Published online May 21, 2017. doi: 10.3748/wjg.v23.i19.3449
Peer-review started: December 9, 2016
First decision: January 19, 2017
Revised: January 23, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: May 21, 2017
Processing time: 162 Days and 22.4 Hours
To investigate the effects of heme oxygenase-1 (HO-1)-modified bone marrow mesenchymal stem cells (BMMSCs) on the microcirculation and energy metabolism of hepatic sinusoids following reduced-size liver transplantation (RLT) in a rat model.
BMMSCs were isolated and cultured in vitro using an adherent method, and then transduced with HO-1-bearing recombinant adenovirus to construct HO-1/BMMSCs. A rat acute rejection model following 50% RLT was established using a two-cuff technique. Recipients were divided into three groups based on the treatment received: normal saline (NS), BMMSCs and HO-1/BMMSCs. Liver function was examined at six time points. The levels of endothelin-1 (ET-1), endothelial nitric-oxide synthase (eNOS), inducible nitric-oxide synthase (iNOS), nitric oxide (NO), and hyaluronic acid (HA) were detected using an enzyme-linked immunosorbent assay. The portal vein pressure (PVP) was detected by Power Lab ML880. The expressions of ET-1, iNOS, eNOS, and von Willebrand factor (vWF) protein in the transplanted liver were detected using immunohistochemistry and Western blotting. ATPase in the transplanted liver was detected by chemical colorimetry, and the ultrastructural changes were observed under a transmission electron microscope.
HO-1/BMMSCs could alleviate the pathological changes and rejection activity index of the transplanted liver, and improve the liver function of rats following 50% RLT, with statistically significant differences compared with those of the NS group and BMMSCs group (P < 0.05). In term of the microcirculation of hepatic sinusoids: The PVP on POD7 decreased significantly in the HO-1/BMMSCs and BMMSCs groups compared with that of the NS group (P < 0.01); HO-1/BMMSCs could inhibit the expressions of ET-1 and iNOS, increase the expressions of eNOS and inhibit amounts of NO production, and maintain the equilibrium of ET-1/NO (P < 0.05); and HO-1/BMMSCs increased the expression of vWF in hepatic sinusoidal endothelial cells (SECs), and promoted the degradation of HA, compared with those of the NS group and BMMSCs group (P < 0.05). In term of the energy metabolism of the transplanted liver, HO-1/BMMSCs repaired the damaged mitochondria, and improved the activity of mitochondrial aspartate aminotransferase (ASTm) and ATPase, compared with the other two groups (P <0.05).
HO-1/BMMSCs can improve the microcirculation of hepatic sinusoids significantly, and recover the energy metabolism of damaged hepatocytes in rats following RLT, thus protecting the transplanted liver.
Core tip: Hepatic sinus is important in the liver microcirculation, which is the basis for transplanted liver regeneration. Transplanted liver grafts with disturbed microcirculation of the hepatic sinus may affect liver energy metabolism. We investigated the protective effects of heme oxygenase-1-modified bone marrow mesenchymal stem cells (HO-1/BMMSCs) on rat reduced-size liver transplantation in terms of the microcirculation and hepatic energy metabolism. HO-1/BMMSCs promoted the equilibrium of ET-1/NO, repaired damaged hepatic sinusoidal endothelial cells, and lowered the portal vein pressure in rats following reduced-size liver transplantation, which improved the microcirculation of hepatic sinusoids and ATPase activity, and recover the energy metabolism of damaged hepatocytes.
- Citation: Yang L, Shen ZY, Wang RR, Yin ML, Zheng WP, Wu B, Liu T, Song HL. Effects of heme oxygenase-1-modified bone marrow mesenchymal stem cells on microcirculation and energy metabolism following liver transplantation. World J Gastroenterol 2017; 23(19): 3449-3467
- URL: https://www.wjgnet.com/1007-9327/full/v23/i19/3449.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i19.3449
Liver transplantation is the only effective treatment for end-stage liver diseases; however, a shortage of donor graft remains the major impediment to the development of liver transplantation. Although reduced-size liver transplantation (RLT), split-liver transplantation, and living donor liver transplantation can make up the shortage of donor grafts to some extent[1,2], transplantation-induced hepatic injury can seriously affect liver function, even leading to small-for-size syndrome (SFSS). SFSS is a clinical syndrome involving multiple factors, such as the volume and quality of the donor graft, recipient characteristics, and surgical techniques. The main pathophysiological characteristic is disturbance in the microcirculation of hepatic sinusoids[3,4]. Hepatic sinusoids play an important role in the liver microcirculation. The integrity of sinusoidal structures and the stability of sinusoidal microcirculation are essential not only for normal liver function, but also for good physiological function and regeneration of a transplanted liver. Preservation of the donor graft, ischemia-reperfusion, and rejection may result in damage to the transplanted liver during transplantation. Ischemia-reperfusion promotes swelling, necrosis, and apoptosis of the sinusoidal endothelial cells (SECs), leading to sinusoidal obstruction. Kupffer cell activation and SECs injury after liver transplantation can lead to disturbance in the microcirculation of hepatic sinusoids, adhesion of leukocytes and platelets, and a series of inflammatory reactions, which cause dysfunction of the sinusoidal microcirculation[5,6]. Furthermore, all these pathological processes would lead to organ ischemia, and due to a lack of oxygen, a large amount of lipid peroxide in mitochondria was produced and ATP was gradually reduced, which caused tissue damage[7]. A low baseline level of hepatic ATP leads to liver necrosis and apoptosis, although ischemic preconditioning and ATP pretreatment can increase the intrahepatic ATP level significantly, providing protective effects on liver function[8,9]. Thus, disturbance in the microcirculation of hepatic sinusoids and disordered energy metabolism are important factors affecting the functions of transplanted livers, which still remain unsolved.
Bone marrow mesenchymal stem cells (BMMSCs), a group of non-hematopoietic stem cells derived from stromal cells, have multi-directional differentiation potential and can be differentiated into endothelia. BMMSCs promote angiogenesis, tissue repair, and paracrine signaling[10-15], which can relieve ischemic reperfusion injury (IRI) of the liver, reduce hepatocyte injury and accelerate liver regeneration, and are involved in anti-inflammation and immunoregulation[16-21]. BMMSCs have been investigated in the field of liver, kidney, small intestine, and islet transplantation[22-27]; however, the proportion of BMMSCs surviving in the recipient’s body for more than a week is less than 1%, which has affected its use in experimental studies[22,24,28]. Thus, improving the survival time of BMMSCs is also a research hotspot.
Heme oxygenase-1 (HO-1) is a multifunctional microsomal oxidase related to heme metabolism, with anti-inflammatory, anti-oxidative stress, anti-apoptosis, anti-ischemia reperfusion injury, and microcirculation regulation effects that protect cells[29-31]. HO-1 has been shown to alleviate rejection, prolong graft survival time, and induce immune tolerance in organ transplantation[32-34]. HO-1 can regulate BMMSCs by reducing the apoptosis of BMMSCs under hypoxia and oxidative stress in vitro[35], and prolonging the protective effects of BMMSCs on transplanted grafts[22,24].
A previous study showed that transplantation of HO-1/BMMSCs could inhibit the apoptosis of hepatocytes and reduce IRI[22]. How HO-1/BMMSCs exert their protective effects, and whether HO-1/BMMSCs can affect the microcirculation of hepatic sinusoids, portal vein pressure (PVP,) and energy metabolism of hepatocytes following RLT have not been studied extensively. Therefore, the aim of this study was to determine whether HO-1/BMMSCs could protect the microcirculation of hepatic sinusoids and energy metabolism of the transplanted liver after RLT in order to provide a reliable experimental basis for solving the shortage of donors.
Specific-pathogen-free (SPF) adult inbred Brown-Norway (BN) rats and Lewis rats were purchased from the Academy of Military Medical Sciences, Beijing, China. Male BN rats (4-5 wk old; 100-120 g) were inbred for the extraction and characterization of BMMSCs. Inbred male Lewis rats (6-8 wk old; 200-220 g) were the liver transplantation donors, and the inbred male BN rats were the recipients. The experimental animals were kept at 23 °C, with 50% humidity, and a 12 h light and dark cycle for 2 wk, with free access to water and food, and regular replacement of cage and clean bedding before the experiments. All experimental procedures were performed in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Institutes of Health, 8th ed. 2011). All protocols were approved by the Animal Care and Research Committee of Tianjin First Central Hospital. All the rats were anesthetized with chloral hydrate to minimize their pain.
The following instruments and reagents were used: Dulbecco’s modified Eagle medium (DMEM)/F12, penicillin-streptomycin solution, and trypsin/EDTA solution (Gibco, Carlsbad, CA, United States); fetal bovine serum (FBS; Biowest, Nuaillé, France); dexamethasone phosphate sodium (5 mg/mL), sodium glycerophosphate (216 mg/mL), insulin (40 U/mL), 1-methyl-isobutyl-xanthine, vitamin C and indomethacin (Sigma Aldrich, St. Louis, MO, United States); Oil Red O powder (Dingguo Changsheng Biotechnology, Beijing, China); von Kossa cell staining kit (Genmed, Shanghai, China); recombinant adenovirus expressing rat HO-1 (Genechem Co., Ltd., Shanghai, China); phosphate buffer solution (PBS), highly sensitive radio immunoprecipitation assay (RIPA), bicinchoninic acid (BCA) protein assay kit (Solarbio, Beijing, China); Western blotting-associated reagents (Boster, Wuhan, China); normal goat serum (Minhai Biotechnology, Lanzhou, China); SuperPicture™ Polymer Detection Kit (Thermo, Waltham, MA, United States); diaminobenzidine (Dako, Glostrup, Denmark); flow cytometry-related antibodies (anti-rat CD34-fluorescein isothiocyanate (FITC), CD29-phycoerythrin (PE), CD45-PE, CD90-FITC, RT1A-PE, and RT1B-FITC; Biolegend, San Diego, CA, United States); rabbit antibodies for inducible nitric oxide synthetase (iNOS), endothelial nitric oxide synthetase (eNOS), von Willebrand factor (vWF), and mouse antibodies for endothelin (Abcam, Cambridge, United Kingdom); rabbit antibodies for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (SAB, College Park, MD, United States); goat anti-rabbit IgG labeled with horseradish peroxidase (HRP) and goat anti-mouse IgG labeled with HRP (Invitrogen, Carlsbad, CA, United States); ELISA kits (Biovalue, Shanghai, China); ATP assay kit (Jiancheng Biotechnology, Nanjing, China); inverted fluorescent microscope (Olympus, Japan); and FACSCalibur flow cytometric analysis (BD FACSAria III, Franklin Lakes, NJ, United States).
BMMSCs were isolated aseptically from the femur and tibia of BN rats after sacrifice by cervical dislocation. After cutting off both ends of the epiphyseal plate, the marrow cavity was rinsed by DMEM/F12 containing 10% FBS. Red blood cells (RBCs) were lysed using 0.1 mol/L NH4Cl, and the remaining cells were washed, resuspended as a single cell suspension, and cultured in T75 culture flask at 37 °C with 5 mL/L CO2 in an incubator. Well-grown third-passage cells were resuspended and then labeled fluorescently with antibodies (anti-CD29-PE, anti-CD34-FITC, anti-CD45-PE, anti-CD90-FITC, anti-RT1A-PE and anti-RT1B-FITC) for 30 min for flow cytometric analysis.
Adipogenic differentiation: Well-grown third-passage BMMSCs were inoculated into 6-well plates at 2 × 105 cells/well. After complete adherence, BMMSCs were cultured in adipogenic differentiation medium (DMEM/F12 containing 10% FBS, 1 μmol/L dexamethasone, 10 μg/mL insulin, 0.5 mmol/L 1-methyl-3-isobutyl xanthine, and 0.1 mmol/L indomethacin). The medium was changed every 72 h. After induction for 8-10 d, BMMSCs were fixed by 4% paraformaldehyde, and stained with Oil Red O for 30 min. The BMMSCs were then rinsed with PBS and positive cells showed orange lipid droplets.
Osteogenic differentiation: Well-grown third-passage BMMSCs were also inoculated into 6-well plates at 2 × 105 cells/well. After complete adherence, BMMSCs were cultured in osteogenic differentiation medium (DMEM/F12 containing 10% FBS, 0.1 μmol/L dexamethasone, 10 mmol/L sodium glycerophosphate, and 50 μg/mL vitamin C). The medium was changed every 72 h. After induction for 13-15 d, BMMSCs were stained using a von Kossa staining kit, and positive cells showed black calcium deposits.
HO-1-bearing recombinant adenovirus (Adv/HO-1) was diluted to 10 pfu/cell with complete culture medium, which was used to replace the original medium of well-grown third-passage BMMSCs. After 6-8 h, the Adv/HO-1 culture medium was replaced with complete culture medium for continued cultivation of the BMMSCs. After 48 h, the proportion of cells containing green fluorescence was observed under a fluorescence microscope.
A 50% RLT rejection model was established with Lewis donor rats and BN recipient rats, using the two-cuff technique by a single operator. The donor rats were anesthetized with 5% chloral hydrate, and incised by abdominal median incision. After dividing the perihepatic ligaments, the portal vein (PV) and infrahepatic vena cava (IHVC) were isolated. The anterior wall of the common bile duct was cut off and a stent was implanted, the hepatic artery was ligated and cut off, the PV was punctured and infused with 4 °C lactated Ringer’s solution, and the IHVC and suprahepatic vena cava (SHVC) were both cut off as the outflow tract. When the liver turned yellowish and the outflow of perfusion fluid became clear, the PV was dissected and the donor liver was removed. The SHVC was trimmed, the PV and IHVC were prepared as vascular cuffs, and then the harvested graft was preserved at 4 °C. The perihepatic ligaments were divided in the recipient rats, the PV and IHVC were blocked, and the PV was punctured and infused with 1 mL normal saline (NS). The PV, SHVC, and IHVC were then dissected, and the liver was removed. The donor liver was placed orthotopically in the abdominal cavity of the recipient. The SHVC was anastomosed using an 8-0 nylon suture. Cuff anastomosis of the PV and IHVC was then performed. The graft was reperfused by opening the PV, SHVC, and IHVC in turn. The bile duct was connected by a stent suture. After checking that all the cuff tubes were not distorted and no leak occurred in the SHVC and IHVC, the abdomen was washed and closed. The detailed surgical procedure was described previously[36].
Experimental rats were divided into three groups: the control group (receiving 1 mL NS), the BMMSCs group (receiving 5 × 106 BMMSCs resuspended in 1 mL), and the HO-1/BMMSCs group (receiving 5 × 106 HO-1/BMMSCs resuspended in 1 mL). All injections in the three groups were administered via the superficial dorsal veins immediately after RSL. Five rats in each of the three groups were euthanized on postoperative day (POD) 0, 1, 5, 7, or 14, respectively, and their peripheral venous blood and transplanted liver tissues (cooled in liquid nitrogen and stored at -80 °C) were collected for further analysis.
The rat serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), and mitochondrial AST (ASTm) were measured using an automatic biochemical analyzer (Hitachi, Japan) according to the manufacturer’s instructions.
The rat serum levels of ET-1, eNOS, iNOS, nitric oxide (NO), and hyaluronic acid (HA) were detected using the ELISA kits according to the manufacturer’s instructions.
The pathological injuries were most obvious on POD 7; therefore, POD 7 was chosen as the time point to measure the PVP. The rats were anesthetized by intraperitoneal injection of phenobarbital, and fixed in the supine position, the abdominal cavity was opened, and the PV was exposed. An ileum mesenteric vein branch in the right side of anterior mesenteric vein near the PV was isolated, its distal end was ligated, a suitable nick to insert a catheter was made, and then the catheter was inserted along the anterior mesenteric vein upstream. After fixing the catheter and connecting it to the pressure transducer, the PVP was measured using a Power Lab ML880 (AD Instrument, Australia).
Liver tissues collected from different POD were treated with RIPA lysis buffer to extract total proteins, and the concentrations of the total proteins were detected using a BCA protein assay kit. The proteins were separated electrophoretically and then transferred to nitrocellulose membranes. After blocking with 5% skimmed milk for 2 h, ET-1 (1:250), eNOS (1:200), iNOS (1:250), vWF (1:250) and internal reference protein GAPDH (1:3000) antibodies were added and incubated at 4 °C overnight. The membranes were then rinsed with Tris Buffered Saline with Tween-20 (TBST), incubated with secondary antibodies (1:5000) for 2 h at room temperature, rinsed with TBST again, the chemiluminescence HRP substrate was added, and the membranes were exposed in a gel imaging analysis system (Alpha Innotech FluorChem FC2, CA, United States). The images were analyzed using the AlphaView SA 3.4.0 software (San Jose, CA, United States) to determine the grey scale. The relative abundance of a target protein was calculated as target protein band brightness value - background brightness value/internal reference protein GAPDH band brightness value - background brightness value. The resulting ratio was the relative abundance of the target protein. The samples were replicated three times in different batches at each time point.
The transplanted livers on POD 0, 1, 5, 7, and 14 were sectioned, fixed, paraffin embedded, sliced, and stained with hematoxylin and eosin (HE). The histopathological changes in the liver tissues were observed in five randomly selected fields under a light microscope. Acute rejection was graded according to the Banff criteria[37].
The transplanted liver was sliced, dewaxed by xylene, dehydrated by gradient ethanol, subjected to antigen retrieval, and blocked by normal goat serum at 37 °C for 1 h. After incubation with primary antibodies (1:500) at 37 °C for 1 h and at 4 °C overnight, the slides were incubated with secondary antibodies at 37 °C for 40 min, developed with DAB and stained with hematoxylin, differentiated using 1% hydrochloric acid ethanol, dehydrated using gradient ethanol, and clarified and encapsulated using xylene. The tissue slices were observed to determine the presence of ET-1, eNOS, iNOS and vWF in the transplanted liver. The immunohistochemical results were analyzed using the Image-Pro Plus 6.0.0.260 software (IPP, Media Cybernetics, Rockville, MD, United States).
The activity of Na+-K+-ATPase in the liver tissues was detected using the chemical colorimetry method according to the instruction manual of the ATPase test kit.
The transplanted liver was double-fixed by glutaraldehyde and osmic acid, dehydrated by gradient acetone, immersed in embedding medium, ultrathin sectioned using an automatic microtome (LeicaRM2235, Leica, Germany), and stained with 1% uranyl acetate. The tissue slices were observed and filmed under a transmission electron microscope (Hitachi, Japan) to observe the status of mitochondria in the hepatocytes.
SPSS 17.0 (SPSS GmbH, Munich, Germany) was used for the statistical analysis. All data were presented as means ± SD. Different groups of data were compared by analysis of variance (ANOVA). Differences were considered to be statistically significant at P < 0.05.
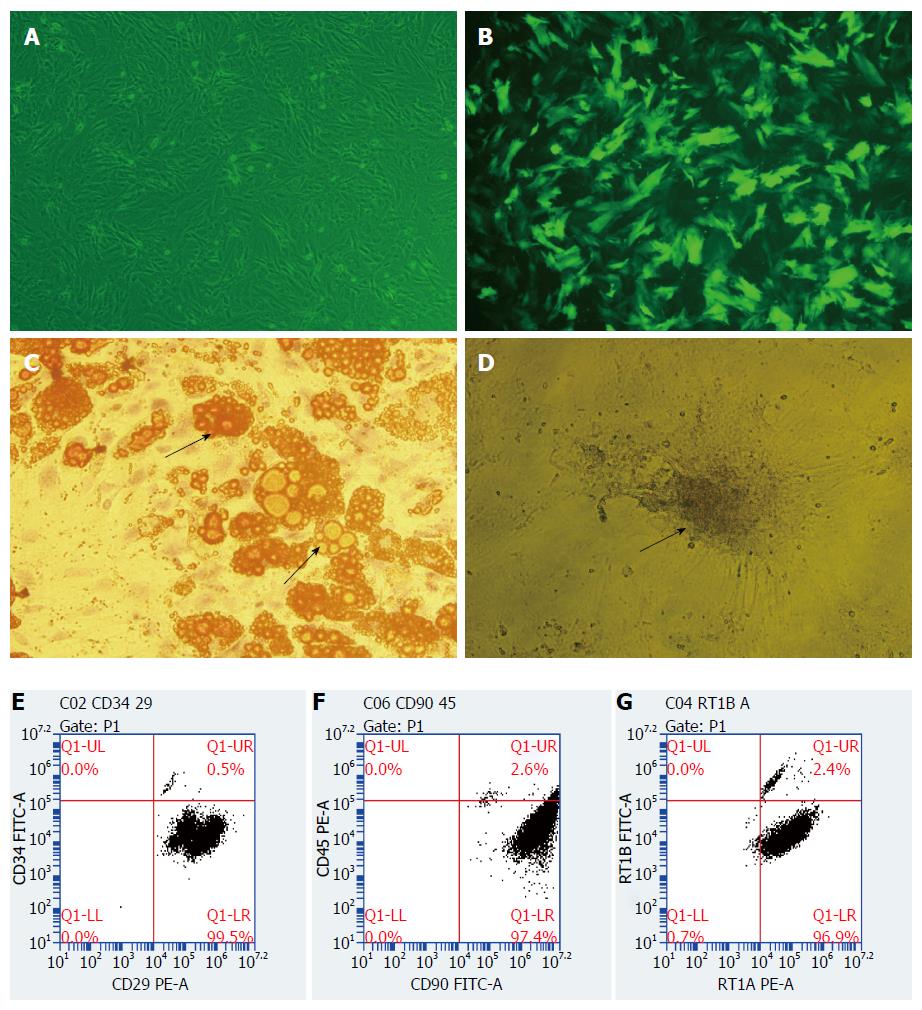
Morphology and phenotype identification of BMMSCs: MSCs are spindle-shaped in morphology like fibroblasts in which the cytoplasm can stretch peripherally and maintain the ability to differentiate into a variety of other cell lines when cultured ex vivo. BMMSCs grew adherently when observed under microscope, and the cells were long spindle-shaped and partially vortexed or chrysanthemum-like, with typical morphological characteristics of MSCs (Figure 1A). Flow cytometry analysis showed that the positive rates of CD29, CD90 and RT1A in the third generation BN rat BMMSCs were 99.5%, 97.4%, and 96.9%, respectively; and the negative rates of CD34, CD45, and RT1B were all above 95% (Figure 1E-G).
After induction by adipogenic differentiation medium for 8-10 d, BMMSCs showed multiple orange lipid droplets in their cytoplasm, which was consistent with the characteristics of adipocytes (Figure 1C). Similarly, BMMSCs showed black granular or lumpy calcium deposits in their cytoplasm after induction by osteogenic differentiation medium for 13-15 d, which was consistent with the characteristics of osteoblasts (Figure 1D). These results showed that the extracted BMMSCs had the potential to differentiate into adipocytes and osteoblasts in vitro.
BMMSCs were infected with recombinant adenovirus expressing HO-1 gene for 48 h, and the cells were observed under a fluorescence microscope. The rate of positive cells expressing green fluorescent protein was more than 80% (Figure 1B).
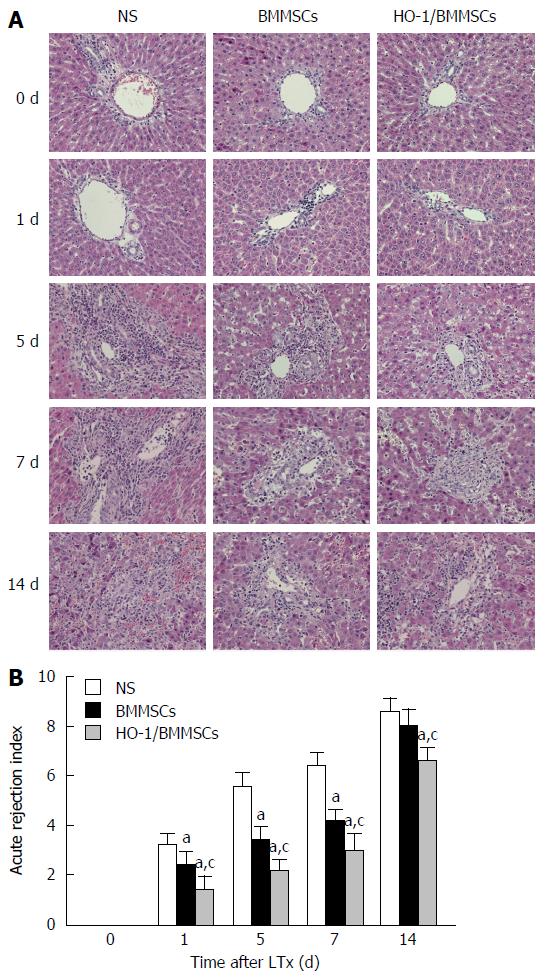
Histopathological manifestations of the transplanted liver in the NS group included obvious congestion in the hepatic sinus, red blood cell deposition in the sinus, peri-central vein and portal area, hepatocyte swelling, eosinophilic degeneration, and punctate necrosis. The infiltration of a large volume of mixed lymphocytes in the central vein and the portal area was accompanied by necrosis of hepatocytes on POD5. Infiltration of inflammatory cells and necrosis of hepatocytes increased on POD7. The mixed lymphocyte infiltration became significant on POD14, with severe hepatic sinus congestion, hepatocyte destruction, disappearance of lobular structure, and significant liver fibrosis. The transplanted livers in the BMMSCs group showed no obvious hepatic sinus congestion, and the endothelial swelling and hepatocyte necrosis was less severe than those in the NS group. The histological changes showed disordered hepatic lobules on POD5, with slightly infiltrated inflammatory cells and mild necrosis of hepatocytes. Inflammatory cell infiltration and hepatocyte necrosis progressed on POD7. On POD14, lymphocyte infiltration became obvious, liver sinus congestion increased, accompanied by liver fibrosis and incomplete hepatic lobule structures. The HO-1/BMMSCs group showed less rejection, and fewer injuries of the liver allografts compared with the BMMSCs and NS groups at all time points, without obvious hepatic sinus congestion, large volume of inflammatory cell infiltration, or disordered lobular structures; only mild hepatic fibrosis was observed (Figure 2A).
The rejection injuries tended to increase in all the groups with time after operation. Rejection in the HO-1/BMMSCs group was the least severe at all time points, and was significantly less severe than that in the BMMSCs and NS groups (P < 0.05). Rejection in the BMMSCs group was significantly less severe than that in the NS group on POD1, 5, and 7 (P < 0.05). There was no significant difference between the BMMSCs group and the NS group on POD14 (Figure 2B).
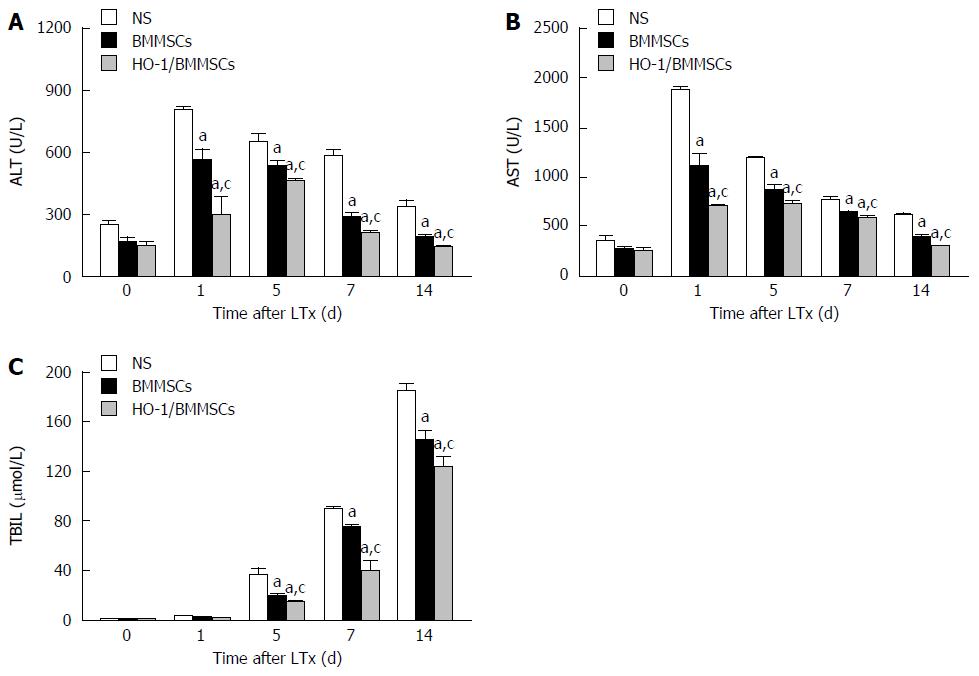
The serum ALT and AST in the three groups increased initially and then decreased gradually after operation, while TBIL tended to increase with time. The serum levels of liver enzymes and TBIL in the HO-1/BMMSCs group were significantly lower than in the BMMSCs and NS groups on POD1, 5, 7, and 14 (P < 0.05). The serum liver enzymes and TBIL in the BMMSCs group were significantly lower than in the NS group (P < 0.05; Figure 3).
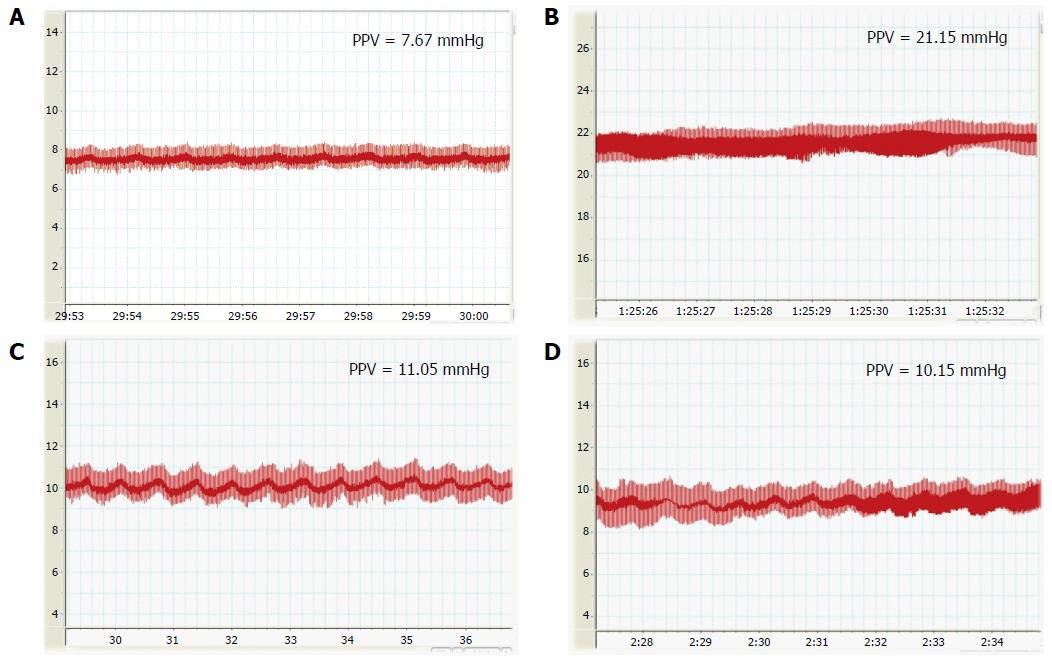
The PVP correlates with the blood flow and resistance of the PV, and plays an important role in maintaining the blood flow in the liver. The PVP was measured on POD7 after RLT. The PVP in the HO-1/BMMSCs and BMMSCs groups was significantly lower than that in the NS group (P < 0.05; Figure 4).
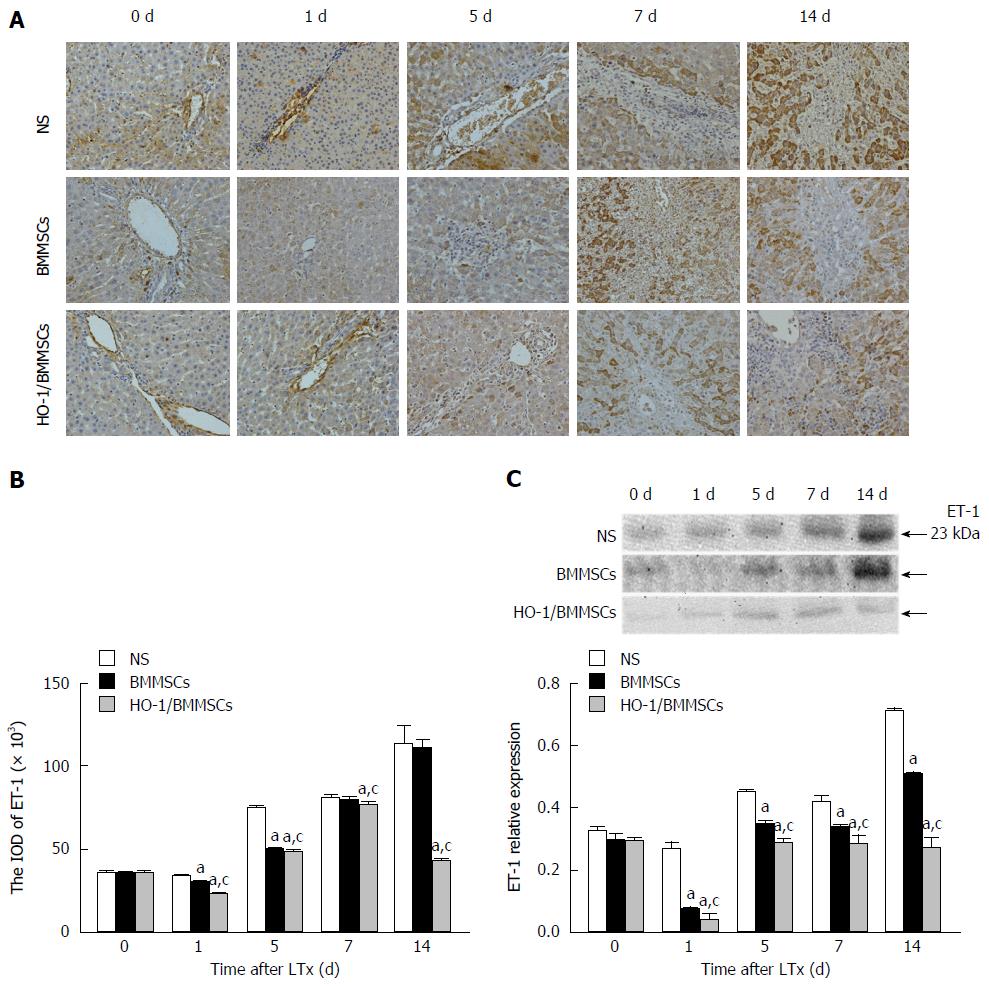
Effects of HO-1/BMMSCs on ET-1 expression in the transplanted liver: ET-1 positive cells were expressed in the hepatic sinusoids of transplanted livers surrounding the Glisson system. The proportions of ET-1 positive cells in the BMMSCs and HO-1/BMMSCs groups were significantly lower than that in the NS group, and HO-1/BMMSCs group showed fewer positive cells than the BMMSCs group. There were relatively more ET-1-positive cells in the NS group, which increased with time after operation (Figure 5A and B).
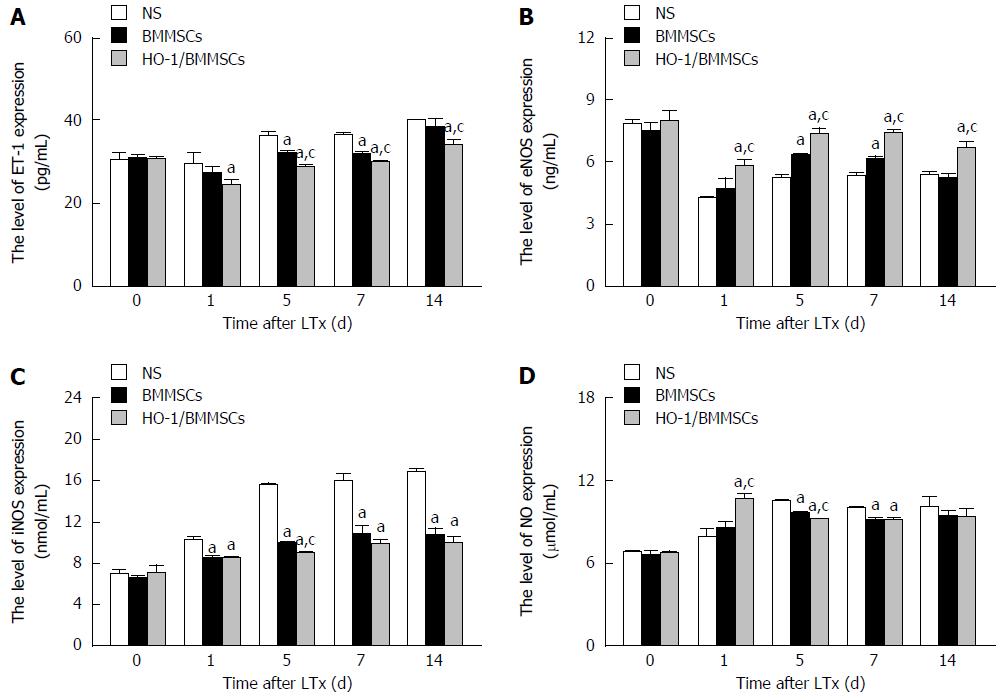
The levels of ET-1 in the transplanted liver decreased initially and then increased with time after operation. The level of ET-1 was the lowest in the HO-1/BMMSCs group, while the NS group showed the highest levels on POD1, 5, 7, and 14 (P <0.05). The results indicated that BMMSCs could inhibit the secretion of ET-1 by SECs, thereby reducing sinusoidal contraction, decreasing sinusoidal vascular resistance, and improving hepatic sinus perfusion. The sinusoidal vasoconstriction alleviating effects of HO-1/BMMSCs were superior to those of BMMSCs (Figure 5C).
The serum ET-1 levels after RLT decreased initially and then increased gradually after operation. The level of ET-1 in the HO-1/BMMSCs group was lower than that in the NS group on POD1 (P < 0.01). The level of ET-1 in the HO-1/BMMSCs group was the lowest among the three groups, while the NS group showed the highest levels on POD5 and POD7 (P < 0.01). The level of ET-1 in the HO-1/BMMSCs group was significantly lower than that in the BMMSCs and NS groups on POD14 (P < 0.01). The results indicated that BMMSCs could inhibit the secretion of ET-1 in rats after liver transplantation, and the inhibitory effects of HO-1/BMMSCs were better than those of BMMSCs (Figure 6A).
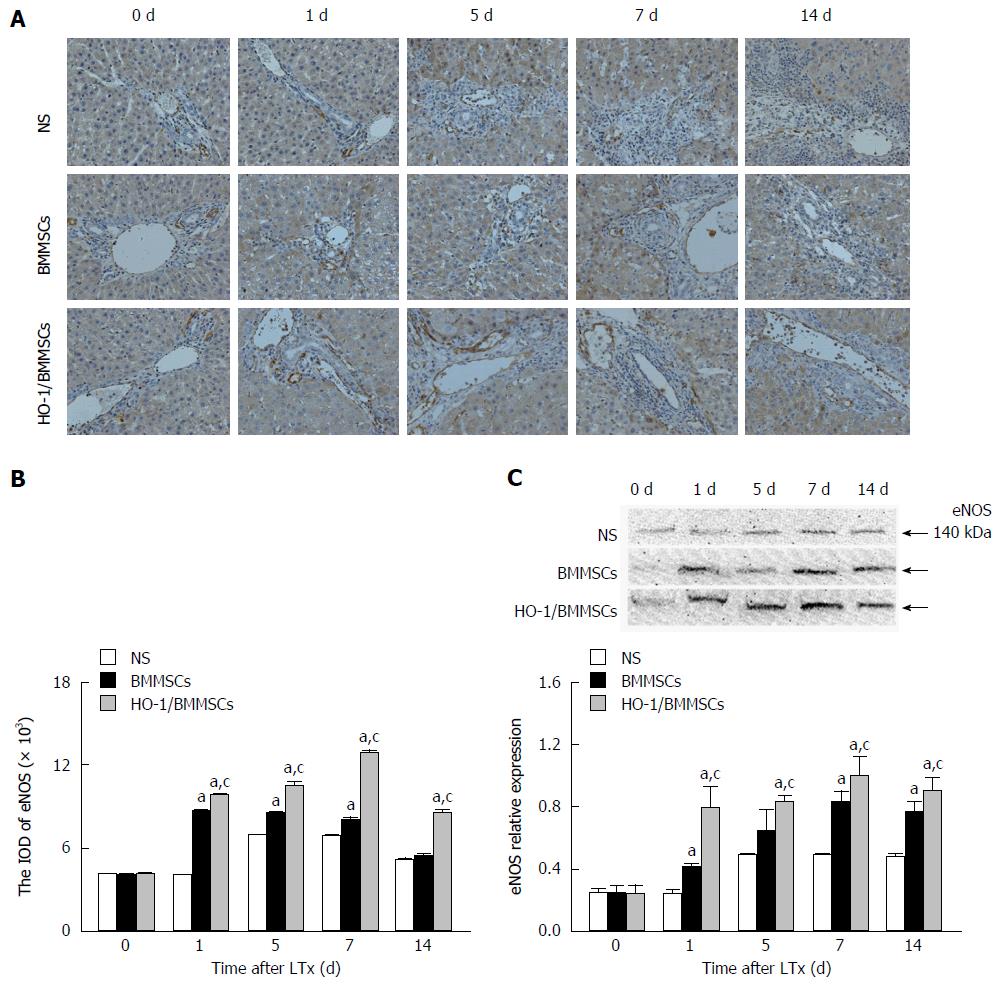
Effects of HO-1/BMMSCs on eNOS expression in the transplanted liver: The immunohistochemical results showed that eNOS positive cells were present in the hepatic sinusoids around the Glisson system. The ratios of eNOS positive cells in the BMMSCs and HO-1/BMMSCs groups were significantly higher than in the NS group. HO-1/BMMSCs group showed more eNOS-positive cells than the BMMSCs group, and the number of eNOS-positive cells was low in the NS group (Figure 7A and B).
The level of eNOS in the transplanted liver tended to increase with time after operation. The level of eNOS was the highest in HO-1/BMMSCs group, and the lowest in the NS group on POD1, 7, and 14 (P < 0.05). The level of eNOS in the HO-1/BMMSCs group was higher than that in the BMMSCs and NS groups on POD5 (P < 0.05). These results suggested that BMMSCs could promote the synthesis of eNOS in the transplanted liver, and more significantly in the HO-1/BMMSCs group (Figure 7C).
The serum levels of eNOS increased from POD1 to POD14 after RLT, but were lower than that on POD0. The levels of eNOS in the HO-1/BMMSCs group were significantly higher than those in the BMMSCs and NS groups on POD1 and POD14 (P <0.01). The level of eNOS was highest in HO-1/BMMSCs group and lowest in the NS group on POD5 and POD7 (P <0.01). This suggested that BMMSCs could promote the synthesis of eNOS in rats after RLT, and the promotive effects of HO-1/BMMSCs were more significant than those of BMMSCs (Figure 6B).
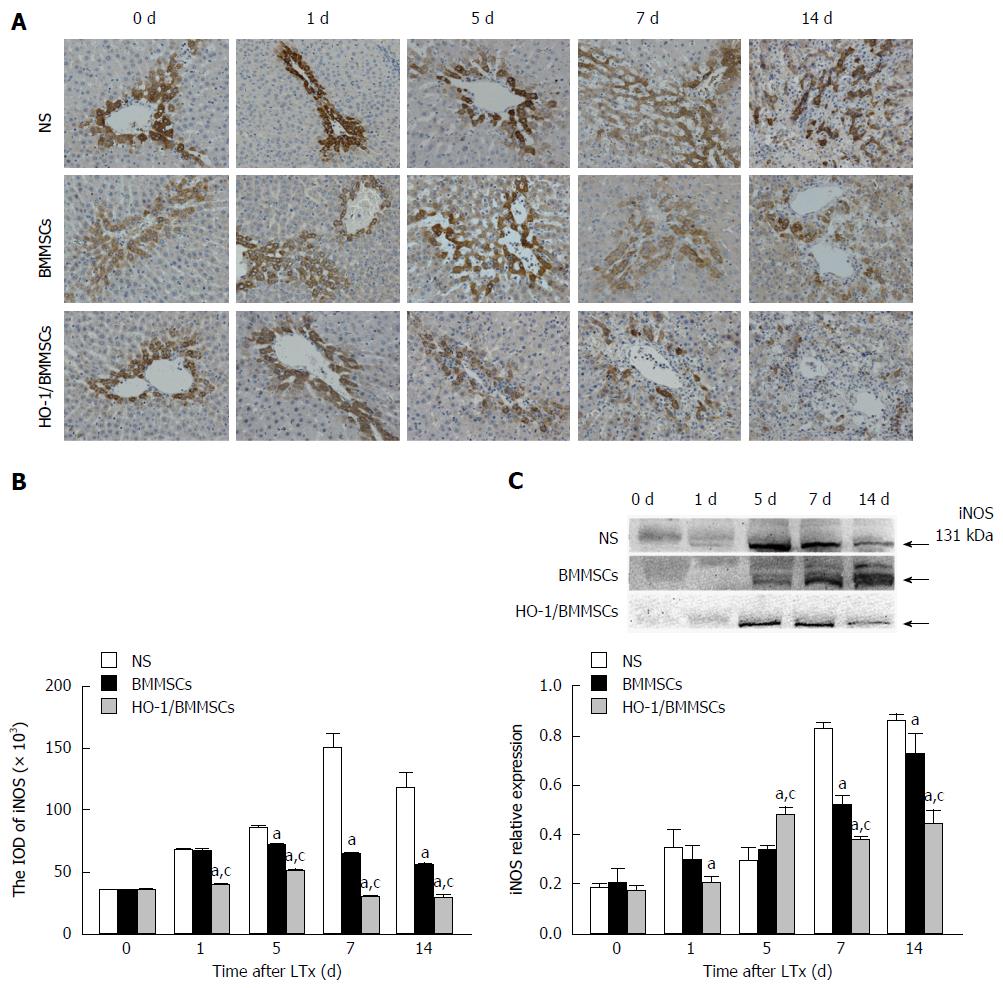
Effects of HO-1/BMMSCs on iNOS expression in the transplanted liver: The immunohistochemical results showed that iNOS positive cells were present in hepatic sinusoids around the Glisson system, and that iNOS was expressed in the cytoplasm of hepatocytes. The ratio and staining intensity of iNOS positive cells in the BMMSCs and HO-1/BMMSCs groups were significantly lower than those in the NS group, and those in the HO-1/BMMSCs group were lower than in the BMMSCs group. The number and staining intensity of iNOS positive cells in the NS group were relatively high (Figure 8A and B).
The level of iNOS in the transplanted liver tended to increase with the extension of post-operative time. The level of iNOS in the HO-1/BMMSCs and BMMSCs groups was lower than that in the NS group at all time points. The level of iNOS in the HO-1/BMMSCs group was significantly higher than that in the NS group on POD1 (P < 0.05), and was significantly higher than that in BMMSCs and NS groups on POD5 (P < 0.05). The expression of iNOS was highest in the HO-1/BMMSCs group and lowest in the NS group on POD7 and POD14 (P < 0.05). This suggested that BMMSCs could inhibit the synthesis of iNOS in the transplanted liver, and that the inhibitory effects of HO-1/BMMSCs were more significant than those of BMMSCs (Figure 8C).
The serum expression of iNOS increased with the extension of post-operative time. The expression of iNOS in HO-1/BMMSCs group and BMMSCs group was significantly lower than that in NS group on POD1, 7, and 14 (P < 0.01). The expression of iNOS was the lowest in HO-1/BMMSCs group and highest in NS group on POD5 (P < 0.01). This suggested that BMMSCs could inhibit the synthesis of iNOS in rats after RLT, and the inhibitory effects of HO-1/BMMSCs were more significant than that of BMMSCs (Figure 6C).
Effects of HO-1/BMMSCs on NO production in rats after RLT: The serum expression of NO tended to increase initially and then decrease with time after operation; however, the decreasing tendency was not significant in all groups. The serum NO in the HO-1/BMMSCs group was higher than that in the BMMSCs and NS groups on POD1 (P < 0.01). The serum NO on POD5 was lowest in the HO-1/BMMSCs group and highest in the NS group (P < 0.01). The serum NO in the HO-1/BMMSCs and BMMSCs groups was significantly lower than that in the NS group on POD7 (P < 0.01). This suggested that BMMSCs could inhibit the synthesis of NO in the transplanted liver, and the inhibitory effects of HO-1/BMMSCs were more significant than those of simple BMMSCs (Figure 6D).
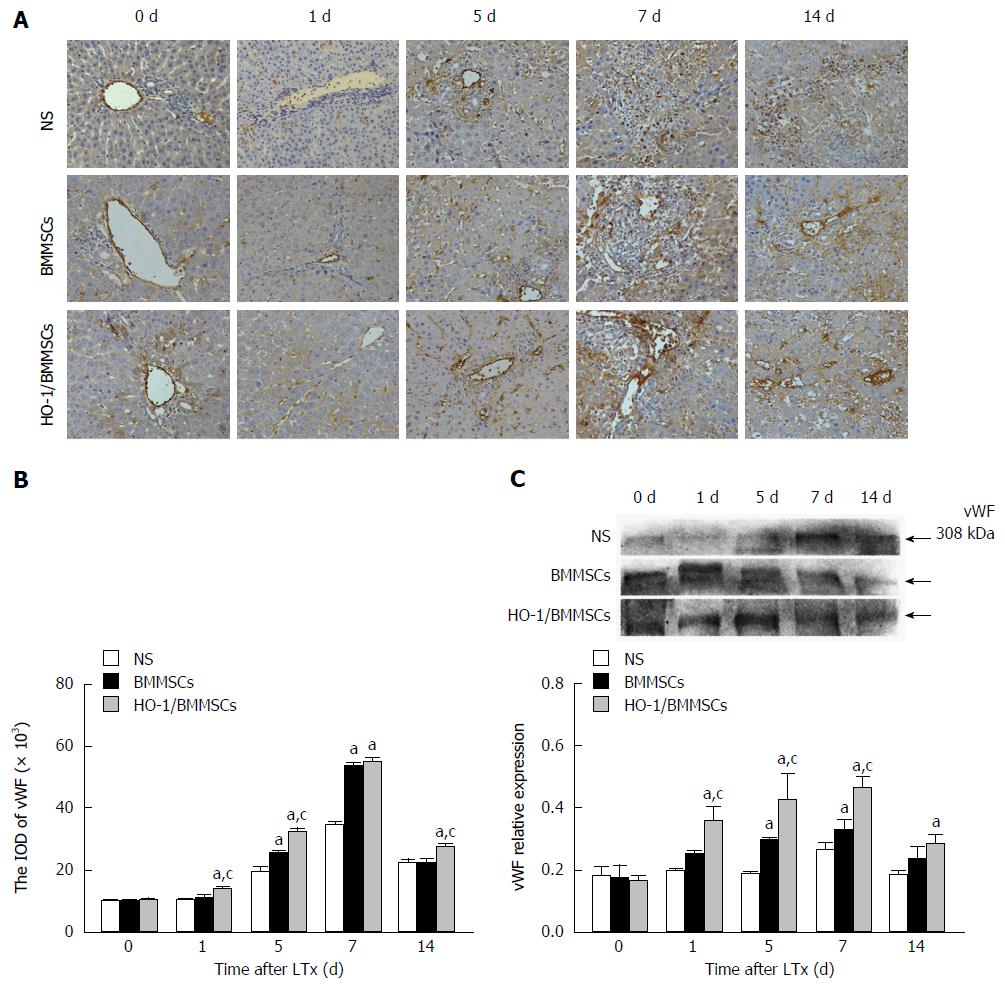
Effects of HO-1/BMMSCs on vWF expression in hepatic sinusoids: vWF was expressed in sinusoidal endothelial cells and SECs of transplanted liver. Immunohistochemical staining for liver vWF showed that the ratios of vWF positive cells in the BMMSCs and HO-1/BMMSCs groups were significantly higher than that in the NS group, and the level in the HO-1/BMMSCs group was higher than that in the BMMSCs group. The number of vWF positive cells in the NS group was relatively low and the cells were scattered. Whereas the vWF positive cells in the BMMSCs and HO-1/BMMSCs groups were arranged regularly and were consistent with hepatic sinusoids. These results showed that HO-1/BMMSCs could promote the proliferation of SECs and the remodeling of hepatic sinusoids more significantly than BMMSCs. The results suggested that HO-1 could enhance the ability of BMMSCs to promote the regeneration of hepatocytes (Figure 9A and B).
Intrahepatic expression of vWF tended to increase initially and then decrease after POD7; however, the NS group showed no significant increasing trend. The expression of vWF in the HO-1/BMMSCs group was significantly higher than in the BMMSCs and NS groups on POD1 (P < 0.05). The level of vWF was highest in the HO-1/BMMSCs group and lowest in the NS group on POD5 and POD7 (P < 0.05). The level of vWF in the HO-1/BMMSCs group was higher than that in NS group on POD14 (P < 0.05). These results suggested that BMMSCs could promote the synthesis of vWF in the transplanted liver graft, and that the effect of HO-1/BMMSCs was more significant than that of BMMSCs. However, the effects of BMMSCs decreased with time after operation (Figure 9C).
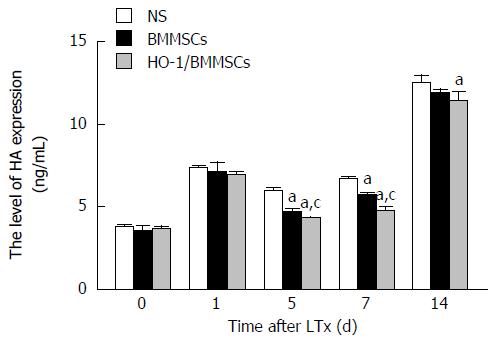
Effects of HO-1/BMMSCs on the degradation of HA by SECs: The serum HA in all groups tended to increase initially, then decrease, and later increase again with increasing post-operative time. Serum HA was lowest in the HO-1/BMMSCs group and highest in the NS group on POD5 and POD7 (P < 0.01). Serum HA in the HO-1/BMMSCs group was lower than that in the NS group on POD14 (P < 0.01). This suggested that BMMSCs could promote the degradation of HA in rats after RLT, and the effect of HO-1/BMMSCs was more significant than that of simple BMMSCs. However, the effects of BMMSCs decreased with increasing post-operative time (Figure 10).
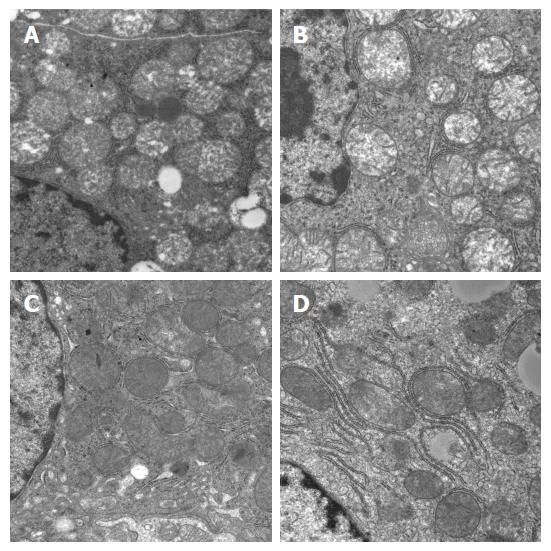
Effects on mitochondrial morphology under electron microscope: There were various degrees of damage to mitochondria in hepatocytes after RLT. The damage was more severe on POD7 in the NS group, with obvious mitochondrial swelling, vacuolization, and disturbed structures of mitochondrial cristae (some even disappeared). Mitochondria in the HO-1/BMMSCs and BMMSCs groups showed mild swelling, no vacuolization, and integrated structures of the mitochondrial ridge. These results suggested that HO-1/BMMSCs could ameliorate the damage to mitochondria (Figure 11).
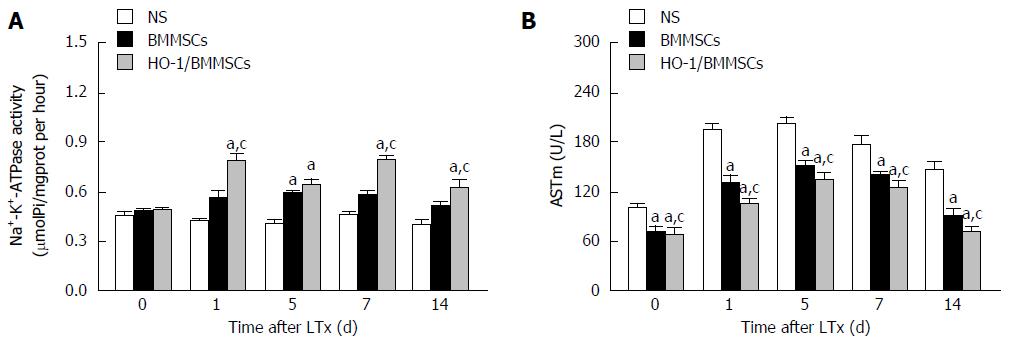
ATPase can degrade ATP to produce ADP and inorganic phosphorus, thus the amount of inorganic phosphorus reflects the activity of ATPase. The activities of ATPase in the transplanted liver tended to increase initially and then decrease after POD 7. The activities of ATPase in the HO-1/BMMSCs group were higher than those of the BMMSCs and NS groups on POD1, 7, and 14 (P < 0.05). The activities of ATPase in the HO-1/BMMSCs and BMMSCs groups were both higher than that in NS group on POD5 (P < 0.05). The results suggested that HO-1/BMMSCs could improve the activity of hepatic Na+-K+-ATPase and promote hepatic energy metabolism (Figure 12A).
ASTm exists in the mitochondria of hepatocytes, and is released into the blood when hepatocytes are severely damaged. Thus, the serum ASTm level can indicate whether the liver function is normal or not, and is also a sign of mitochondrial damage. In the present study, ASTm showed a tendency to increase initially and then decrease with increasing post-operative time. The expression of ASTm was lowest in the HO-1/BMMSCs group and highest in the NS group on POD0, 1, 5, 7 and 14 (P < 0.05; Figure 12B).
Ischemia-reperfusion, reduction of liver volume, and rejection of the transplanted liver can lead to disturbance of hepatic microcirculation: the main changes are stasis in the hepatic sinusoids and collapse of Disse’s space[38]. Transient or sustained hypertension of the PV will cause mechanical damage to the transplanted liver, and induce the activation and release of a variety of cytokines, resulting in a vicious circle. Shear stress caused by excessive blood flow of the PV will break the balance between vasoconstrictor factors and vasodilator factors, leading to microcirculation disturbance in hepatic sinusoids[39,40]. Reduction of the PVP and improvement of hepatic venous perfusion are important to improve liver function after RLT[39]. BMMSCs have tissue repair, regulation of inflammation, and immune response functions, and have protective effects on transplanted livers[22,27,41,42]. To solve the problem that BMMSCs have low activity in damaged tissue[43], and have a short survival time[22,24,44,45], adenovirus-transduced BMMSCs expressing HO-1 were used to study their protective effects on transplanted livers, sinusoidal microcirculation, and energy metabolism after RLT.
Hepatic microcirculation comprises the circulation of the terminal PV and hepatic artery through sinusoids to central veins. The central structure is the sinusoid. The wall of a hepatic sinusoid comprises SECs, Kupffer cells, hepatic stellate cells, and crypt cells. Among these cells, SECs account for 70%, protecting the hepatic parenchymal cells and maintaining the structural and functional integrity of hepatic lobules. SECs also show secretory functions of vascular endothelia, secreting ET-1 and NO to regulate the vascular tone and hepatic stellate cells, which plays an important role in maintaining hepatic sinusoidal microcirculation and intrahepatic homeostasis[46,47]. Impaired SECs, and an imbalance in vasodilator substances, can lead to dysfunction of hepatic microcirculation, such as sinusoidal stasis, ET-1/NO imbalance, and increased reactive oxygen species (ROS)[48]. ET-1 is produced mainly by vascular endothelial cells, and is the strongest endogenous vasoconstrictor discovered to date. Hypoxia is an important stimulating factor of ET-1 upregulation[49]. When the liver is damaged, the expression of ET-1 in SECs increases, the production of NO is reduced, and the balance between vasoconstriction and vasodilatation is broken. The vasoconstrictive effects of ET-1 are dominant, leading to hepatic sinusoid stasis, vasoconstriction, increased intrahepatic vascular resistance, upregulation of leukocyte-endothelia interaction, and portal hypertension[50,51]. IRI induces hepatic ischemia and hypoxia; the ET-1 secreted by all kinds of cells in the liver can lead to extensive sinusoidal vasoconstriction, decreased sinusoidal diameters, and dysfunction of hepatic sinusoidal microcirculation[52]. The results of this study showed that the expression of ET-1 increased initially and then decreased gradually as the degree of liver injury and rejection increased. The levels of ET-1 in the BMMSCs and HO-1/BMMSCs groups were significantly lower than in the NS group. The level of ET-1 increased gradually with the disappearance of BMMSCs, and the level of ET-1 was the lowest and the duration of the effect was the longest in the HO-1/BMMSCs group (P < 0.05). Taken together, these results suggested that HO-1/BMMSCs could downregulate the expression of ET-1, and alleviate the damage to hepatic sinusoids induced by ET-1, indicating indirectly that HO-1 could prolong the effects of BMMSCs.
NO has a protective effect against ET-1 by inhibiting the synthesis of ET, relaxing the smooth muscle to dilate blood vessels, improving microcirculation, and inhibiting platelet, leukocyte adhesion and antioxidation[53,54]. Endogenous NO is produced by NOS-catalyzed oxidation of ammonia in the guanidine terminus of L-arginine. There are three isoenzymes of NOS (eNOS, iNOS, and nNOS). eNOS is only expressed continuously in the vascular endothelia of the liver, where it relaxes blood vessels, inhibits inflammation, scavenges free radicals, and inhibits platelet activation, adhesion and aggregation, and plays a protective role on the transplanted liver by improving the sinusoidal microcirculation and inhibiting hepatocytes apoptosis[55]. iNOS is expressed in various kinds of intrahepatic cells, and can produce large amounts of NO under pathological conditions[56]. Selective iNOS inhibitors were found to improve liver blood flow and reduce liver IRI, but aggravate liver injury under ischemia-reperfusion and sepsis conditions; and eNOS-derived NO could alleviate IRI, while iNOS-derived NO promoted IRI[57,58]. We found that HO-1/BMMSCs and BMMSCs promoted the synthesis of eNOS, and inhibited the synthesis of iNOS, while HO-1/BMMSCs had more significant effects than BMMSCs. As mentioned above, BMMSCs were potent in anti-inflammation activity, and could differentiate into endothelial cells, and secrete vascular endothelial growth factor (VEGF)[11,12]. We hypothesized that BMMSCs could promote endothelial proliferation and angiogenesis to improve the ET-1/NO and vasodilation balance, thereby improving sinusoidal microcirculation.
ET-1 plays a key role in the regulation of liver microcirculation, and dysfunction of SECs leads to the upregulation of ET-1 to promote hepatic stellate cell contraction and portal hypertension[59]. Plasma ET-1 levels in the early stage after reperfusion of a transplantated liver correlated with the PVP[60]. We monitored the PVP on POD7 in this study, and the results suggested that both BMMSCs and HO-1/BMMSCs could reduce the PVP after liver transplantation, which was associated with an improved ET-1/NO balance, leading to downregulation of ET-1 expression and upregulation of eNOS expression. This dual regulation of vasodilatory effects could decrease the PVP and improve hepatic sinusoidal perfusion.
As a glycoprotein present in plasma and on the surface of endothelial cells, vWF is a marker of endothelial cell activation[61,62]. In this study, rejection and liver damage increased with the extension of post-operative time in the RLT model. In addition, SECs injury gradually increased and vWF expression decreased. BMMSCs showed a potent ability to differentiate into endothelial cells, and promote angiogenesis, tissue repair and secretion of VEGF[10-15]; therefore, injection of BMMSCs after RLT could reduce apoptosis of hepatocytes and SECs, and promote the proliferation of hepatocytes and SECs, mainly through the effects of VEGF secreted by BMMSCs[13]. VEGF regulates the recruitment of hepatic sinusoidal endothelial progenitor cells and promotes the proliferation of SECs, which play an important role in the postoperative sinusoidal regeneration[63,64]. Therefore, BMMSCs might relieve sinusoidal injury by recruiting hepatic sinusoidal endothelial progenitor cells and secreting VEGF to promote SECs proliferation. The expression of the vWF protein in HO-1/BMMSCs group was significantly higher than that in BMMSCs group (P < 0.05), which suggested that HO-1/BMMSCs could improve the proliferation of SECs and promote the angiogenesis of hepatic sinusoids, leading to improved blood circulation in hepatic sinusoids and delayed sinusoidal injury.
To evaluate the degree of SECs injury accurately, we examined the level of HA. As a macromolecular mucopolysaccharide synthesized by liver interstitial cells, HA is mainly metabolized by SECs. SECs bind HA through the HA receptor, ingest HA by pinocytosis, and catabolize it using hyaluronidase in lysosomes. About 85%-95% of HA in the blood is absorbed and metabolized by SECs, thus serum HA levels can reflect the severity of SECs injury accurately[65,66]. Our results showed that the SECs damage was more severe and the degradation of HA was reduced with increasing post-operative time. The concentration of HA in the HO-1/BMMSCs group was significantly lower than that in the BMMSCs group, suggesting less injury to SECs. Thus, we speculated that BMMSCs have a protective effect on SECs in the short-term after allogeneic RLT, leading to improved hepatic sinusoidal microcirculation, and HO-1 might prolong the effects of BMMSCs.
Disturbance of hepatic sinusoidal microcirculation can lead directly to intrahepatic ischemia and hypoxia, thereby affecting liver energy metabolism. Mitochondrial dysfunction results in excessive production of ROS, which can affect the activity of mitochondrial oxidation-respiratory chain complexes, thus disturbing mitochondrial oxidative phosphorylation and reducing intracellular ATP synthesis[67]. The concentration of ATP in the liver is critical for the maintenance of transplanted liver function. Necrosis and apoptosis of hepatocytes are associated with low baseline levels of intrahepatic ATP to some extent, ischemic preconditioning and liposomally-entrapped ATP pretreatment could significantly improve the hepatic ATP level after ischemia-reperfusion, thus increasing the operation success rate and graft survival rate[8,9,68]. The serum level of ASTm is a sign of mitochondrial damage, and Na+-K+-ATPase activity can assess the energy metabolism of the transplanted liver. In our study, the ultrastructure of the transplanted liver tissue was observed, and the mitochondria was damaged significantly in the NS group on POD7 after RLT, while they were recovered in BMMSCs group and HO-1/BMMSCs group at the same time point. ASTm and Na+-K+-ATPase were also measured, and the results showed that the ATPase activities in the BMMSCs and HO-1/BMMSCs groups were higher than those in the NS group, which may be related to the significant decrease of ATP during rejection and disordered energy metabolism[69]. The level of ASTm was lowest in HO-1/BMMSCs group, and was relatively high in the NS group. We found that the activity of Na+-K+-ATPase after treatment with BMMSCs was higher than that in the NS group, which was consistent with the results for ASTm in the HO-1/BMMSCs group and BMMSCs group, which were lower than those in the NS group. These results indicated that mitochondrial injury was lowest in HO-1/BMMSCs group, which was beneficial to the metabolism of the transplanted liver. The results suggested that BMMSCs and HO-1/BMMSCs not only ameliorated the effects on ATPase activity and mitochondrial damage, but also had protective effects on the energy metabolism of the transplanted liver, and the effects of HO-1/BMMSCs were more significant.
In conclusions, in the context of liver donor graft shortage, RLT is a research hotspot in clinical liver transplantation. HO-1/BMMSCs could improve the hepatic sinusoidal microcirculation in rats after RLT, and promote liver energy metabolism to protect the transplanted liver. These results provide a basis to improve the quality of transplanted livers by gene therapy combined with stem cells transplantation, and offer a reliable method to expand the source of donor liver.
We would like to thank Professor Fu-Hui Zhang for his guidance of this experimental study. We also thank Tianjin Key Laboratory of Organ Transplantation for kindly providing the research platform.
Graft injury after reduced-size liver transplantation (RLT) can affect the quality of donor liver seriously. Disturbance of the hepatic microcirculation and disorder of the hepatic energy metabolism are important factors affecting liver function after LT. Bone marrow mesenchymal stem cells (BMMSCs) can alleviate hepatic ischemia-reperfusion injury, accelerate liver regeneration, and have anti-inflammatory and immunoregulatory effects; however, their survival rate is low and survival time is short. Heme oxygenase-1 (HO-1) can regulate BMMSCs by enhancing the regulatory effects of BMMSCs under the state of hypoxia and oxidative stress, and can prolong the survival time of BMMSCs. The aim of this study was to explore the effects of HO-1/BMMSCs on hepatic microcirculation and energy metabolism of the transplanted liver in a rat model of rejection following RLT.
BMMSCs have the potential for multipotent differentiation, regeneration promotion, and anti-inflammatory and immunoregulatory effects, and have been used widely in a variety of cell therapy research. However, the survival rate and survival time of BMMSCs in the diseased tissue are low. Therefore, how to improve the survival time of BMMSCs in vivo, and improve the microcirculation and energy metabolism of the transplanted liver at the same time, is a research hotspot.
HO-1/BMMSCs could improve the hepatic microcirculation after RLT significantly, and they decreased SECs injury and restored the energy metabolism of the damaged hepatocytes, showing a good protective effect on the transplanted liver.
In this study, BMMSCs modified by HO-1 could prolong the survival time and improve the activity of BMMSCs, and further demonstrated the protective effect of HO-1/BMMSCs on the transplantated liver after RLT. These results provided the basis for improving the quality of reduced-size transplanted livers by gene therapy combined with stem cells transplantation, and provide a reliable method to expand the source of donor livers.
Sinusoids are central structures of the hepatic microcirculation, and the sinusoidal wall consists of SECs, Kupffer cells, hepatic stellate cells, and crypt cells, which play important roles in maintaining sinusoidal microcirculation and intrahepatic homeostasis. BMMSCs are non-hematopoietic stem cells derived from bone marrow, with multi-directional differentiation potential, and function in tissue repair, paracrine signaling, anti-inflammation, and immunoregulation. HO-1 is a microsomal oxidase in mammals, with protective effects, including anti-inflammation, anti-oxidative stress, anti-apoptosis, anti-ischemia reperfusion injury, and microcirculatory modulation.
This article demonstrates the protective effects of HO-1/BMMSCs on the transplanted liver in a rat model of rejection following RLT, which was studied from hepatic microcirculation and energy metabolism, hepatic microcirculation is very important and not well studied in liver transplantation. This study will be of interest and the paper is clearly written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gencdal G, Sert I S- Editor: Ma YJ L- Editor: Ma JY E- Editor: Wang CH
| 1. | Merion RM. Current status and future of liver transplantation. Semin Liver Dis. 2010;30:411-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Song AT, Avelino-Silva VI, Pecora RA, Pugliese V, D'Albuquerque LA, Abdala E. Liver transplantation: fifty years of experience. World J Gastroenterol. 2014;20:5363-5374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 3. | Taniguchi M, Shimamura T, Todo S, Furukawa H. Small-for-size syndrome in living-donor liver transplantation using a left lobe graft. Surg Today. 2015;45:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Jadlowiec CC, Taner T. Liver transplantation: Current status and challenges. World J Gastroenterol. 2016;22:4438-4445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 238] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (2)] |
| 5. | Imamura H, Brault A, Huet PM. Effects of extended cold preservation and transplantation on the rat liver microcirculation. Hepatology. 1997;25:664-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Romanque U P, Uribe M M, Videla LA. Molecular mechanisms in liver ischemic-reperfusion injury and ischemic preconditioning. Rev Med Chil. 2005;133:469-476. [PubMed] |
| 7. | Kohli V, Gao W, Camargo CA, Clavien PA. Calpain is a mediator of preservation-reperfusion injury in rat liver transplantation. Proc Natl Acad Sci USA. 1997;94:9354-9359. [PubMed] |
| 8. | Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39:55-61. [PubMed] |
| 9. | Chaïb S, Charrueau C, Neveux N, Nakib S, Chaumeil JC, Cynober L, De Bandt JP. Effect of apoE/ATP-containing liposomes on hepatic energy state. Liver Int. 2003;23:379-385. [PubMed] |
| 10. | Gardner OF, Alini M, Stoddart MJ. Mesenchymal Stem Cells Derived from Human Bone Marrow. Methods Mol Biol. 2015;1340:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 569] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 12. | Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C. Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol. 2007;292:F1626-F1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 467] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 13. | Du Z, Wei C, Cheng K, Han B, Yan J, Zhang M, Peng C, Liu Y. Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J Surg Res. 2013;183:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 14. | Zhou TB, Yang GS. Roles of vascular endothelial growth factor in acute rejection reaction following liver transplantation. Transpl Immunol. 2011;25:207-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Zhang C, Yang G, Lu D, Ling Y, Chen G, Zhou T. Expression of vascular endothelial growth factor and basic fibroblast growth factor in acute rejection reaction following rat orthotopic liver transplantation. Exp Ther Med. 2014;8:483-487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Lee MW, Ryu S, Kim DS, Sung KW, Koo HH, Yoo KH. Strategies to improve the immunosuppressive properties of human mesenchymal stem cells. Stem Cell Res Ther. 2015;6:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Casiraghi F, Perico N, Cortinovis M, Remuzzi G. Mesenchymal stromal cells in renal transplantation: opportunities and challenges. Nat Rev Nephrol. 2016;12:241-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Kim KH, Blasco-Morente G, Cuende N, Arias-Santiago S. Mesenchymal stromal cells: properties and role in management of cutaneous diseases. J Eur Acad Dermatol Venereol. 2017;31:414-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Zou Z, Cai Y, Chen Y, Chen S, Liu L, Shen Z, Zhang S, Xu L, Chen Y. Bone marrow-derived mesenchymal stem cells attenuate acute liver injury and regulate the expression of fibrinogen-like-protein 1 and signal transducer and activator of transcription 3. Mol Med Rep. 2015;12:2089-2097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Sang JF, Shi XL, Han B, Huang X, Huang T, Ren HZ, Ding YT. Combined mesenchymal stem cell transplantation and interleukin-1 receptor antagonism after partial hepatectomy. World J Gastroenterol. 2016;22:4120-4135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 21. | Eom YW, Kim G, Baik SK. Mesenchymal stem cell therapy for cirrhosis: Present and future perspectives. World J Gastroenterol. 2015;21:10253-10261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Wu B, Song HL, Yang Y, Yin ML, Zhang BY, Cao Y, Dong C, Shen ZY. Improvement of Liver Transplantation Outcome by Heme Oxygenase-1-Transduced Bone Marrow Mesenchymal Stem Cells in Rats. Stem Cells Int. 2016;2016:9235073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Perico N, Casiraghi F, Gotti E, Introna M, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P. Mesenchymal stromal cells and kidney transplantation: pretransplant infusion protects from graft dysfunction while fostering immunoregulation. Transpl Int. 2013;26:867-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Yang Y, Song HL, Zhang W, Wu BJ, Fu NN, Zheng WP, Dong C, Shen ZY. Reduction of acute rejection by bone marrow mesenchymal stem cells during rat small bowel transplantation. PLoS One. 2014;9:e114528. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Wu H, Wen D, Mahato RI. Third-party mesenchymal stem cells improved human islet transplantation in a humanized diabetic mouse model. Mol Ther. 2013;21:1778-1786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Doğan SM, Kılınç S, Kebapçı E, Tuğmen C, Gürkan A, Baran M, Kurtulmuş Y, Olmez M, Karaca C. Mesenchymal stem cell therapy in patients with small bowel transplantation: single center experience. World J Gastroenterol. 2014;20:8215-8220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Zhang W, Shen ZY, Song HL, Yang Y, Wu BJ, Fu NN, Liu T. Protective effect of bone marrow mesenchymal stem cells in intestinal barrier permeability after heterotopic intestinal transplantation. World J Gastroenterol. 2014;20:7442-7451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Li X, Xiao Y, Cui Y, Tan T, Narasimhulu CA, Hao H, Liu L, Zhang J, He G, Verfaillie CM. Cell membrane damage is involved in the impaired survival of bone marrow stem cells by oxidized low-density lipoprotein. J Cell Mol Med. 2014;18:2445-2453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Liu B, Qian JM. Cytoprotective role of heme oxygenase-1 in liver ischemia reperfusion injury. Int J Clin Exp Med. 2015;8:19867-19873. [PubMed] |
| 30. | Gao Z, Han Y, Hu Y, Wu X, Wang Y, Zhang X, Fu J, Zou X, Zhang J, Chen X. Targeting HO-1 by Epigallocatechin-3-Gallate Reduces Contrast-Induced Renal Injury via Anti-Oxidative Stress and Anti-Inflammation Pathways. PLoS One. 2016;11:e0149032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 31. | Lawal AO, Marnewick JL, Ellis EM. Heme oxygenase-1 attenuates cadmium-induced mitochondrial-caspase 3- dependent apoptosis in human hepatoma cell line. BMC Pharmacol Toxicol. 2015;16:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Petersen B, Ramackers W, Lucas-Hahn A, Lemme E, Hassel P, Queisser AL, Herrmann D, Barg-Kues B, Carnwath JW, Klose J. Transgenic expression of human heme oxygenase-1 in pigs confers resistance against xenograft rejection during ex vivo perfusion of porcine kidneys. Xenotransplantation. 2011;18:355-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 33. | Lee HS, Lee JG, Yeom HJ, Chung YS, Kang B, Hurh S, Cho B, Park H, Hwang JI, Park JB. The Introduction of Human Heme Oxygenase-1 and Soluble Tumor Necrosis Factor-α Receptor Type I With Human IgG1 Fc in Porcine Islets Prolongs Islet Xenograft Survival in Humanized Mice. Am J Transplant. 2016;16:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Schumacher A, Zenclussen AC. Effects of heme oxygenase-1 on innate and adaptive immune responses promoting pregnancy success and allograft tolerance. Front Pharmacol. 2014;5:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Hamedi-Asl P, Halabian R, Bahmani P, Mohammadipour M, Mohammadzadeh M, Roushandeh AM, Jahanian-Najafabadi A, Kuwahara Y, Roudkenar MH. Adenovirus-mediated expression of the HO-1 protein within MSCs decreased cytotoxicity and inhibited apoptosis induced by oxidative stresses. Cell Stress Chaperones. 2012;17:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Omura T, Ascher NL, Emond JC. Fifty-percent partial liver transplantation in the rat. Transplantation. 1996;62:292-293. [PubMed] |
| 37. | Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1016] [Cited by in RCA: 1002] [Article Influence: 35.8] [Reference Citation Analysis (1)] |
| 38. | Schmucker DL, Curtis JC. A correlated study of the fine structure and physiology of the perfused rat liver. Lab Invest. 1974;30:201-212. [PubMed] |
| 39. | Palmes D, Minin E, Budny T, Uhlmann D, Armann B, Stratmann U, Herbst H, Spiegel HU. The endothelin/nitric oxide balance determines small-for-size liver injury after reduced-size rat liver transplantation. Virchows Arch. 2005;447:731-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Li J, Liang L, Ma T, Yu X, Chen W, Xu G, Liang T. Sinusoidal microcirculatory changes after small-for-size liver transplantation in rats. Transpl Int. 2010;23:924-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 41. | Tsolaki E, Yannaki E. Stem cell-based regenerative opportunities for the liver: State of the art and beyond. World J Gastroenterol. 2015;21:12334-12350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Yang Y, Shen ZY, Wu B, Yin ML, Zhang BY, Song HL. Mesenchymal stem cells improve the outcomes of liver recipients via regulating CD4+ T helper cytokines in rats. Hepatobiliary Pancreat Dis Int. 2016;15:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 43. | Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 44. | Parekkadan B, Milwid JM. Mesenchymal stem cells as therapeutics. Annu Rev Biomed Eng. 2010;12:87-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 533] [Cited by in RCA: 576] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 45. | Lee JW, Gupta N, Serikov V, Matthay MA. Potential application of mesenchymal stem cells in acute lung injury. Expert Opin Biol Ther. 2009;9:1259-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 46. | Oda M, Han JY, Yokomori H. Local regulators of hepatic sinusoidal microcirculation: recent advances. Clin Hemorheol Microcirc. 2000;23:85-94. [PubMed] |
| 47. | Maslak E, Gregorius A, Chlopicki S. Liver sinusoidal endothelial cells (LSECs) function and NAFLD; NO-based therapy targeted to the liver. Pharmacol Rep. 2015;67:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 48. | Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med. 2002;33:1200-1208. [PubMed] |
| 49. | Davenport AP, Hyndman KA, Dhaun N, Southan C, Kohan DE, Pollock JS, Pollock DM, Webb DJ, Maguire JJ. Endothelin. Pharmacol Rev. 2016;68:357-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 572] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 50. | Feng HQ, Weymouth ND, Rockey DC. Endothelin antagonism in portal hypertensive mice: implications for endothelin receptor-specific signaling in liver disease. Am J Physiol Gastrointest Liver Physiol. 2009;297:G27-G33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Rosado E, Rodríguez-Vilarrupla A, Gracia-Sancho J, Monclús M, Bosch J, García-Pagán JC. Interaction between NO and COX pathways modulating hepatic endothelial cells from control and cirrhotic rats. J Cell Mol Med. 2012;16:2461-2470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Gandhi CR, Behal RH, Harvey SA, Nouchi TA, Olson MS. Hepatic effects of endothelin. Receptor characterization and endothelin-induced signal transduction in hepatocytes. Biochem J. 1992;287:897-904. [PubMed] |
| 53. | Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7858] [Cited by in RCA: 7386] [Article Influence: 194.4] [Reference Citation Analysis (0)] |
| 54. | Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Datta G, Luong TV, Fuller BJ, Davidson BR. Endothelial nitric oxide synthase and heme oxygenase-1 act independently in liver ischemic preconditioning. J Surg Res. 2014;186:417-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4983] [Cited by in RCA: 4459] [Article Influence: 247.7] [Reference Citation Analysis (0)] |
| 57. | Wang Y, Lawson JA, Jaeschke H. Differential effect of 2-aminoethyl-isothiourea, an inhibitor of the inducible nitric oxide synthase, on microvascular blood flow and organ injury in models of hepatic ischemia-reperfusion and endotoxemia. Shock. 1998;10:20-25. [PubMed] |
| 58. | Isobe M, Katsuramaki T, Hirata K, Kimura H, Nagayama M, Matsuno T. Beneficial effects of inducible nitric oxide synthase inhibitor on reperfusion injury in the pig liver. Transplantation. 1999;68:803-813. [PubMed] |
| 59. | Iwakiri Y. Endothelial dysfunction in the regulation of cirrhosis and portal hypertension. Liver Int. 2012;32:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 60. | Man K, Fan ST, Lo CM, Liu CL, Fung PC, Liang TB, Lee TK, Tsui SH, Ng IO, Zhang ZW. Graft injury in relation to graft size in right lobe live donor liver transplantation: a study of hepatic sinusoidal injury in correlation with portal hemodynamics and intragraft gene expression. Ann Surg. 2003;237:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 148] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 61. | Lenting PJ, Christophe OD, Denis CV. von Willebrand factor biosynthesis, secretion, and clearance: connecting the far ends. Blood. 2015;125:2019-2028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 62. | Nishigori N, Matsumoto M, Koyama F, Hayakawa M, Hatakeyayama K, Ko S, Fujimura Y, Nakajima Y. von Willebrand Factor-Rich Platelet Thrombi in the Liver Cause Sinusoidal Obstruction Syndrome following Oxaliplatin-Based Chemotherapy. PLoS One. 2015;10:e0143136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 63. | Shimizu H, Mitsuhashi N, Ohtsuka M, Ito H, Kimura F, Ambiru S, Togawa A, Yoshidome H, Kato A, Miyazaki M. Vascular endothelial growth factor and angiopoietins regulate sinusoidal regeneration and remodeling after partial hepatectomy in rats. World J Gastroenterol. 2005;11:7254-7260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Wang L, Wang X, Wang L, Chiu JD, van de Ven G, Gaarde WA, Deleve LD. Hepatic vascular endothelial growth factor regulates recruitment of rat liver sinusoidal endothelial cell progenitor cells. Gastroenterology. 2012;143:1555-1563.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 65. | Fraser JR, Laurent TC, Laurent UB. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27-33. [PubMed] |
| 66. | McCourt PA, Smedsrød BH, Melkko J, Johansson S. Characterization of a hyaluronan receptor on rat sinusoidal liver endothelial cells and its functional relationship to scavenger receptors. Hepatology. 1999;30:1276-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 67. | Kajikawa M, Fujimoto S, Tsuura Y, Mukai E, Takeda T, Hamamoto Y, Takehiro M, Fujita J, Yamada Y, Seino Y. Ouabain suppresses glucose-induced mitochondrial ATP production and insulin release by generating reactive oxygen species in pancreatic islets. Diabetes. 2002;51:2522-2529. [PubMed] |
| 68. | Neveux N, De Bandt JP, Fattal E, Hannoun L, Poupon R, Chaumeil JC, Delattre J, Cynober LA. Cold preservation injury in rat liver: effect of liposomally-entrapped adenosine triphosphate. J Hepatol. 2000;33:68-75. [PubMed] |
| 69. | Marni A, Ferrero ME, Gaja G. Metabolic function of grafted liver in rats. Transplantation. 1988;46:830-835. [PubMed] |