Published online May 7, 2017. doi: 10.3748/wjg.v23.i17.3133
Peer-review started: September 23, 2016
First decision: December 19, 2016
Revised: January 16, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: May 7, 2017
Processing time: 226 Days and 2.6 Hours
To evaluate the diagnostic value of gadobenate dimeglumine (Gd-BOPTA)-enhanced hepatocyte-phase magnetic resonance imaging (MRI) in evaluating hepatic fibrosis and hepatitis.
Hepatocyte-phase images of Gd-BOPTA-enhanced MRI were retrospectively evaluated in 76 patients with chronic liver disease. These patients were classified into five groups according to either the histopathological fibrosis stage (S0-S4) or the histopathological hepatitis grade (G0-G4). The relative enhancement ratio (RE) of the liver parenchyma in the T1-vibe sequence was calculated by measuring the signal intensity before (SI pre) and 90 min after (SI post) intravenous injection of Gd-BOPTA using the following formula: RE = (SI post - SI pre)/SI pre. One-way analysis of variance was used to compare the difference between the relative RE in the hepatocyte phase (REh) and the stage of hepatic fibrosis and the grade of hepatitis. Pearson’s product-moment correlation analysis was used to evaluate the relationship between the REh and the levels of serologic liver functional parameters.
According to histopathological hepatic fibrosis stage, the 76 patients were classified into five groups: 16 in S0, 15 in S1, 21 in S2, 9 in S3, and 15 in S4 group. According to histopathological hepatitis grade, the 76 patients were also classified into five groups: 0 in G0, 44 in G1, 22 in G2, 8 in G3, and 2 in G3 group. With regard to the stage of hepatic fibrosis, REh showed significant differences between the S2 and S3 groups and between the S2 and S4 groups (P < 0.05), but no significant difference was observed between the other groups. With regard to the grade of hepatitis, REh showed significant differences between the G1 and G2 groups and between the G1 and G4 groups (P < 0.05), but no significant difference was observed between the other groups. Increased REh showed correlations with decreased serum levels of TB, ALT and AST (P < 0.05).
To some extent, measuring the REh using Gd-BOPTA-enhanced MRI might be a noninvasive technique for assessing the stage of hepatic fibrosis. This method is able to differentiate no/mild hepatitis from advanced hepatitis. TB, ALT and AST levels can predict the degree of liver enhancement in the hepatocyte phase of Gd-BOPTA-enhanced MRI.
Core tip: A crucial issue in the prognosis and management of chronic liver diseases is the extent and the progression of hepatic fibrosis. The percutaneous liver biopsy is a widely adopted classical method to diagnose hepatic fibrosis, but it is invasive. We use the degree enhancement in the hepatocyte phase of Gd-BOPTA-enhanced magnetic resonance imaging to evaluate the liver condition of patients with chronic liver diseases. This method is recommended because of advantages such as no injury to the patient and possibility to assess the stage of hepatic fibrosis, the degree of hepatitis and the liver function.
- Citation: Li XM, Chen Z, Xiao EH, Shang QL, Ma C. Diagnostic value of gadobenate dimeglumine-enhanced hepatocyte-phase magnetic resonance imaging in evaluating hepatic fibrosis and hepatitis. World J Gastroenterol 2017; 23(17): 3133-3141
- URL: https://www.wjgnet.com/1007-9327/full/v23/i17/3133.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i17.3133
Hepatic fibrosis is an unavoidable process when chronic liver disease (CLD) develops into cirrhosis. Effective and scientific anti-fibrotic treatments can contribute to the regression of fibrosis[1-3], but no treatments for cirrhosis have been reported in previous research findings[4]. It is therefore essential to diagnose early fibrosis in the therapeutic process of CLD because it can delay or reverse the fibrotic process, thereby reducing the morbidity and mortality of terminal liver disease. With the recent development and application of new imaging techniques such as ultrasound elasticity imaging[5], magnetic resonance (MR) diffusion-weighted imaging[6], and liver magnetic resonance elastography[7], early diagnosis of hepatic fibrosis and cirrhosis is possible by imageology. However, these techniques are still in the early phases of research.
Several studies have shown that MR imaging (MRI) with administration of hepatocyte-specific MR contrast media[8-11] is more accurate than dynamic helical computed tomography (CT) and unenhanced MRI for the detection of focal lesions. The hepatocyte-specific MR contrast agents gadoxetic acid disodium (Gd-EOB-DTPA; Primovist, Schering, Berlin, Germany) and gadobenate dimeglumine (Gd-BOPTA; MultiHance; Bracco Imaging, Milan, Italy), with the dual properties of an extracellular agent and a hepatobiliary contrast agent, are gradually taken up by functional hepatocytes and excreted into bile[12,13] and could display liver parenchymal enhancement in the hepatocyte phase[8]. Impaired hepatobiliary function may severely influence the hepatic uptake of Gd-EOB-DTPA[14], but fewer studies have focused on Gd-BOPTA in the assessment of CLD.
This study investigated the relationship between the enhancement degree in the hepatocyte phase of Gd-BOPTA-enhanced MRI and the stage of hepatic fibrosis, the grade of hepatitis and the levels of functional serum markers. The aim of this study was to evaluate the diagnostic value of Gd-BOPTA-enhanced hepatocyte-phase MRI in evaluating hepatic fibrosis and hepatitis.
This retrospective study was approved by the ethics committee of our hospital, and the requirement for informed consent was waived. The study population was selected from a database of 512 CLD patients who underwent Gd-BOPTA-enhanced hepatocyte-phase MRI examination from December 2014 to March 2016. Seventy-six patients were enrolled in this study group based on the following criteria: (1) They undersent ultrasound-guided percutaneous right liver biopsy within one week after the MRI examination, and the biopsy report included the stage of hepatic fibrosis and the grade of hepatitis; (2) They took the serologic liver functional test within one week of the MRI examination; (3) They did not undergo hepatobiliary surgery or liver transplantation and there were no hepatic lesions with a diameter > 3 cm; and (4) They had no renal insufficiency. The information of 76 patients, including age, gender, pathogeny and the liver functional serum markers which contained total bilirubin (TB), albumin, prothrombin time (PT), alanine aminotransferase (ALT), and aspartate aminotransferase (AST), was collected.
All MRI examinations were performed on a single 3 Tesla unit (Magnetom Verio, A Tim System; Siemens Healthcare, Erlangen, Germany) using an eight-channel body phased-array coil and a spine array coil. The standard sequences performed before Gd-BOPTA administration were T1-weighted in-phase and T1-weighted out-phase imaging, respiratory triggered fat-suppressed turbo spin-echo T2-weighted and diffusion weight imaging. Then, a transverse T1-weighted volume interpolated breath hold examination (T1-vibe) sequence with fat suppression was acquired before and after contrast medium injection in the arterial phase (25 s), portal venous phase (60 s), hepatic vein phase (120 s) and hepatocyte phase (90 min). The T1-vibe image parameters were as follows: repetition time, 3.92 ms; echo time, 1.39 ms; flip angle, 9°; slice thickness, 3 mm; number of partitions, 80; bandwidth, 400 Hz/pixel; field of view (FOV), 380 mm × 308 mm; matrix, 182 × 320; acceleration factor, 2; and acquisition time, 17 s.
All patients received a bolus injection of 0.2 mmol/kg body weight Gd-BOPTA into a cubital or antecubital vein at 2-3 mL/s using a power injector, followed by a 20-mL saline flush at the same speed.
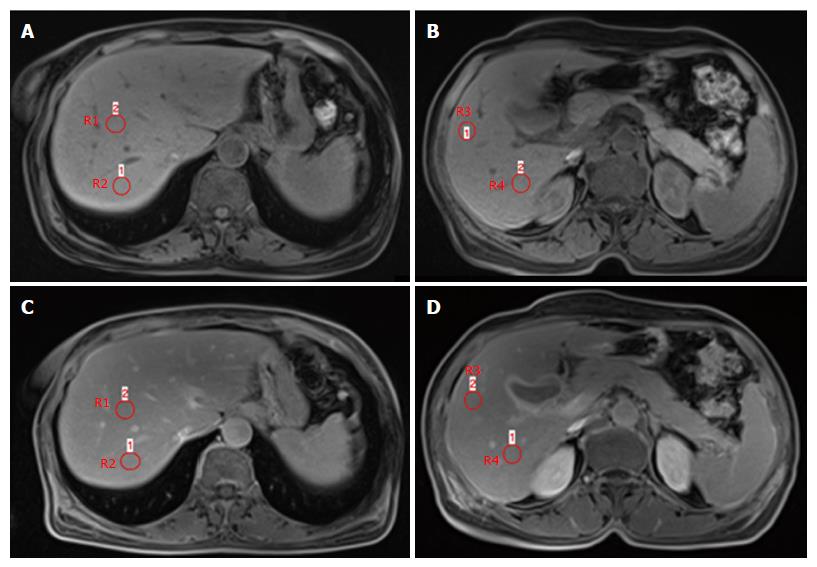
The signal intensity (SI) measurements were performed on a secondary workstation of the SIEMENS healthcare system by a radiologist with more than 20 years of experience in abdominal MRI. The expert radiologist was blinded to the patients’ clinical history, laboratory data, and histopathology characteristics. Measurements were performed by positioning four circular regions of interest (ROIs) of a minimum of 1.0 cm2-2.0 cm2 in the four segments of the right liver lobe in a position similar to that of the right liver biopsy location. SI was obtained by using the averages from the four ROIs located in the right liver. ROIs were drawn to avoid vascular motion and abdominal wall artefacts, as well as visible vascular and biliary structures. Quantitative measurements of the SI of the liver were performed on unenhanced (SI pre) and Gd-BOPTA-enhanced hepatocyte-phase axial images (SI post). Normalized enhanced liver SI was calculated as the relative enhancement (RE) according to the following formula: RE = (SI post - SI pre)/SI pre (Figure 1).
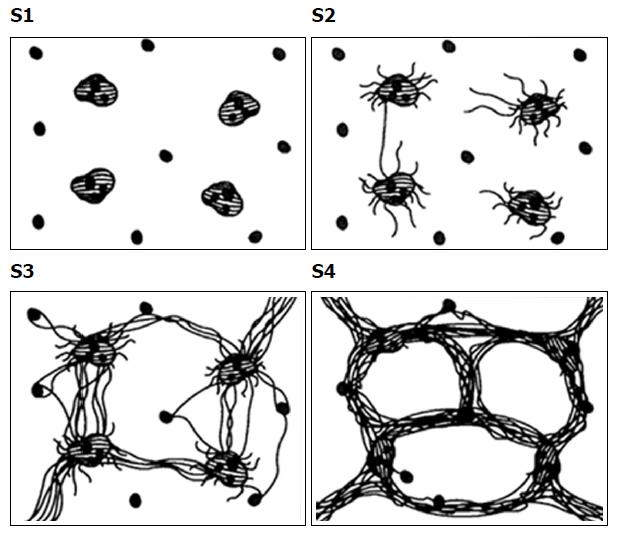
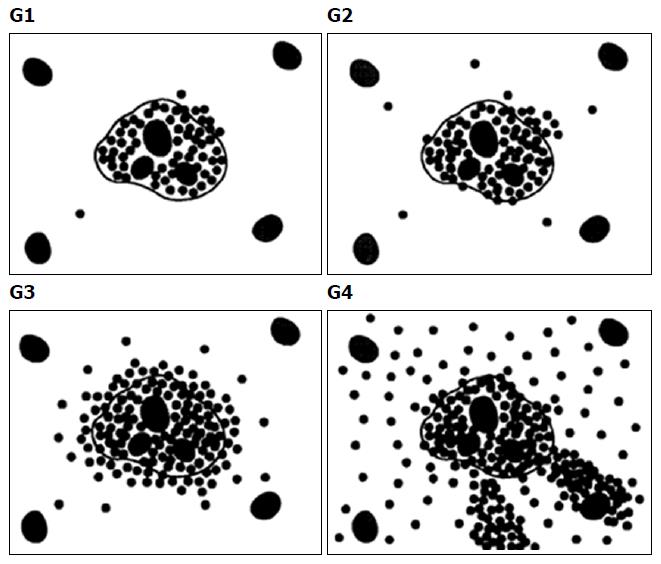
The 76 patients underwent ultrasound-guided percutaneous right liver biopsy within one week after the Gd-BOPTA enhanced MRI examination. According to the stage of hepatic fibrosis and the grade of hepatitis in the Batts and Ludwig system[15], hepatic fibrosis was classified into five stages (Table 1 and Figure 2) and hepatitis was classified into five grades (Table 2 and Figure 3). All biopsies were read by the same liver pathologist who was blinded to the patient details and the MR examination results (Table 3).
| Stage | Description | Criteria |
| S0 | No fibrosis | Normal connective tissue |
| S1 | Portal fibrosis | Fibrous portal expansion |
| S2 | Periportal fibrosis | Periportal or rare portal-portal septa |
| S3 | Septal fibrosis | Fibrous septa with architectural distortion; no obvious cirrhosis |
| S4 | Early cirrhosis | Cirrhosis |
| Grade | Description | Criteria |
| G0 | No activity | No lobular inflammation and necrosis |
| G1 | Minimal activity | Minimal, occasional spotty necrosis |
| G2 | Mild activity | Mild, little hepatocellular damage |
| G3 | Moderate activity | Moderate, noticeable hepatocellular change |
| G4 | Severe activity | Severe, prominent diffuse hepatocellular damage |
| Pathogeny | Number | Percent |
| Chronic hepatitis B | 47 | 61.80% |
| Primary biliary cirrhosis | 10 | 13.20% |
| Non-alcoholic fatty liver disease | 5 | 6.60% |
| Chronic drug hepatitis | 4 | 5.30% |
| Autoimmune hepatitis | 2 | 2.60% |
| Alcoholic fatty liver disease | 2 | 2.60% |
| Chronic hepatitis EB | 2 | 2.60% |
| Hemochromatosis | 1 | 1.30% |
| Hyperthyroid liver damage | 1 | 1.30% |
| Hyperbilirubinemia | 1 | 1.30% |
| Portal hypertension | 1 | 1.30% |
Data entry and analysis were performed by using the SPSS version 18.0. One-way analysis of variance was used to compare the differences between the degree of the relative enhancement in the hepatocyte phase (REh) and the stage of liver fibrosis and the grade of hepatitis. Pearson’s product-moment correlation analysis was used to evaluate the relationship between the REh and the levels of serologic liver functional parameters. P values < 0.05 were considered statistically significant.
Of the 76 patients prospectively enrolled, 47 were male and 29 were female, with a mean age of 42.7 years. The diagnostic work-up resulted in the following 11 definite or plausible causes of CLD in the 76 patients (Table 3).
According to the stage of liver fibrosis in the pathological reports, the 76 patients were classified into five groups: 16 in S0, 15 in S1, 21 in S2, 9 in S3, and 15 in S4 group. According to the grade of hepatitis in the pathological reports, the 76 patients were also classified into five groups: 0 in G0, 44 in G1, 22 in G2, 8 in G3, and 2 in G4 group.
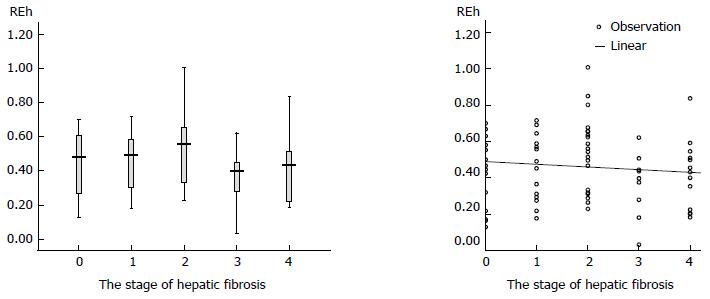
One-way analysis of variance (Table 4) demonstrated that for REh, there were significant differences between the S2 and S3 groups (0.542 ± 0.205 vs 0.364 ± 0.177, P = 0.021) and between the S2 and S4 groups (0.542 ± 0.205 vs 0.411 ± 0.184, P = 0.044), and there were no significant differences between the other groups. Figure 4 illustrates that REh decreased as the stage of hepatic fibrosis progressed.
| Stage | REh (mean ± SD) | Minimum value | Maximum value |
| S0 (n = 16) | 0.450 ± 0.195 | 0.129 | 0.698 |
| S1 (n = 15) | 0.460 ± 0.176 | 0.178 | 0.715 |
| S2 (n = 21) | 0.542 ± 0.204 | 0.229 | 1.006 |
| S3 (n = 9) | 0.364 ± 0.177 | 0.323 | 0.621 |
| S4 (n = 15) | 0.411 ± 0.184 | 0.184 | 0.836 |
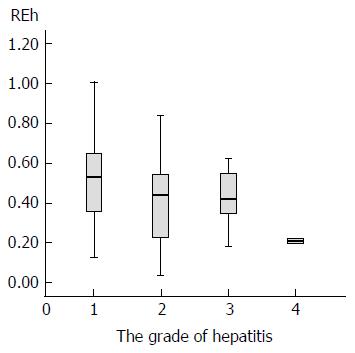
One-way analysis of variance (Table 5) demonstrated that for REh, there were significant differences between the G1 and G2 groups (0.505 ± 0.194 vs 0.402 ± 0.190, P = 0.039) and between the G1 and G4 groups (0.505 ± 0.194 vs 0.209 ± 0.017, P = 0.032), and there were no significant differences between the other groups (Figure 5).
| Grade | REh (mean ± standard) | Minimum value | Maximum value |
| G1 (n = 44) | 0.505 ± 0.194 | 0.129 | 1.006 |
| G2 (n = 22) | 0.402 ± 0.190 | 0.033 | 0.836 |
| G3 (n = 8) | 0.428 ± 0.147 | 0.182 | 0.621 |
| G4 (n = 2) | 0.209 ± 0.170 | 0.197 | 0.221 |
Pearson’s product-moment correlation analysis indicated that the TB, ALT, and AST were significantly negatively correlated with the REh (r = -0.346, P = 0.002; r = -0.290, P = 0.011; r = -0.299, P = 0.009), but albumin and PT were not significantly correlated with the REh (P = 0.227, P = 0.263) (Table 6).
The prognosis and management of CLD greatly depend on the extent and the progression of hepatic fibrosis[16]. Early detection of hepatic fibrosis and accurate assessment of the curative effect of anti-fibrotic therapy depend on the reliable, repeatable, safe diagnosis of hepatic fibrosis[17]. In recent years, due to technical advances in MRI and the application of hepatocyte-specific MR contrast agents, hepatic disease diagnosis by MRI has been further improved. Gd-BOPTA enters hepatocytes and are subsequently excreted into bile with the help of organic anion transporting polypeptides (OATPs) on the cytomembrane[18-21]. In humans, 95% of the dose of Gd-BOPTA are excreted in urine and only approximately 3%-5% of the dose are excreted into bile[8,22]. Although Gd-BOPTA through the liver metabolism is little, its hepatic elimination is slow and it can maintain the liver enhancement for 40 to 120 min[23]. Previous research findings have indicated that the degree of enhancement of GD-EOB-DTPA decreased as the stage of hepatic fibrosis progressed[24]. Similar to Gd-EOB-DTPA, with the depletion in the number of hepatocytes or the failure of the hepatocellular function in hepatic fibrosis patients, the enhancement in the hepatocyte phase of Gd-BOPTA-enhanced MRI was insufficient. It is feasible to use the degree of REh of Gd-BOPTA-enhanced MRI to quantify the stage of hepatic fibrosis and the degree of hepatitis.
The main finding of our study was that there were significant differences in the REh between the S2 and S3 groups and between the S2 and S4 groups. There are likely multiple mechanisms responsible for this finding. First, the number of organic anion carriers of Gd-BOPTA in the hepatocytes was depleted with hepatic fibrosis progression[25]. Second, the S2 group represented mild hepatic fibrosis and the S3 and S4 groups represented severe hepatic fibrosis; therefore, the uptake of Gd-BOPTA was obviously decreased in the severe group compared with the mild group. The curative effect is satisfactory when hepatic fibrosis is in the S1 or S2 stage. In these stages, the course of fibrosis can be completely reversed by yielding a normal liver organization structure. The curative effect is unsatisfactory when hepatic fibrosis is in the stage of S3 or S4, when it is difficult to reverse the course of fibrosis[26]. Therefore, distinguishing S2 from S3 and S4 makes sense. Our results also showed that the degree of REh was decreased in more advanced fibrosis stages, in agreement with a previous study[27]. Some possible causes may contribute to this result. On one hand, hepatic fibrosis causes hepatocellular hypofunction. On the other hand, a liver which is in the process of hepatic fibrosis and regeneration has a reduced number of functional hepatocytes. As a result, the intake and excretion of GD-BOPTA in unhealthy livers are reduced and the degree of REh is decreased. The value of REh overlapped among different stages of fibrosis. The likely reasons are two-fold. First, hepatic fibrosis progression varies in different segments of the liver, and pathological sampling might therefore not represent the right liver fibrosis. Second, hepatic fibrosis progression is a continuous process that cannot be clearly segmented. Fibrosis progression was artificially separated into five stages according to the change in histomorphology; therefore, there was a deviation from the established histopathological fibrosis stages.
Hepatitis is accompanied by hepatic fibrosis. The results for hepatitis grading are therefore unsurprising: there were significant differences in the REh between the G1 and G2 groups and between the G1 and G4 groups. Therefore, the calculation of the hepatocyte-specific contrast agent uptake may differentiate no/minimal hepatitis from advanced hepatitis. However, this needs to be confirmed further. There was no significant difference between the G1 and G3 groups, probably because the number of subjects in the G3 group was limited (only 8).
Generally, it is well known that biochemical tests, such as TB, PT, AST, and ALT, are commonly used to evaluate liver function[28]. The present study revealed that the decreased REh of the hepatic parenchyma was correlated with the elevated serum levels of TB, ALT and AST. The reason for these findings may be that: (1) both Gd-BOPTA and bilirubin share a common hepatic transport supporter - OATP - for hepatic uptake, which is the key factor underlying why the degree of REh is correlated with serum levels of TB[29]. In decompensated liver, the bilirubin excretion is increasing, while Gd-BOPTA excretion is decreasing, which will lead to decreased REh of the hepatic parenchyma; and (2) hepatic enzymes such as ALT and AST reflect the degree of hepatocyte injury. Previous studies showed that the decreases in RE in the hepatocyte phase of Gd-EOB-DTPA-enhanced MRI was related to the impairment of liver function[30]. As they did with Gd-EOB-DTPA, functional hepatocytes also take in and excrete Gd-BOPTA; if hepatocellular function is damaged, hepatic enzyme levels are elevated, and the degree of REh of Gd-BOPTA is decreased.
This study had some limitations. First, the pathogenesis was different and the number of patients from any kind of pathogenesis was also different. Thus, these factors would influence the result of REh measurements. Second, the study data could not distinguish every stage of hepatic fibrosis. Hopefully, future studies could address these issues.
The study showed that with the depletion of the number of the hepatocytes or the failure of hepatocellular function in hepatic fibrosis patients, the enhancement in the hepatocyte phase of Gd-BOPTA-enhanced MRI was insufficient. In conclusion, our prospective study demonstrated that it is possible to stage hepatic fibrosis by Gd-BOPTA-enhanced hepatocyte-phase MRI, which makes the assessment of hepatitis and liver function possible.
Effective and scientific anti-fibrotic treatments can contribute to the regression of fibrosis, but no treatments for cirrhosis have been reported in previous research findings. It is therefore essential to diagnose early fibrosis in the therapeutic process of chronic liver disease (CLD) because at this stage we can delay or reverse the fibrotic process, thereby reducing the morbidity and mortality of terminal liver disease. However, to date, there are still no ideal methods to diagnose early fibrosis. With the recent development and application of new imaging techniques such as ultrasound elasticity imaging, magnetic resonance (MR) diffusion-weighted imaging, and liver magnetic resonance elastography, early diagnosis of hepatic fibrosis and cirrhosis is possible by imageology. However, these techniques are still in the early phases of research.
Several studies have shown that MR imaging with administration of hepatocyte-specific MR contrast media (Gd-EOB-DTPA and Gd-BOPTA) is more accurate than dynamic helical computed tomography (CT) and unenhanced MR imaging for the detection of focal lesions. Impaired hepatobiliary function may severely influence the hepatic uptake of Gd-EOB-DTPA. Similar to Gd-EOB-DTPA, with the depletion in the number of hepatocytes or the failure of the hepatocellular function in hepatic fibrosis patients, the enhancement in the hepatocyte phase of Gd-BOPTA-enhanced MRI was insufficient.
Previous research findings have indicated that the degree of enhancement of GD-EOB-DTPA decreased as the stage of hepatic fibrosis progressed, but fewer studies have focused on Gd-BOPTA in the assessment of CLD. This study investigated the relationship between the enhancement degree in the hepatocyte phase of Gd-BOPTA-enhanced MR and the stage of hepatic fibrosis, the grade of hepatitis and the levels of functional serum markers. The aim of this study was to evaluate the diagnostic value of Gd-BOPTA-enhanced hepatocyte-phase MRI in evaluating hepatic fibrosis and hepatitis.
The study showed that it is possible to stage hepatic fibrosis by Gd-BOPTA-enhanced hepatocyte-phase MRI, which makes the assessment of hepatitis and liver function possible.
Hepatic fibrosis is an unavoidable process when chronic liver disease (CLD) develops into cirrhosis. The hepatocyte-specific MR contrast agents gadoxetic acid disodium (Gd-EOB-DTPA; Primovist, Schering, Berlin, Germany) and gadobenate dimeglumine (Gd-BOPTA; MultiHance; Bracco Imaging, Milan, Italy), with the dual properties of an extracellular agent and a hepatobiliary contrast agent, are gradually taken up by functional hepatocytes and excreted into bile and could display liver parenchymal enhancement in the hepatocyte phase. Gd-BOPTA enters hepatocytes and are subsequently excreted into bile with the help of organic anion transporting polypeptides in the cytomembrane. Gd-BOPTA can maintain the liver enhancement for 40 to 120 min.
This is a meaningful clinical trial study in which the authors used the degree enhancement in the hepatocyte phase of Gd-BOPTA-enhanced magnetic resonance imaging to evaluate the liver condition of patients with chronic liver disease. This method is recommended because of advantages such as no injury to the patient and possibility to assess the stage of hepatic fibrosis, the degree of hepatitis and the liver function.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Razek AKA, Strom SC S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 574] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 2. | Poynard T, McHutchison J, Manns M, Trepo C, Lindsay K, Goodman Z, Ling MH, Albrecht J. Impact of pegylated interferon alfa-2b and ribavirin on liver fibrosis in patients with chronic hepatitis C. Gastroenterology. 2002;122:1303-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 828] [Cited by in RCA: 803] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 3. | Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 539] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 4. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 5. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 1936] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 6. | Girometti R, Furlan A, Bazzocchi M, Soldano F, Isola M, Toniutto P, Bitetto D, Zuiani C. Diffusion-weighted MRI in evaluating liver fibrosis: a feasibility study in cirrhotic patients. Radiol Med. 2007;112:394-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 500] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 8. | Kirchin MA, Pirovano GP, Spinazzi A. Gadobenate dimeglumine (Gd-BOPTA). An overview. Invest Radiol. 1998;33:798-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 169] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Cavagna FM, Maggioni F, Castelli PM, Daprà M, Imperatori LG, Lorusso V, Jenkins BG. Gadolinium chelates with weak binding to serum proteins. A new class of high-efficiency, general purpose contrast agents for magnetic resonance imaging. Invest Radiol. 1997;32:780-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 213] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Brismar TB, Dahlstrom N, Edsborg N, Persson A, Smedby O, Albiin N. Liver vessel enhancement by Gd-BOPTA and Gd-EOB-DTPA: a comparison in healthy volunteers. Acta Radiol. 2009;50:709-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Park G, Kim YK, Kim CS, Yu HC, Hwang SB. Diagnostic efficacy of gadoxetic acid-enhanced MRI in the detection of hepatocellular carcinomas: comparison with gadopentetate dimeglumine. Br J Radiol. 2010;83:1010-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Motosugi U, Ichikawa T, Sou H, Sano K, Tominaga L, Kitamura T, Araki T. Liver parenchymal enhancement of hepatocyte-phase images in Gd-EOB-DTPA-enhanced MR imaging: which biological markers of the liver function affect the enhancement? J Magn Reson Imaging. 2009;30:1042-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 199] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 13. | Higaki A, Tamada T, Sone T, Kanki A, Sato T, Tanimoto D, Higashi H, Ito K. Potential clinical factors affecting hepatobiliary enhancement at Gd-EOB-DTPA-enhanced MR imaging. Magn Reson Imaging. 2012;30:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Dahlqvist Leinhard O, Dahlström N, Kihlberg J, Sandström P, Brismar TB, Smedby O, Lundberg P. Quantifying differences in hepatic uptake of the liver specific contrast agents Gd-EOB-DTPA and Gd-BOPTA: a pilot study. Eur Radiol. 2012;22:642-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Batts KP, Ludwig J. Chronic hepatitis. An update on terminology and reporting. Am J Surg Pathol. 1995;19:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 847] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 16. | Norén B, Forsgren MF, Dahlqvist Leinhard O, Dahlström N, Kihlberg J, Romu T, Kechagias S, Almer S, Smedby Ö, Lundberg P. Separation of advanced from mild hepatic fibrosis by quantification of the hepatobiliary uptake of Gd-EOB-DTPA. Eur Radiol. 2013;23:174-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1315] [Article Influence: 119.5] [Reference Citation Analysis (0)] |
| 18. | Pastor CM, Planchamp C, Pochon S, Lorusso V, Montet X, Mayer J, Terrier F, Vallee JP. Kinetics of gadobenate dimeglumine in isolated perfused rat liver: MR imaging evaluation. Radiology. 2003;229:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Pascolo L, Cupelli F, Anelli PL, Lorusso V, Visigalli M, Uggeri F, Tiribelli C. Molecular mechanisms for the hepatic uptake of magnetic resonance imaging contrast agents. Biochem Biophys Res Commun. 1999;257:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Pascolo L, Petrovic S, Cupelli F, Bruschi CV, Anelli PL, Lorusso V, Visigalli M, Uggeri F, Tiribelli C. Abc protein transport of MRI contrast agents in canalicular rat liver plasma vesicles and yeast vacuoles. Biochem Biophys Res Commun. 2001;282:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | de Haën C, Lorusso V, Tirone P. Hepatic transport of gadobenate dimeglumine in TR-rats. Acad Radiol. 1996;3 Suppl 2:S452-S454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 22. | Bruce R, Wentland AL, Haemel AK, Garrett RW, Sadowski DR, Djamali A, Sadowski EA. Incidence of Nephrogenic Systemic Fibrosis Using Gadobenate Dimeglumine in 1423 Patients With Renal Insufficiency Compared With Gadodiamide. Invest Radiol. 2016;51:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Gatto A, De Gaetano AM, Giuga M, Ciresa M, Siciliani L, Miele L, Riccardi L, Pizzolante F, Rapaccini GL, Gasbarrini A. Differentiating hepatocellular carcinoma from dysplastic nodules at gadobenate dimeglumine-enhanced hepatobiliary-phase magnetic resonance imaging. Abdom Imaging. 2013;38:736-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Tamada T, Ito K, Higaki A, Yoshida K, Kanki A, Sato T, Higashi H, Sone T. Gd-EOB-DTPA-enhanced MR imaging: evaluation of hepatic enhancement effects in normal and cirrhotic livers. Eur J Radiol. 2011;80:e311-e316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 25. | Geier A, Dietrich CG, Voigt S, Kim SK, Gerloff T, Kullak-Ublick GA, Lorenzen J, Matern S, Gartung C. Effects of proinflammatory cytokines on rat organic anion transporters during toxic liver injury and cholestasis. Hepatology. 2003;38:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 26. | Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, Bedossa P. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 27. | Ma C, Liu A, Wang Y, Geng X, Hao L, Song Q, Sun B, Wang H, Zhao G. The hepatocyte phase of Gd-EOB-DTPA-enhanced MRI in the evaluation of hepatic fibrosis and early liver cirrhosis in a rat model: an experimental study. Life Sci. 2014;108:104-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Sakka SG. Assessing liver function. Curr Opin Crit Care. 2007;13:207-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Zhao X, Huang M, Zhu Q, Wang T, Liu Q. The relationship between liver function and liver parenchymal contrast enhancement on Gd-BOPTA-enhanced MR imaging in the hepatocyte phase. Magn Reson Imaging. 2015;33:768-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Chen BB, Hsu CY, Yu CW, Wei SY, Kao JH, Lee HS, Shih TT. Dynamic contrast-enhanced magnetic resonance imaging with Gd-EOB-DTPA for the evaluation of liver fibrosis in chronic hepatitis patients. Eur Radiol. 2012;22:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |