Published online May 7, 2017. doi: 10.3748/wjg.v23.i17.3122
Peer-review started: October 14, 2016
First decision: December 28, 2016
Revised: January 16, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: May 7, 2017
Processing time: 205 Days and 20 Hours
To investigate whether the preoperative neutrophil-to-lymphocyte ratio (NLR) could predict the prognosis of hepatocellular carcinoma (HCC) patients with portal/hepatic vein tumor thrombosis (PVTT/HVTT) after hepatectomy.
The study population included 81 HCC patients who underwent hepatectomy and were diagnosed with PVTT/HVTT based on pathological examination. The demographics, laboratory analyses, and histopathology data were analyzed.
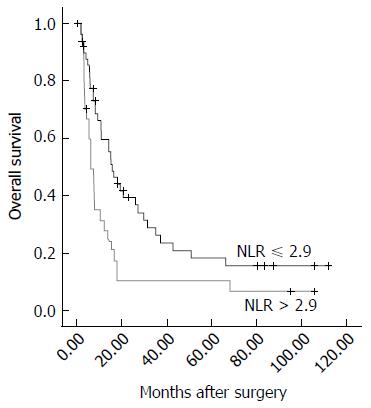
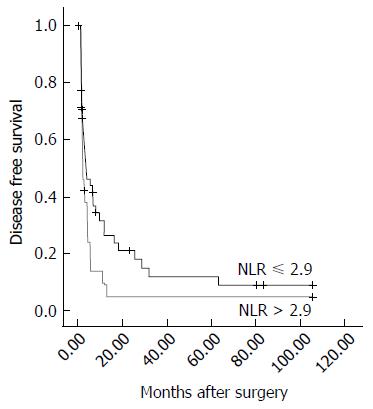
Overall survival (OS) and disease-free survival (DFS) were determined in the patients with a high (> 2.9) and low (≤ 2.9) NLR. The median OS and DFS duration in the high NLR group were significantly shorter than those in the low NLR group (OS: 6.2 mo vs 15.7 mo, respectively, P = 0.007; DFS: 2.2 mo vs 3.7 mo, respectively, P = 0.039). An NLR > 2.9 was identified as an independent predictor of a poor prognosis of OS (P = 0.034, HR = 1.866; 95%CI: 1.048-3.322) in uni- and multivariate analyses. Moreover, there was a significantly positive correlation between the NLR and the Child-Pugh score (r = 0.276, P = 0.015) and the maximum diameter of the tumor (r = 0.435, P < 0.001). Additionally, the NLR could enhance the prognostic predictive power of the CLIP score for DFS in these patients.
The preoperative NLR is a prognostic predictor after hepatectomy for HCC patients with PVTT/HVTT. NLR > 2.9 indicates poorer OS and DFS.
Core tip: The systemic inflammatory response generated by tumors has been shown to cause the upregulation of cytokines and inflammatory mediators, leading to the promotion of angiogenesis and DNA damage and the inhibition of apoptosis. The presence of a systemic inflammatory response can be detected by the elevation of the neutrophil-to-lymphocyte ratio (NLR), which has been shown to be associated with poorer prognosis in patients with various types of malignant tumors. Our findings confirm that the NLR can be used as a potential prognostic predictor for hepatocellular carcinoma patients with portal/hepatic vein tumor thrombosis after resection. The results of the present study may help identify a new serum marker for predicting the post-operation survival of these patients.
- Citation: Li SH, Wang QX, Yang ZY, Jiang W, Li C, Sun P, Wei W, Shi M, Guo RP. Prognostic value of the neutrophil-to-lymphocyte ratio for hepatocellular carcinoma patients with portal/hepatic vein tumor thrombosis. World J Gastroenterol 2017; 23(17): 3122-3132
- URL: https://www.wjgnet.com/1007-9327/full/v23/i17/3122.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i17.3122
Hepatocellular carcinoma (HCC) is one of the most common malignant tumors worldwide[1]. Portal/hepatic vein tumor thrombosis (PVTT/HVTT) is a common complication of HCC[2] and is widely accepted as a sign of advanced stage[3]. PVTT/HVTT frequently leads to intrahepatic or distant metastasis with a poor prognosis[4]. The median survival of untreated HCC with PVTT/HVTT has been reported to be 2.7 mo, whereas the survival in those without PVTT/HVTT has been reported to be 24.4 mo[5,6]. A large body of evidence has shown that surgery can improve the survival of HCC patients with PVTT/HVTT[7-9]. However, the median survival duration varies from 9.0 to 26.0 mo[7-9], which is still unsatisfactory. The reasons for this remain unclear and seem to be complex and multifactorial.
The systemic inflammatory response generated by tumors has been shown to cause the upregulation of cytokines and inflammatory mediators, leading to the promotion of angiogenesis, DNA damage, and inhibition of apoptosis[10-12]. The presence of a systemic inflammatory response can be detected by the elevation of the neutrophil-to-lymphocyte ratio (NLR), which has been shown to be associated with poorer prognosis in patients with various types of malignant tumors, including colorectal cancer, intrahepatic cholangiocellular carcinoma, pancreatic ductal adenocarcinoma, gastric cancer, non-small cell lung cancer, renal cell carcinoma, breast cancer, and soft tissue sarcoma[13-18]. Recently, an increasing number of reports has shown that the NLR can be used as a predictor of poor survival after curative hepatectomy, radio-frequency ablation (RFA), transarterial chemoembolization (TACE), liver transplantation (LT), and sorafenib therapy for HCC[19-29]. To date, however, few studies have mentioned the role of the NLR in predicting the prognosis of HCC patients with PVTT/HVTT after hepatectomy.
The current study aimed to evaluate the relationship between systemic inflammation, as represented by the preoperative NLR, and long-term outcomes in HCC patients with PVTT/HVTT after hepatectomy, determining whether the NLR can be used as a predictor of survival in these patients.
The present study population included 81 HCC patients who underwent hepatectomy at the Department of Hepatobiliary Surgery, Cancer Center of Sun Yat-Sen University, Guangzhou, China and were diagnosed with PVTT/HVTT via pathological examination between January 2004 and July 2009. During this period, there were 931 hepatocellular carcinoma patients who underwent hepatic resection at our department.
The patients were excluded from the analysis if they had extrahepatic disease, thrombus extending to the level of the superior mesenteric vein, or any antitumor treatments before operation.
The preoperative diagnosis and tumor evaluation were made using ultrasonography, contrast-enhanced magnetic resonance (MR), and/or tri-phase contrast-enhanced helical computed tomography (CT). Liver function was evaluated based on the Child-Pugh classification system[30] and/or the indocyanine green (ICG) clearance test performed routinely before operation. The neutrophil and lymphocyte counts were routinely measured within three days before operation. NLR was calculated by dividing the neutrophil measurement by the lymphocyte measurement.
The selection criteria for the operative procedure depended on the tumor location and extent, liver function, and future liver remnant volume. Hepatectomy was defined as major if three or more Couinaud segments were resected and minor if fewer than three segments were resected[31]. The diagnosis of HCC and PVTT/HVTT was confirmed by histopathological examination of the resected specimens.
Operative mortality was defined as death within 30 d after operation. Operative complication was defined as any deviation from the normal course of recovery with the need for any medical interventions.
All patients were followed up as a routine protocol one month after operation by enhanced CT of the chest and upper abdomen, serum α-fetoprotein (AFP) examination, and serum examination of liver function. Then, follow-up was carried out every 2-3 mo with enhanced CT of the chest and upper abdomen or combined CDUS and chest X-ray; and serum examination for the first year. Thereafter, all patients were followed up every 3-6 mo with CDUS, chest X-ray, and serum tests. Abdominal enhanced CT, abdominal enhanced MR, and/or contrast-enhanced ultrasonography (CEUS) were performed when intrahepatic recurrence was suspected, and thoracic enhanced CT, whole-body bone scintigraphy, or/and other relevant radiological examination was performed when extrahepatic recurrence was suspected.
Patients with recurrence were treated with the following therapies based on their liver function and pattern of recurrence as a routine practice: hepatectomy, TACE, transarterial infusion (TAI), percutaneous microwave tumor coagulation therapy, radiofrequency ablation (RFA), systemic chemotherapy, percutaneous ethanol injection therapy (PEI), sealed source radiotherapy, sorafenib therapy, cytokine-induced killer (CIK) cell therapy, and/or supportive care.
Comparisons between categorical variables were performed using Pearson’s χ2 test or Fisher’s exact test where appropriate. Continuous variables were compared using Student’s t test (when values were normally distributed) or the Mann-Whitney test (when the values had a distribution that departed significantly from normal). Survival analysis was performed using the Kaplan-Meier method and comparison were made using the log-rank test. Univariate and multivariate analyses using Cox’s proportional hazard models were performed to evaluate the prognostic factors. The correlation between two variables was examined by Pearson’s correlation analysis (when the variables were normally distributed) or Spearman’s correlation analysis (when the variables had a distribution that departed significantly from normal). A value of P < 0.05 was considered statistically significant. All data were analyzed using SPSS statistical software for Windows (ver. 18.0; SPSS Inc., Chicago, IL, United States).
All continuous variable data were expressed as mean ± standard error (when the values were normally distributed) or medians (range) (when the values had a distribution that departed significantly from normal). All data regarding categorical variables are shown as n (proportion).
To determine whether an elevated NLR was correlated with the postoperative survival of HCC patients with PVTT/HVTT, we performed survival analysis, and the results are shown in Table 1. Using NLR cut-offs from 1 to 5 and comparing the 1-, 2-, 3-, and 5-year overall survival (OS) rates, several NLRs were found statistically correlated with the postoperative OS of HCC patients with PVTT/HVTT. Among these, an NLR of 2.9 was the most significant, with a χ2 value of 7.227 and a P value of 0.007. We therefore utilized an NLR cut-off of 2.9 as a risk factor of the poorer prognosis of these patients.
| Cut-off value | Group | Cases | 1-yr OS | 2-yr OS | 3-yr OS | 5-yr OS | χ2 | P value |
| 1.0 | NLR ≤ 1 vs > 1 | 5 vs 76 | 80.0% vs 46.9% | 40.0% vs 27.7% | 40.0% vs 19.0% | 0.0% vs 17.3% | 0.272 | 0.602 |
| 1.5 | NLR ≤ 1.5 vs > 1.5 | 16 vs 65 | 80.0% vs 41.5% | 46.7% vs 24.2% | 26.7% vs 19.8% | 6.7% vs 19.8% | 1.575 | 0.210 |
| 2.0 | NLR ≤ 2 vs > 2 | 28 vs 53 | 72.3% vs 37.3% | 44.2% vs 20.7% | 28.1% vs 17.8% | 16.1% vs 17.8% | 3.657 | 0.056 |
| 2.5 | NLR ≤ 2.5 vs > 2.5 | 39 vs 42 | 64.5% vs 35.1% | 39.2% vs 18.9% | 25.2% vs 18.9% | 16.8% vs 18.9% | 3.935 | 0.047 |
| 2.6 | NLR ≤ 2.6 vs > 2.6 | 42 vs 39 | 62.1% vs 35.4% | 38.5% vs 17.7% | 24.7% vs 17.7% | 16.5% vs 17.7% | 3.987 | 0.046 |
| 2.7 | NLR ≤ 2.7 vs > 2.7 | 44 vs 37 | 59.1% vs 37.3% | 36.6% vs 18.7% | 23.6% vs 18.7% | 15.7% vs 18.7% | 2.254 | 0.133 |
| 2.8 | NLR ≤ 2.8 vs > 2.8 | 49 vs 32 | 60.2% vs 32.7% | 39.1% vs 13.1% | 26.1% vs 13.1% | 18.3% vs 13.1% | 6.007 | 0.014 |
| 2.9 | NLR ≤ 2.9 vs > 2.9 | 51 vs 30 | 59.8% vs 31.5% | 39.5% vs 10.5% | 26.3% vs 10.5% | 18.4% vs 10.5% | 7.227 | 0.007 |
| 3.0 | NLR ≤ 3 vs > 3 | 53 vs 28 | 59.8% vs 32.1% | 38.8% vs 10.7% | 25.9% vs 10.7% | 18.1% vs 10.7% | 6.158 | 0.013 |
| 3.5 | NLR ≤ 3.5 vs > 3.5 | 62 vs 19 | 56.7% vs 26.3% | 34.4% vs 10.5% | 23.7% vs 10.5% | 17.2% vs 10.5% | 2.843 | 0.092 |
| 4.0 | NLR ≤ 4 vs > 4 | 66 vs 15 | 56.2% vs 20.0% | 33.8% vs 6.7% | 23.9% vs 6.7% | 17.9% vs 6.7% | 5.284 | 0.022 |
| 4.5 | NLR ≤ 4.5 vs > 4.5 | 72 vs 9 | 51.1% vs 33.3% | 30.7% vs 11.1% | 21.7% vs 11.1% | 16.3% vs 11.1% | 1.724 | 0.189 |
| 5.0 | NLR ≤ 5 vs > 5 | 75 vs 6 | 50.3% vs 33.3% | 29.4% vs 16.7% | 20.7% vs 16.7% | 15.6% vs 16.7% | 0.527 | 0.468 |
The characteristics of the 81 HCC patients with PVTT/HVTT are summarized in Table 2. Of the 81 patients, 51 had an NLR ≤ 2.9 and 30 had an NLR > 2.9. Most of the characteristics of the two groups were similar. Patients in the high-NLR group had significantly higher preoperative HBV DNA (P = 0.025), serum AFP level (P = 0.038), and maximum diameter of tumor (P = 0.003), worse Child-Pugh score (P = 0.017), longer operative time (P = 0.011), and shorter surgical margin (P = 0.002).
| NLR ≤ 2.9 (n = 51) | NLR > 2.9 (n = 30) | P value | |
| Age in years | 48.49 ± 1.52 | 48.47 ± 2.28 | 0.993 |
| Gender | 0.292 | ||
| Male | 48 (94.1) | 30 (100.0) | |
| Female | 3 (5.9) | 0 (0.0) | |
| HBsAg status | 0.281 | ||
| Negative | 3 (5.9) | 0 (0.0) | |
| Positive | 45 (88.2) | 30 (100.0) | |
| Unknown | 3 (5.9) | 0 (0.0) | |
| Preoperative HBV DNA | 0.025 | ||
| < 1 × 103 | 19 (37.3) | 4 (13.3) | |
| ≥ 1 × 103 | 23 (45.1) | 19 (63.3) | |
| Unknown | 9 (17.6) | 7 (23.3) | |
| Preoperative AFP level | 0.038 | ||
| < 400 ng/mL | 19 (37.3) | 5 (16.7) | |
| ≥ 400 ng/mL | 30 (58.8) | 25 (83.3) | |
| Unknown | 2 (3.9) | 0 (0.0) | |
| Preoperative ALT level (U/L) | 41 (10-713) | 42.5 (21-146.5) | 0.697 |
| Preoperative Hgb level (g/L) | 147.79 ± 2.43 | 148.52 ± 4.37 | 0.884 |
| Preoperative PLT level (109/L) | 185.84 ± 12.73 | 201.97 ± 13.97 | 0.417 |
| Preoperative Child-Pugh score | 0.017 | ||
| Child A (5) | 21 (41.2) | 7 (23.3) | |
| Child A (6) | 22 (43.1) | 14 (46.7) | |
| Child B (7) | 4 (7.8) | 5 (16.7) | |
| Child B (8) | 1 (2.0) | 2 (6.7) | |
| Child B (9) | 0 (0.0) | 1 (3.3) | |
| Unknown | 3 (5.9) | 1 (3.3) | |
| Preoperative ICGR15 (%) | 6.42 ± 0.79 | 5.37 ± 0.94 | 0.404 |
| Number of tumors | 0.474 | ||
| Solitary | 28 (54.9) | 14 (46.7) | |
| Multiple | 23 (45.1) | 16 (53.3) | |
| Maximum diameter of tumor (cm) | 8.93 ± 0.58 | 11.88 ± 0.80 | 0.003 |
| Uni/bilobular disease | 0.414 | ||
| Unilobular disease | 48 (94.1) | 26 (86.7) | |
| Bilobular disease | 3 (5.9) | 4 (13.3) | |
| Adjacent organ invasion | 0.722 | ||
| Negative | 32 (62.7) | 20 (66.7) | |
| Positive | 19 (37.3) | 10 (33.3) | |
| Operative procedure | 0.215 | ||
| Minor | 45 (88.2) | 23 (76.7) | |
| Major | 6 (11.8) | 7 (23.3) | |
| Total occlusion time of the hepatic inflow (min) | 17.89 ± 1.58 | 18.18 ± 2.53 | 0.918 |
| Total operative time (min) | 168.43 ± 7.19 | 205.00 ± 13.77 | 0.011 |
| Blood loss (mL) | 579.41 ± 61.82 | 891.67 ± 171.61 | 0.095 |
| Blood transfusion (mL) | 0.061 | ||
| No | 33 (64.7) | 13 (43.3) | |
| Yes | 18 (35.3) | 17 (56.7) | |
| Surgical margin | 0.002 | ||
| ≤ 1 cm | 33 (64.7) | 25 (83.3) | |
| > 1 cm | 18 (35.3) | 1 (3.3) | |
| Unknown | 0 (0.0) | 4 (13.3) | |
| Histological grade of tumor cells | 0.958 | ||
| I-II | 19 (37.3) | 11 (36.7) | |
| III-IV | 32 (62.7) | 19 (63.3) | |
| Postoperative complication | 0.792 | ||
| Negative | 42 (82.4) | 24 (80.0) | |
| Positive | 9 (17.6) | 6 (20.0) | |
| Postoperative hospital stay (d) | 11 (8-28) | 11.5 (8-83) | 0.468 |
The patterns of recurrence and postoperative treatments in the patients of the two groups are shown in Table 3. The recurrence patterns were not significantly different between the two groups.
| NLR ≤ 2.9 | NLR > 2.9 | P value | |
| Recurrence | n = 51 | n = 30 | 1.000 |
| Negative | 3 (5.9) | 1 (3.3) | |
| Positive | 39 (76.5) | 23 (76.7) | |
| Unknown | 9 (17.6) | 6 (20) | |
| Recurrence pattern | n = 39 | n = 23 | 0.832 |
| Intrahepatic only | 26 (66.7) | 15 (65.2) | |
| Extrahepatic only | 2 (5.1) | 2 (8.7) | |
| Both | 11 (28.2) | 6 (26.1) | |
| Treatments | n = 51 | n = 30 | |
| TACE | 23 | 15 | |
| Hepatectomy | 2 | 0 | |
| PMCT | 4 | 0 | |
| Systemic chemotherapy | 3 | 2 | |
| RFA | 5 | 0 | |
| PEI | 2 | 0 | |
| CIK | 0 | 2 | |
| TAI | 1 | 1 | |
| Sorafenib | 0 | 1 | |
| Radiotherapy | 1 | 0 | |
| Sealed source radiotherapy | 1 | 2 | |
| Traditional Chinese medicine | 4 | 0 | |
| Supportive care only | 21 | 12 |
As shown in Figures 1 and 2, when we compared the survival outcomes in the two groups, we found that the 1-, 2-, 3-, and 5-year OS rates were significantly lower in the high (31.5%, 10.5%, 10.5%, and 10.5%, respectively) than in the low (59.8%, 39.5%, 26.3%, and 18.4%, respectively) NLR group (P = 0.007). Similarly, we found that the 1-, 2-, 3-, and 5-year disease-free survival (DFS) rates were significantly lower in the high (9.4%, 4.7%, 4.7%, and 4.7%, respectively) than in the low (29.1%, 21.1%, 12.1%, and 9.1%, respectively) NLR group (P = 0.039).
Univariate and multivariate analyses of the factors affecting OS are shown in Table 4. An NLR > 2.9, AFP ≥ 400 ng/mL, multiple tumors, bilobular disease, and surgical margin ≤ 1 cm found to be significant on univariate analysis were then included in multivariate regression analysis, and the results revealed that an NLR > 2.9 [P = 0.034, a hazard ratio (HR): 1.866; 95%CI: 1.048-3.322], AFP ≥ 400 ng/mL (P = 0.042, HR = 1.863; 95%CI: 1.024-3.392), and bilobular disease (P = 0.019, HR = 3.292; 95%CI: 1.215-8.918) were independent predictors of the poorer prognosis of OS.
| Variables | Univariate analysis for OS | Multivariate analysis for OS | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| NLR > 2.9 | 1.969 | 1.190-3.259 | 0.008 | 1.866 | 1.048-3.322 | 0.034 |
| Age ≤ 50 yr | 1.532 | 0.913-2.569 | 0.106 | |||
| Female | 2.035 | 0.625-6.623 | 0.238 | |||
| HBsAg (+) | 1.055 | 0.327-3.402 | 0.929 | |||
| HBV-DNA > 1 × 103 | 1.258 | 0.709-2.234 | 0.432 | |||
| AFP ≥ 400 ng/mL | 2.026 | 1.163-3.527 | 0.013 | 1.863 | 1.024-3.392 | 0.042 |
| ALT ≤ 40 U/L | 1.122 | 0.684-1.839 | 0.649 | |||
| Hgb > 130 g/L | 1.964 | 0.987-3.909 | 0.054 | |||
| PLT > 100 × 109/L | 1.168 | 0.467-2.916 | 0.740 | |||
| Child A | 1.090 | 0.564-2.106 | 0.798 | |||
| ICGR15 ≤ 10% | 1.642 | 0.746-3.610 | 0.218 | |||
| Multiple tumors | 1.676 | 1.022-2.748 | 0.041 | 1.084 | 0.627-1.876 | 0.772 |
| Maximum diameter of tumor > 5 cm | 1.869 | 0.973-3.593 | 0.061 | |||
| Bilobular disease | 2.764 | 1.153-6.628 | 0.023 | 3.292 | 1.215-8.918 | 0.019 |
| Adjacent organ invaded | 1.268 | 0.756-2.127 | 0.369 | |||
| Major hepatectomy | 1.145 | 0.597-2.195 | 0.683 | |||
| Pringle maneuver | 1.937 | 0.950-3.952 | 0.069 | |||
| Operation time > 180 min | 1.352 | 0.819-2.233 | 0.238 | |||
| Intraoperative blood loss > 1000 mL | 1.721 | 0.957-3.096 | 0.070 | |||
| Intraoperative blood transfusion | 1.485 | 0.901-2.448 | 0.121 | |||
| Surgical margin ≤ 1 cm | 1.868 | 1.024-3.408 | 0.042 | 1.195 | 0.596-2.394 | 0.616 |
| Histologic grade III-IV | 1.220 | 0.736-2.020 | 0.440 | |||
| Postoperative complication | 1.467 | 0.807-2.667 | 0.209 | |||
| Postoperative hospital stay ≤ 10 d | 1.078 | 0.646-1.798 | 0.774 | |||
Similarly, univariate and multivariate analyses of the factors affecting DFS are shown in Table 5. NLR > 2.9, AFP ≥ 400 ng/mL, Hgb > 130 g/L, ICGR15 ≤ 10%, and intraoperative blood loss > 1000 mL were found to be significant on univariate analysis and were then included in multivariate regression analysis. However, none was identified as an independent predictor of the poorer prognosis of DFS.
| Variables | Univariate analysis for DFS | Multivariate analysis for DFS | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| NLR > 2.9 | 1.720 | 1.017-2.907 | 0.043 | 1.553 | 0.850-2.837 | 0.153 |
| Age ≤ 50 yr | 1.424 | 0.842-2.409 | 0.188 | |||
| Female | 1.593 | 0.384-6.610 | 0.522 | |||
| HBsAg (+) | 1.314 | 0.407-4.249 | 0.648 | |||
| HBV-DNA > 1 × 103 | 1.426 | 0.772-2.634 | 0.257 | |||
| AFP ≥ 400 ng/mL | 2.099 | 1.196-3.684 | 0.010 | 1.732 | 0.954-3.146 | 0.071 |
| ALT ≤ 40 U/L | 1.272 | 0.771-2.098 | 0.346 | |||
| Hgb > 130 g/L | 2.629 | 1.266-5.460 | 0.010 | 2.051 | 0.940-4.476 | 0.071 |
| PLT ≤ 100 × 109/L | 1.147 | 0.413-3.186 | 0.793 | |||
| Child B | 1.254 | 0.631-2.492 | 0.518 | |||
| ICGR15 ≤ 10% | 2.470 | 1.052-5.796 | 0.038 | 2.134 | 0.870-5.236 | 0.098 |
| Multiple tumors | 1.189 | 0.709-1.992 | 0.512 | |||
| Maximum diameter of tumor > 5 cm | 1.865 | 0.944-3.686 | 0.073 | |||
| Bilobular disease | 1.778 | 0.753-4.199 | 0.189 | |||
| Adjacent organ not invaded | 1.119 | 0.659-1.899 | 0.677 | |||
| Major hepatectomy | 1.253 | 0.635-2.474 | 0.516 | |||
| Pringle maneuver | 1.922 | 0.933-3.960 | 0.076 | |||
| Operation time ≤ 180 min | 1.067 | 0.638-1.785 | 0.805 | |||
| Intraoperative blood loss > 1000 mL | 1.854 | 1.012-3.396 | 0.046 | 1.258 | 0.649-2.437 | 0.497 |
| Intraoperative blood transfusion | 1.655 | 0.972-2.817 | 0.064 | |||
| Surgical margin ≤ 1 cm | 1.492 | 0.827-2.690 | 0.183 | |||
| Histologic grade I-II | 1.083 | 0.648-1.808 | 0.761 | |||
| Postoperative complication | 1.173 | 0.608-2.264 | 0.634 | |||
| Postoperative hospital stay > 10 d | 1.041 | 0.616-1.759 | 0.882 | |||
The relationship between the NLR and Child-Pugh score, preoperative AFP level, preoperative HBV DNA level, and maximum tumor diameter, which may impact the prognosis of HCC patients, was evaluated. There was a significantly positive correlation between the NLR and Child-Pugh score (r = 0.276, P = 0.015). Additionally, the NLR had a significantly positive correlation with the maximum tumor diameter (r = 0.435, P < 0.001). NLR was not associated with the preoperative AFP level and HBV DNA level (data not shown).
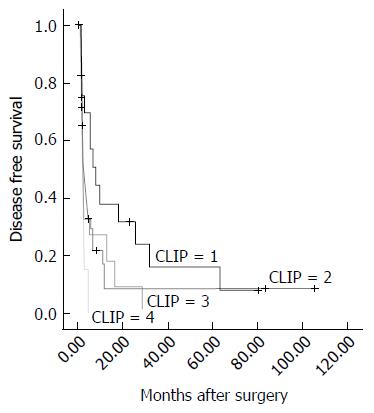
The Cancer of the Liver Italian Program (CLIP) score was calculated as described previously[32] in 76 of the 81 cases (the score could not be calculated in 5 cases due to missing data). The CLIP stages assigned in the present study population ranged from 1 to 4 because all of the cases enrolled in the current study population had PVTT/HVTT. Survival analysis revealed significant differences in DFS (χ2 = 7.870, P = 0.049) for each group, as shown in Figure 3. However, the result was opposite for OS (P = 0.055, data not shown).
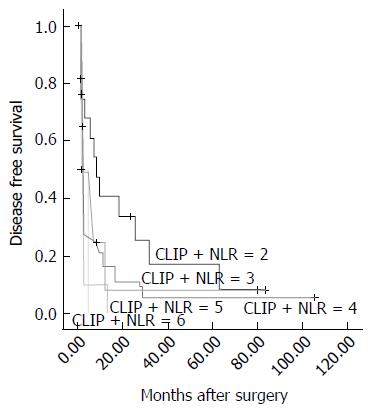
We added the NLR to the CLIP score in accordance with the following scheme: a low NLR (NLR ≤ 2.9) was given a score of 1, and a high NLR (NLR > 2.9) was given a score of 2. Next, all of the cases were divided into 5 groups (from 2 to 6). Survival analysis revealed significant differences in DFS (χ2 = 11.371, P = 0.023) for the 5 groups, as shown in Figure 4. A larger χ2 value and a smaller P value suggested the NLR could enhance the prognostic predictive power of the CLIP score for DFS in these patients. Similarly, based on the results using CLIP score alone, there was no significant difference in OS (P = 0.055, data not shown).
NLR, a simple, cheap, safe and effective marker of inflammation, is easily calculated from routinely available data. Many studies have shown that a higher NLR is correlated with adverse survival outcomes in patients with various tumors[13-15]. NLR was first linked to hepatic malignant tumors by Halazun et al[22,33]. They observed poor DFS and OS in patients with colorectal liver metastasis and a higher preoperative NLR, with the NLR being an independent predictor of both recurrence and death[33]. Recently, increasing reports have shown that NLR can be used as a predictor of poor survival after various types of treatments for HCC[19-29,34,35]. To expand these findings, we assessed whether NLR can evaluate the prognosis of HCC patients with PVTT/HVTT after hepatectomy. Moreover, we investigated the best cut-off value for the NLR in prediction of prognosis for these patients. We also found that the NLR could enhance the predictive power of the CLIP score for DFS in these patients. To the best of our knowledge, this is the first study to describe the important role of the NLR in prediction of prognosis for HCC with PVTT/HVTT after hepatectomy.
We found that the OS of the patients whose preoperative NLR > 2.9 was shorter than that of those with NLR ≤ 2.9. An NLR > 2.9 was also identified as an independent predictor on multivariate analysis. These results are consistent with those of previously published articles in HCC patients who had undergone other treatments with various cut-offs[20-23,25,27-29,35,36]. Moreover, preoperative AFP ≥ 400 ng/mL and bilobular disease were identified as independent predictors of OS after operation in patients with HCC and PVTT/HVTT on multivariate analysis. The former is consistent with the results of previously published studies in a similar subpopulation of HCC patients who had undergone resection[9,37], while the latter was also recognized to be an independent prognostic factor for unresectable patients[38,39].
In the present study, poor prognostic indicators influencing DFS included an NLR > 2.9, AFP ≥ 400 ng/mL, Hgb > 130 g/L, ICGR15 ≤ 10%, and intraoperative blood loss > 1000 mL. These patients had poorer DFS on univariate analysis, although none of these factors were identified as independent predictors on multivariate analysis. In fact, no factors were identified as independent predictors on multivariate analysis. Moreover, the rate and pattern of recurrence in the NLR > 2.9 group showed no significant differences compared with those in the NLR ≤ 2.9 group. These results may suggest that tumor recurrence in the remnant liver after surgery was common and nearly inevitable in HCC patients with PVTT/HVTT, which was a major cause of unsatisfactory prognosis[9,40,41]. Therefore, adjuvant treatment such as TACE and TAI could significantly improve the prognosis of HCC patients with PVTT/HVTT[40,41]. However, this result did not mean that the NLR was not associated with recurrence. Our results showed a significant association between the elevated NLR and tumor size. Previous studies have shown that tumor size > 3 cm on imaging is an independent predictor of microvascular invasion[42]. Additionally, several studies have indicated that preoperative elevated NLR can reflect tumor burden, malignancy, invasion, and metastasis[19,22-24,27,28,36].
The CLIP score consists of 4 variables, the Child-Pugh score, tumor morphology, serum AFP level, and portal vein invasion, which account for both liver function and tumor characteristics relevant to the prognostic assessment for patients with HCC[32]. It was confirmed that the CLIP score could reveal a class of HCC patients with an impressively more favorable prognosis and another class with a relatively shorter life expectancy in various population cohorts[32,43]. To the best of our knowledge, the present study is the first study confirming the prognostic predictive power of the CLIP score for HCC with PVTT/HVTT that could be enhanced by combining the NLR.
Inflammatory markers have long been linked with malignancy. Virchow first observed leukocytes appearing in neoplastic tissue in the mid 1800s[11]. Recently, consistent lines of evidence have suggested that there is a close relationship between the development of cancer and inflammation. As an inflammatory marker, NLR reflects an immune microenvironment that both favors tumor vascular invasion and suppresses the host immune surveillance[19].
A high NLR means relatively fewer lymphocytes and more neutrophil leucocytes, reflecting the impairment of the host immune response to tumors and a large reservoir of vascular endothelial growth factor (VEGF)[13-15,22,24,34]. Circulating VEGF, whose primary sources are recognized as neutrophil leukocytes, has been established as a major contributor to tumor-related angiogenesis[44]. Elevated VEGF expression correlates with increased vascular density, higher rates of vascular invasion, and an increased tendency for seeding. Therefore, increased neutrophil leukocytes are related to an increased risk of recurrence in HCC patients[22,44]. Many studies have demonstrated that once the T-lymphocyte-mediated antitumor response is impaired and the cytotoxic CD81 lymphocyte subpopulation is dysfunctional, the lymphocytes may diminish, possibly leading to impaired defense against the tumor[22,45]. Okano et al[45] found that the extent of lymphocytic infiltration between the metastatic nodule and normal hepatic tissue may reflect host defensive activity in the liver and is associated with the outcome in patients who underwent hepatectomy for liver metastases from colorectal cancer. Patients with dense tumor-infiltrating lymphocytes (TILs) had better outcomes than those with weak TILs after operation. Our results showed that an elevated NLR had significant associations with tumor size and liver function. Taken together, it was confirmed that an elevated NLR can indirectly reflect the tumor burden, vascular invasion, a high risk of tumor recurrence, and shorter survival after resection.
Tumor-associated macrophages (TAMs) have been shown to have tumor-promoting effects, with a high density of TAMs in tumors reported to be associated with a poor prognosis. Some reports have indicated that macrophage infiltration into HCC is related to the aggressiveness of the tumor[46]. Maniecki et al[47] showed that a high infiltration of TAMs in HCC was related to a high NLR[28]. TAMs express certain cytokines, such as IL-6 and IL-8, within the tumor, and these cytokines may promote systemic neutrophilia. Ubukata et al[48] demonstrated that a high NLR is significantly correlated with a high level of Th2 cells, which can polarize macrophages to TAMs through expressing certain cytokines, such as IL-4 and IL-10. A high NLR is associated with high infiltration of TAMs and high inflammatory cytokine production in the tumor. Some studies have reported that TAMs are closely related to proinflammatory cytokine IL-17[49]. Peritumoral IL-17 may enhance systematic neutrophil leukocytes and play an important role in tumor progression[28,50]. Therefore, a similar mechanism may be one of the reasons for the NLR elevation in HCC patients. Some researchers have suggested that a high infiltration of TAMs is a first and important step of NLR elevation[28]. However, further examination is necessary to elucidate the mechanism.
The power of the present research was limited by its retrospective nature, single-center data, and relatively small sample size. More molecular experiments are needed to clarify the detailed molecular mechanism of the role that NLR plays in HCC with PVTT/HVTT. In consideration of these limitations, we believe that cross-validation in independent and larger patient cohorts, possibly in a prospective setting, should be mandatory before the NLR can be confidently incorporated as a validated biomarker to guide treatment decisions.
Although only 81 cases were included in our study, achieving this number of cases was difficult given the rarity of HCC patients with PVTT/HVTT who are suitable for hepatic surgery. We are the first to demonstrate that the NLR could predict the prognosis of HCC patients with PVTT/HVTT after hepatectomy. As described previously, the NLR may reflect the complex interplay between inflammatory mediators and angiogenic factors that are known to influence the survival of HCC patients. Moreover, unlike other complex molecular markers, the NLR is easy to compute and is universally available because it is derived from laboratory measures that are routinely assessed before operation. NLR could be used as a potential prognostic predictor for HCC patients with PVTT/HVTT after hepatectomy.
Portal/hepatic vein tumor thrombosis (PVTT/HVTT) in hepatocellular carcinoma (HCC) is a sign of advanced-stage disease and is associated with a poor prognosis. This study investigated whether the preoperative neutrophil-to-lymphocyte ratio (NLR) could predict the prognosis of these patients after hepatectomy.
PVTT/HVTT in HCC is a sign of advanced-stage disease and is associated with a poor prognosis. Substantial evidence has recently shown that hepatectomy can improve the survival of HCC patients with PVTT/HVTT, although the median survival duration is still unsatisfactory. The reasons for this remain unclear and seem to be complex and multifactorial.
The presence of a systemic inflammatory response can be detected by the elevation of the NLR, which has been shown to be associated with a poorer prognosis in patients with various types of malignant tumors. This study confirmed that the NLR could be used as a potential prognostic predictor for HCC patients with PVTT/HVTT after hepatectomy.
The results of the present study may identify a new serum marker for predicting the post-operative survival of these patients.
Although the power of the present research was limited by its retrospective nature, single center data, and relatively small sample size, the paper is well written and surely gives new ideas in the preoperative evaluation of such complex patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Marzano C S- Editor: Yu J L- Editor: Ma JY E- Editor: Zhang FF
| 1. | Okuda K. Hepatocellular carcinoma: recent progress. Hepatology. 1992;15:948-963. [PubMed] |
| 2. | Amitrano L, Guardascione MA, Brancaccio V, Margaglione M, Manguso F, Iannaccone L, Grandone E, Balzano A. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol. 2004;40:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 391] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Calvet X, Bruix J, Brú C, Ginés P, Vilana R, Solé M, Ayuso MC, Bruguera M, Rodes J. Natural history of hepatocellular carcinoma in Spain. Five year’s experience in 249 cases. J Hepatol. 1990;10:311-317. [PubMed] |
| 4. | Kim JM, Kwon CH, Joh JW, Park JB, Ko JS, Lee JH, Kim SJ, Park CK. The effect of alkaline phosphatase and intrahepatic metastases in large hepatocellular carcinoma. World J Surg Oncol. 2013;11:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 907] [Article Influence: 34.9] [Reference Citation Analysis (1)] |
| 6. | Pawarode A, Voravud N, Sriuranpong V, Kullavanijaya P, Patt YZ. Natural history of untreated primary hepatocellular carcinoma: a retrospective study of 157 patients. Am J Clin Oncol. 1998;21:386-391. [PubMed] |
| 7. | Ohkubo T, Yamamoto J, Sugawara Y, Shimada K, Yamasaki S, Makuuchi M, Kosuge T. Surgical results for hepatocellular carcinoma with macroscopic portal vein tumor thrombosis. J Am Coll Surg. 2000;191:657-660. [PubMed] |
| 8. | Chen XP, Qiu FZ, Wu ZD, Zhang ZW, Huang ZY, Chen YF, Zhang BX, He SQ, Zhang WG. Effects of location and extension of portal vein tumor thrombus on long-term outcomes of surgical treatment for hepatocellular carcinoma. Ann Surg Oncol. 2006;13:940-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Chen JS, Wang Q, Chen XL, Huang XH, Liang LJ, Lei J, Huang JQ, Li DM, Cheng ZX. Clinicopathologic characteristics and surgical outcomes of hepatocellular carcinoma with portal vein tumor thrombosis. J Surg Res. 2012;175:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10123] [Cited by in RCA: 11272] [Article Influence: 490.1] [Reference Citation Analysis (2)] |
| 11. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5763] [Article Influence: 240.1] [Reference Citation Analysis (0)] |
| 12. | Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res. 2000;60:184-190. [PubMed] |
| 13. | Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 844] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 14. | Gomez D, Morris-Stiff G, Toogood GJ, Lodge JP, Prasad KR. Impact of systemic inflammation on outcome following resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2008;97:513-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 15. | Bhatti I, Peacock O, Lloyd G, Larvin M, Hall RI. Preoperative hematologic markers as independent predictors of prognosis in resected pancreatic ductal adenocarcinoma: neutrophil-lymphocyte versus platelet-lymphocyte ratio. Am J Surg. 2010;200:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 16. | Yamanaka T, Matsumoto S, Teramukai S, Ishiwata R, Nagai Y, Fukushima M. The baseline ratio of neutrophils to lymphocytes is associated with patient prognosis in advanced gastric cancer. Oncology. 2007;73:215-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 338] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 17. | Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Pichler M, Hutterer GC, Stoeckigt C, Chromecki TF, Stojakovic T, Golbeck S, Eberhard K, Gerger A, Mannweiler S, Pummer K. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br J Cancer. 2013;108:901-907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 208] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 19. | Wang GY, Yang Y, Li H, Zhang J, Jiang N, Li MR, Zhu HB, Zhang Q, Chen GH. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS One. 2011;6:e25295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Pinato DJ, Sharma R. An inflammation-based prognostic index predicts survival advantage after transarterial chemoembolization in hepatocellular carcinoma. Transl Res. 2012;160:146-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Zhang J, Gong F, Li L, Zhao M, Song J. Diabetes mellitus and the neutrophil to lymphocyte ratio predict overall survival in non-viral hepatocellular carcinoma treated with transarterial chemoembolization. Oncol Lett. 2014;7:1704-1710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Halazun KJ, Hardy MA, Rana AA, Woodland DC, Luyten EJ, Mahadev S, Witkowski P, Siegel AB, Brown RS, Emond JC. Negative impact of neutrophil-lymphocyte ratio on outcome after liver transplantation for hepatocellular carcinoma. Ann Surg. 2009;250:141-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Limaye AR, Clark V, Soldevila-Pico C, Morelli G, Suman A, Firpi R, Nelson DR, Cabrera R. Neutrophil-lymphocyte ratio predicts overall and recurrence-free survival after liver transplantation for hepatocellular carcinoma. Hepatol Res. 2013;43:757-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Motomura T, Shirabe K, Mano Y, Muto J, Toshima T, Umemoto Y, Fukuhara T, Uchiyama H, Ikegami T, Yoshizumi T. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 357] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 25. | Li X, Chen ZH, Ma XK, Chen J, Wu DH, Lin Q, Dong M, Wei L, Wang TT, Ruan DY. Neutrophil-to-lymphocyte ratio acts as a prognostic factor for patients with advanced hepatocellular carcinoma. Tumour Biol. 2014;35:11057-11063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Dan J, Zhang Y, Peng Z, Huang J, Gao H, Xu L, Chen M. Postoperative neutrophil-to-lymphocyte ratio change predicts survival of patients with small hepatocellular carcinoma undergoing radiofrequency ablation. PloS one. 2013;8:e58184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 27. | Liao W, Zhang J, Zhu Q, Qin L, Yao W, Lei B, Shi W, Yuan S, Tahir SA, Jin J. Preoperative Neutrophil-to-Lymphocyte Ratio as a New Prognostic Marker in Hepatocellular Carcinoma after Curative Resection. Transl Oncol. 2014;7:248-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | Mano Y, Shirabe K, Yamashita Y, Harimoto N, Tsujita E, Takeishi K, Aishima S, Ikegami T, Yoshizumi T, Yamanaka T. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 2013;258:301-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 274] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 29. | Harimoto N, Shirabe K, Nakagawara H, Toshima T, Yamashita Y, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, Maehara Y. Prognostic factors affecting survival at recurrence of hepatocellular carcinoma after living-donor liver transplantation: with special reference to neutrophil/lymphocyte ratio. Transplantation. 2013;96:1008-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 31. | Yamada R, Sato M, Kawabata M, Nakatsuka H, Nakamura K, Takashima S. Hepatic artery embolization in 120 patients with unresectable hepatoma. Radiology. 1983;148:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 693] [Cited by in RCA: 673] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 32. | Investigators. A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 963] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 33. | Halazun KJ, Aldoori A, Malik HZ, Al-Mukhtar A, Prasad KR, Toogood GJ, Lodge JP. Elevated preoperative neutrophil to lymphocyte ratio predicts survival following hepatic resection for colorectal liver metastases. Eur J Surg Oncol. 2008;34:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 331] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 34. | Gomez D, Farid S, Malik HZ, Young AL, Toogood GJ, Lodge JP, Prasad KR. Preoperative neutrophil-to-lymphocyte ratio as a prognostic predictor after curative resection for hepatocellular carcinoma. World J Surg. 2008;32:1757-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 295] [Cited by in RCA: 336] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 35. | Zheng YB, Zhao W, Liu B, Lu LG, He X, Huang JW, Li Y, Hu BS. The blood neutrophil-to-lymphocyte ratio predicts survival in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac J Cancer Prev. 2013;14:5527-5531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Bertuzzo VR, Cescon M, Ravaioli M, Grazi GL, Ercolani G, Del Gaudio M, Cucchetti A, D’Errico-Grigioni A, Golfieri R, Pinna AD. Analysis of factors affecting recurrence of hepatocellular carcinoma after liver transplantation with a special focus on inflammation markers. Transplantation. 2011;91:1279-1285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 37. | Shi J, Lai EC, Li N, Guo WX, Xue J, Lau WY, Wu MC, Cheng SQ. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann Surg Oncol. 2010;17:2073-2080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 38. | Mondazzi L, Bottelli R, Brambilla G, Rampoldi A, Rezakovic I, Zavaglia C, Alberti A, Idèo G. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19:1115-1123. [PubMed] |
| 39. | Yamashita Y, Takahashi M, Koga Y, Saito R, Nanakawa S, Hatanaka Y, Sato N, Nakashima K, Urata J, Yoshizumi K. Prognostic factors in the treatment of hepatocellular carcinoma with transcatheter arterial embolization and arterial infusion. Cancer. 1991;67:385-391. [PubMed] |
| 40. | Peng BG, He Q, Li JP, Zhou F. Adjuvant transcatheter arterial chemoembolization improves efficacy of hepatectomy for patients with hepatocellular carcinoma and portal vein tumor thrombus. Am J Surg. 2009;198:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 41. | Shaohua L, Qiaoxuan W, Peng S, Qing L, Zhongyuan Y, Ming S, Wei W, Rongping G. Surgical Strategy for Hepatocellular Carcinoma Patients with Portal/Hepatic Vein Tumor Thrombosis. PLoS One. 2015;10:e0130021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Vibert E, Azoulay D, Hoti E, Iacopinelli S, Samuel D, Salloum C, Lemoine A, Bismuth H, Castaing D, Adam R. Progression of alphafetoprotein before liver transplantation for hepatocellular carcinoma in cirrhotic patients: a critical factor. Am J Transplant. 2010;10:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 43. | Ueno S, Tanabe G, Sako K, Hiwaki T, Hokotate H, Fukukura Y, Baba Y, Imamura Y, Aikou T. Discrimination value of the new western prognostic system (CLIP score) for hepatocellular carcinoma in 662 Japanese patients. Cancer of the Liver Italian Program. Hepatology. 2001;34:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 44. | Kusumanto YH, Dam WA, Hospers GA, Meijer C, Mulder NH. Platelets and granulocytes, in particular the neutrophils, form important compartments for circulating vascular endothelial growth factor. Angiogenesis. 2003;6:283-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 428] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 45. | Okano K, Maeba T, Moroguchi A, Ishimura K, Karasawa Y, Izuishi K, Goda F, Usuki H, Wakabayashi H, Maeta H. Lymphocytic infiltration surrounding liver metastases from colorectal cancer. J Surg Oncol. 2003;82:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Zhou J, Ding T, Pan W, Zhu LY, Li L, Zheng L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int J Cancer. 2009;125:1640-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 47. | Maniecki MB, Etzerodt A, Ulhøi BP, Steiniche T, Borre M, Dyrskjøt L, Orntoft TF, Moestrup SK, Møller HJ. Tumor-promoting macrophages induce the expression of the macrophage-specific receptor CD163 in malignant cells. Int J Cancer. 2012;131:2320-2331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Ubukata H, Motohashi G, Tabuchi T, Nagata H, Konishi S, Tabuchi T. Evaluations of interferon-γ/interleukin-4 ratio and neutrophil/lymphocyte ratio as prognostic indicators in gastric cancer patients. J Surg Oncol. 2010;102:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 49. | Kuang DM, Peng C, Zhao Q, Wu Y, Chen MS, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma promote expansion of memory T helper 17 cells. Hepatology. 2010;51:154-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 50. | Kuang DM, Zhao Q, Wu Y, Peng C, Wang J, Xu Z, Yin XY, Zheng L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J Hepatol. 2011;54:948-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 389] [Article Influence: 27.8] [Reference Citation Analysis (0)] |