Gastroenterology. 2006;130:2060-2073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 335] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 28. | Zhang D, Guo Z, Zhang P, Li Y, Su X, You L, Gao M, Liu C, Wu H, Zhang X. Simplified quantification method for in vivo SPECT/CT imaging of asialoglycoprotein receptor with (99m)Tc-p(VLA-co-VNI) to assess and stage hepatic fibrosis in mice. Sci Rep. 2016;6:25377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Pinson C, Dolores M, Cruypeninck Y, Koning E, Dacher JN, Savoye G, Savoye-Collet C. Magnetization transfer ratio for the assessment of perianal fistula activity in Crohn’s disease. Eur Radiol. 2017;27:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 30. | Aarntzen EH, Hermsen R, Drenth JP, Boerman OC, Oyen WJ. 99mTc-CXCL8 SPECT to Monitor Disease Activity in Inflammatory Bowel Disease. J Nucl Med. 2016;57:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Neurath MF. Molecular Endoscopy and in vivo Imaging in Inflammatory Bowel Diseases. Dig Dis. 2015;33 Suppl 1:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Vis R, Malviya G, Signore A, Grutters JC, Meek B, van de Garde EM, Keijsers RG. 99mTc-anti-TNF-α antibody for the imaging of disease activity in pulmonary sarcoidosis. Eur Respir J. 2016;47:1198-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |