Published online May 7, 2017. doi: 10.3748/wjg.v23.i17.3092
Peer-review started: October 26, 2016
First decision: December 19, 2016
Revised: January 30, 2017
Accepted: March 31, 2017
Article in press: March 31, 2017
Published online: May 7, 2017
Processing time: 194 Days and 17.3 Hours
To establish the ability of magnetic resonance (MR) and computer tomography (CT) to predict pathologic dimensions of pancreatic neuroendocrine tumors (PanNET) in a caseload of a tertiary referral center.
Patients submitted to surgery for PanNET at the Surgical Unit of the Pancreas Institute with at least 1 preoperative imaging examination (MR or CT scan) from January 2005 to December 2015 were included and data retrospectively collected. Exclusion criteria were: multifocal lesions, genetic syndromes, microadenomas or mixed tumors, metastatic disease and neoadjuvant therapy. Bland-Altman (BA) and Mountain-Plot (MP) statistics were used to compare size measured by each modality with the pathology size. Passing-Bablok (PB) regression analysis was used to check the agreement between MR and CT.
Our study population consisted of 292 patients. Seventy-nine (27.1%) were functioning PanNET. The mean biases were 0.17 ± 7.99 mm, 1 ± 8.51 mm and 0.23 ± 9 mm, 1.2 ± 9.8 mm for MR and CT, considering the overall population and the subgroup of non-functioning- PanNET, respectively. Limits of agreement (LOA) included the vast majority of observations, indicating a good agreement between imaging and pathology. The MP further confirmed this finding and showed that the two methods are unbiased with respect to each other. Considering ≤ 2 cm non-functioning-PanNET, no statistical significance was found in the size estimation rate of MR and CT (P = 0.433). PBR analysis did not reveal significant differences between MR, CT and pathology.
MR and CT scan are accurate and interchangeable imaging techniques in predicting pathologic dimensions of PanNET.
Core tip: Radiological tumor size estimation of pancreatic neuroendocrine tumors is of utmost importance for therapeutic decision-making, especially for non-functioning ones. This study showed that both magnetic resonance and computer tomography are accurate and interchangeable in predicting pathologic tumor size.
- Citation: Paiella S, Impellizzeri H, Zanolin E, Marchegiani G, Miotto M, Malpaga A, De Robertis R, D'Onofrio M, Rusev B, Capelli P, Cingarlini S, Butturini G, Davì MV, Amodio A, Bassi C, Scarpa A, Salvia R, Landoni L. Comparison of imaging-based and pathological dimensions in pancreatic neuroendocrine tumors. World J Gastroenterol 2017; 23(17): 3092-3098
- URL: https://www.wjgnet.com/1007-9327/full/v23/i17/3092.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i17.3092
Pancreatic neuroendocrine tumors (PanNET) account for 5% of all pancreatic tumors, with an incidence of 1/100000 per year[1]. Compared with pancreatic carcinoma, patients with PanNET have a better prognosis and the indolent biology of these tumors plays a crucial role in therapeutic decision making. Considering the presence of clinical symptoms of hormonal hypersecretion, they are divided into 2 subgroups, functioning (F-PanNET) and non-functioning (NF-PanNET). The surgical treatment of F-PanNET is the treatment of choice, whereas for NF-PanNET surgery is recommended for tumors greater than 2 cm, in selected cases of MEN-1 syndromes and in cases of symptoms or abdominal discomfort as PanNET compress surrounding organs[2]. Magnetic resonance (MR) imaging and computer tomography (CT) are the imaging techniques of choice for tumor localization and staging, hence the treatment decision-making often depends on the tumor size assessed by these imaging techniques. When referred to a tertiary referral center, most patients from country hospital have already performed cross-sectional imaging studies. To the best of our knowledge, no studies have ever evaluated size-measurement aspects of MR and CT examinations. Hence, we set out to investigate the properties of MR and CT Scan in measuring the size of PanNET, using the pathological final dimensions as reference standard, in a high-volume referral hospital.
The prospectively maintained database of the Surgical Unit of the Pancreas Institute of Verona, Italy was queried. Data were collected from patients referred to our Institution from January 2005 to December 2015. All patients submitted to surgery with a preoperative and a final pathological diagnosis of PanNET, and with at least 1 pre-operative imaging examination (either MR or CT) were included.
Exclusion criteria were considered as follows: (1) multifocal lesions; (2) MEN-1 or Von Hippel- Lindau syndromes; (3) microadenomas [maximum tumor diameter (MTD) < 5 mm] or mixed tumors; (4) presence of metastasis at the time of surgery; and (5) neoadjuvant therapy.
The study received local Ethics Committee approval [#42757] and it was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki.
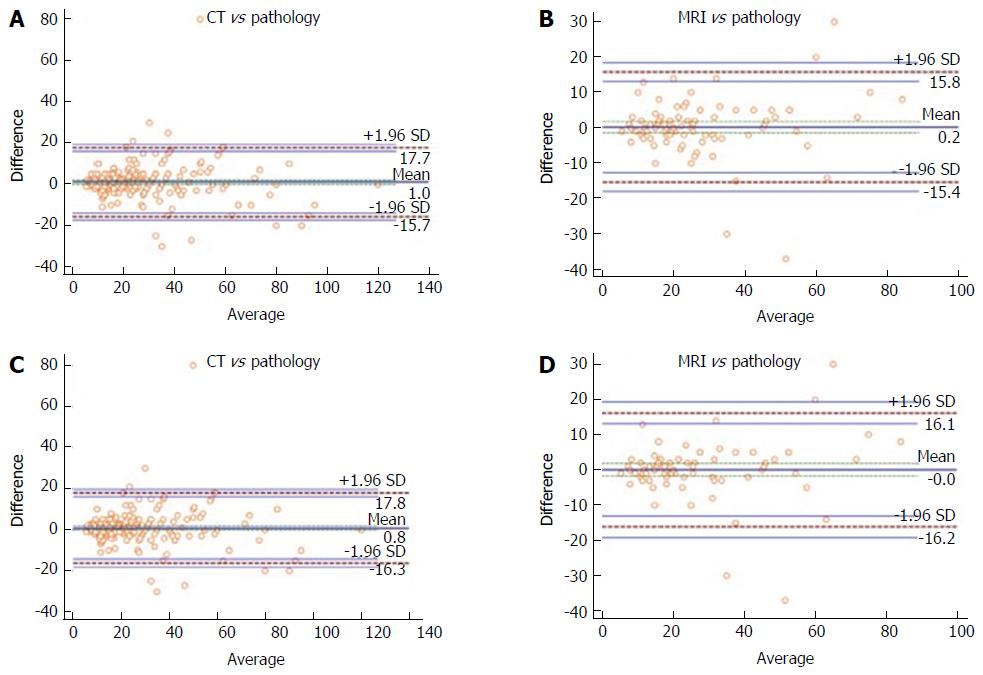
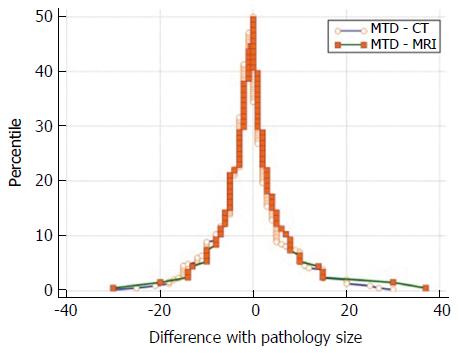
The size discrepancy from imaging to pathology was calculated subtracting pathological MTD to radiological MTD. The differences were summarized as means and SD. The difference between the measured values against their means was represented with scatterplots. A Bland-Altman analysis was used to compare 2 methods of measurement with scatter plots (MR vs pathology and CT vs pathology) where the difference between the measured values against their means was represented[3]. The Bland-Altman analysis calculates the mean difference between 2 methods of measurement (the “bias”) and 95% LOA as the mean difference (1.96 SD). The 95%CI for the limits were reported too. In this visual method, was used considering that the smaller range between these two limits the better the agreement is[4]. A subgroup analysis of NF-PanNET was also performed. In addition, a folded empirical cumulative distribution plot, the Mountain-Plot, was drawn. A Mountain-Plot is usually considered as a complementary plot to Bland-Altman method, especially for not Normally distributed data[5]. Finally, the agreement of MR and CT vs pathology was further investigated using the Passing-Bablok (PB) regression analysis[6,7].
The sensitivity in predicting dimensions of MR and CT for NF-PanNET was calculated using a Fisher’s exact test. As data were not normally distributed, a Kruskal-Wallis test was performed to check for size differences according to tumor site.
All the statistical analyses were performed using MedCalc, version 15.0 (MedCalc Software, Ostend, Belgium).
A total of 292 patients fulfilled the inclusion and exclusion criteria and were included in the analysis during the study period. The subgroup of NF-PanNET included 213 patients (72.9%) and 91 out of 213 (42.7%) were 2 cm. MR was performed in 101 patients (34.5%), whereas CT in 277 (94.8%). Eighty-seven patients out of 292 (29.8%) underwent both MR and CT. The clinical and surgical characteristics of the population are listed in Table 1.
| Variable | n (%) |
| Age (mean, SD, range) | 54.4 (13.7; 18-85) |
| Sex | |
| Male | 147 (50.3) |
| Female | 145 (49.8) |
| Functioning, yes | 79 (27.1) |
| CT, yes | 276 (94.5) |
| MR, yes | 103 (35.3) |
| Location | |
| Head | 125 (42.8) |
| Body | 115 (39.4) |
| Tail | 52 (17.8) |
| Type of surgery | |
| Distal splenopancreatectomy | 108 (37.0) |
| Spleen preserving distal pancreatectomy | 12 (4.1) |
| Pancreaticoduodenectomy | 78 (26.7) |
| Middle pancreatectomy | 25 (8.6) |
| Enucleation | 64 (21.9) |
| Total pancreatectomy | 5 (1.7) |
| Grading | |
| G1 | 190 (65.1) |
| G2 | 96 (32.9) |
| G3 | 6 (2.1) |
| MR, mean MTD (SD, range) | 26.3 (17.1, 5-88) |
| CT, mean MTD (SD, range) | 25.6 (18.5, 5-120) |
| Pathology, mean MTD (SD, range) | 24.6 (19, 5-120) |
Dimension estimates from MR and CT were compared with pathology, that was considered the gold standard. Considering the overall population, the mean discrepancy between radiological and pathologic dimensions (mean bias), calculated subtracting the mean value of the size of the imaging modalities minus the mean pathologic size, was 0.17 ± 7.99 mm for MR and 1 ± 8.51 mm for CT (Table 2). When considering NF-PanNET the mean bias was 0.23 ± 9 mm and 1.2 ± 9.8 mm for MR and CT, respectively (Table 2). The Bland-Altman plots showed that MR had the narrowest LOA, when considering both the overall population and the NF-PanNET subgroup (Figure 1). The Mountain-Plot showed that the two methods are unbiased as the plot is centered over zero and presented similar differences with respect to the gold standard as the tail are superimposable (Figure 2). In fact, the median of the differences is close to zero for both MR and CT, compared with pathologic size.
| MR vs pathology | CT vs pathology | |
| Overall | ||
| Mean bias, mm | 0.17 ± 7.99 | 1 ± 8.51 |
| BA - LOA (95%CI) | -15.4 (-12.7, -18) | -15.7 (-13.9, -17.4) |
| 15.8 (13.1, 18.4) | 17.7 (15.9, 19.4) | |
| NF-PanNET | ||
| Mean bias, mm | 0.23 ± 9 | 1.2 ± 9.8 |
| BA - LOA (95%CI) | 16.1 (12.4, 19.8) | 17.8 (16.4, 19.7) |
| -16.2 (-12.5, -19.9) | -16.3 (-14.1, -18.8) |
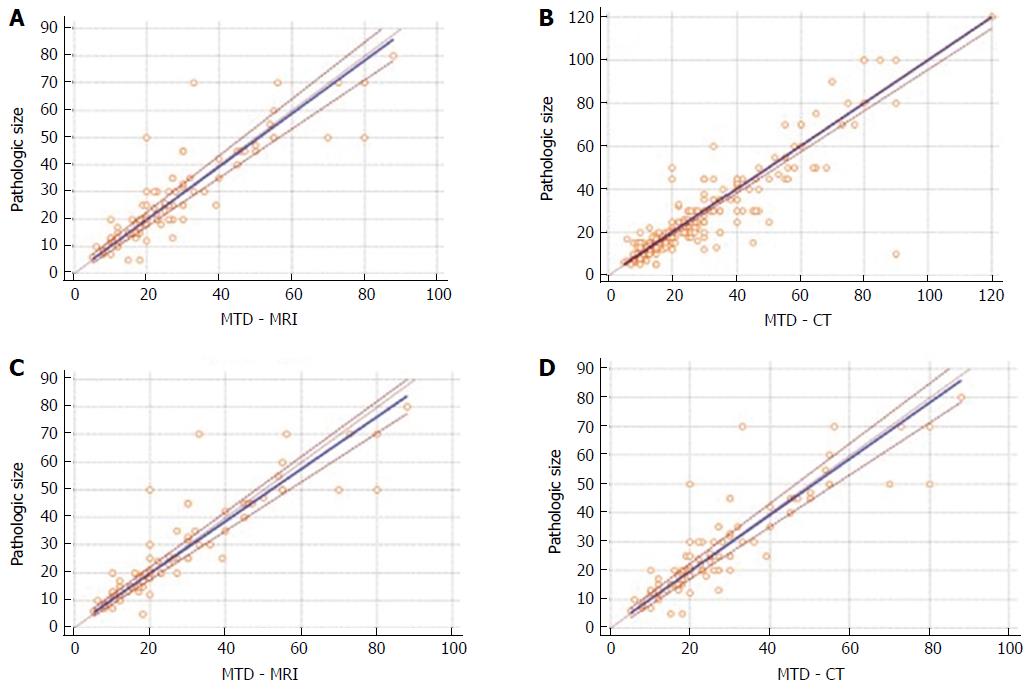
The results of PB regression analysis for measurement of agreement between MR and CT vs pathology are shown in Figure 3. The resulting equation of PB regression analysis for MR (overall population) was y = 0.39 + 0.97 × (95%CI of intercept -0.76-1.77, and slope 0.9-1.03) (Figure 3A). The resulting equation of PB regression analysis for CT (overall population) was y = 0 + 1 × (95%CI of intercept 0-0.52, and slope 0.95-1) (Figure 3B). PB regression analysis for MR (NF-PanNET) was y = 0.84 + 0.94 × (95%CI of intercept 0-2.05, and slope 0.88-1) (Figure 3C). PB regression analysis for CT (NF-PanNET) was y = 0 + 1 × (95%CI of intercept 0-0.57, and slope 0.95-1) (Figure 3D). For all the regressions, the intercepts were not significantly different from zero, pointing out that there was no constant difference between the methods. Also, all the regression slopes were not significantly different from one, indicating the absence of a proportional difference between the methods. There was no significant deviation from linearity in any of the analyzed data set.
The sensitivity of MR and CT in predicting final tumor dimensions for ≤ 2 cm NF-PanNET was 91.4% and 95%, respectively, and there was no statistically significant difference between them (Fisher's exact test, P = 0.433). Considering size discrepancy between radiology and pathology with respect to tumor site (head, body and tail), the Kruskal-Wallis test did not show any statistical significance for both MR (P = 0.933) and CT (P = 0.906).
The biological behavior of a PanNET is unpredictable, yet relatively favorable, especially if compared with pancreatic cancer. Given this various biology an appropriate staging is of utmost importance to tailor the treatment to avoid possible over- or under-treatment. Surgery remains the only chance of cure for PanNET and even debulking procedures are advocated to reduce the burden of the disease in selected metastatic PanNET[8,9]. While for F-PanNET the decision-making is easily based on surgery, on the other hand the surgical management of NF-PanNET has been put under scrutiny when the diameter is ≤ 2 cm[10-12]. The 2016 ENETS Consensus Guidelines Update on F-PanNET and NF-PanNET suggested a conservative management of small NF- PanNET, at least unless more follow-up data are not available[13]. Recently, Regenet et al[14], analyzing a retrospective cohort of 66 patients, claimed that a cut-off size of 1.7 cm is adequate to drive the management of NF-PanNET.
Hence, a precise preoperative size estimation is of paramount importance to drive the management of PanNET, either surgical or conservative. In our study, small and clinically acceptable mean biases either for MR and CT were found, the greater being of 1.2 mm (CT Scan in NF-PanNET). In addition, in the Bland-Altman analysis, the vast majority of the observations were within the LOA, indicating a good agreement between each radiological method size estimation and pathological dimensions. Comparing the two methods, the reported LOA are almost equally larger and this allows substituting a method with the other. These considerations were furtherly confirmed by PB regression analysis, showing no significant systematic or proportional differences between each method and pathology, regarding either MR or CT.
Our results demonstrate that both MR and CT scan are equally accurate in predicting tumor dimensions, even in NF-PanNET, with only a slight overall more accuracy for MR (smaller mean bias and narrower LOA). The interchangeability of the techniques was confirmed by the Mountain-Plot (no systematic biases between the two methods). Interestingly, the sensitivity in predicting small NF-PanNET was slightly higher for CT Scan than for MR.
Considering that MR and CT are interchangeable in the preoperative size estimation of PanNET, therefore, we can speculate that endoultrasonography (EUS), more invasive and less widely available than conventional imaging, could not be necessary for this purpose, and that can be used in doubtful cases (suspected cystic PanNET or pancreatic metastases from renal cell carcinoma), when an evident dimensional discrepancy is present at cross-sectional imaging or when the Ki-67 assessment is required[15-17].
From a logistic point of view our findings are important. First, our results depict the scenario of a high-volume center, to whom patients are referred from country hospitals where they already have been imaged, mostly with CT. Hence, no other cross-sectional imaging techniques are needed to assess preoperative tumor dimensions when just one has been performed. Second, considering that MR is still not as widely available as CT, a first-level cross-sectional imaging with CT could be considered sufficient and adequate to assess tumor size; nevertheless, since MR is the best imaging technique for the identification of liver metastases and may provide ancillary information on the biological behavior of PanNET[18], this technique should be always considered for the evaluation of PanNETs, whenever available. Third, the interchangeability of the two techniques allows a radiation-free follow-up program of 2 cm NF-PanNET using MR.
As far as we know, this is the first study that has evaluated the accuracy of MR and CT in predicting dimensions of PanNET. Despite our results are supported by a large cohort of patients, this study has major limitations that must be considered. First, this is a retrospective study; second, different equipment has been used during the period of inclusion and this could have influenced our results; third, for patients before 2010, no radiological images were available for analysis.
In conclusion, this study provides an insight on the ability of MR and CT in detecting size measurements of PanNET and on how this can impact on the clinical practice of a tertiary referral center. MR provides a precise assessment of tumor size and is the radiological gold standard for the detection of liver metastases, therefore - when available - it should be considered for first for the evaluation of PanNETs; when MR is not available, diametric analysis by CT can provide reliable tumor dimensions.
Preoperative size estimation of pancreatic neuroendocrine tumors (PanNET) is an important parameter that drives the therapeutic decision-making.
This is the first study that explores the properties of magnetic resonance (MR) and computer tomography (CT) scan on the size measurement of PanNET.
In this study, both MR and CT scan provided preoperative accurate estimations of PanNET, even in small NF-PanNET. MR showed a slightly greater accuracy, even for NF-PANNET. However, CT scan was more sensitive in predicting the dimensions of small (≤ 2 cm) NF-PanNET.
This study suggests that both MR and CT scan can be interchangeably adopted for preoperative size estimations of PanNET. When available, MR should be preferred to assess eventual metastases.
This is a retrospectively study trying to establish the ability of MR and CT to predict pathologic dimensions of PanNET with methods of Bland-Altman and Mountain-Plot statistics in a caseload of a tertiary referral center, as a precise preoperative size detecting is of paramount importance to drive the management of PanNET, either surgical or conservative. It was found that both MR and CT scan were equally accurate in predicting tumor dimensions, even in NF-PanNET from a logistic point of view. There is some interest and value to study, the ability of MR and CT in detecting size measurements, and the impact on the clinical practice of PanNET were needed to be verified with much more cases and prospective clinical trials.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fu DL S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063-3072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3022] [Cited by in RCA: 3244] [Article Influence: 190.8] [Reference Citation Analysis (0)] |
| 2. | Falconi M, Bartsch DK, Eriksson B, Klöppel G, Lopes JM, O’Connor JM, Salazar R, Taal BG, Vullierme MP, O’Toole D. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms of the digestive system: well-differentiated pancreatic non-functioning tumors. Neuroendocrinology. 2012;95:120-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 358] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 3. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. [PubMed] |
| 4. | Myles PS, Cui J. Using the Bland-Altman method to measure agreement with repeated measures. Br J Anaesth. 2007;99:309-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 439] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 5. | Krouwer JS, Monti KL. A simple, graphical method to evaluate laboratory assays. Eur J Clin Chem Clin Biochem. 1995;33:525-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Passing H. A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 439] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 7. | Ludbrook J. Linear regression analysis for comparing two measurers or methods of measurement: but which regression? Clin Exp Pharmacol Physiol. 2010;37:692-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Fendrich V, Langer P, Celik I, Bartsch DK, Zielke A, Ramaswamy A, Rothmund M. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg. 2006;244:845-851; discussion 852-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 108] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Sarmiento JM, Heywood G, Rubin J, Ilstrup DM, Nagorney DM, Que FG. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg. 2003;197:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 534] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 10. | Bettini R, Partelli S, Boninsegna L, Capelli P, Crippa S, Pederzoli P, Scarpa A, Falconi M. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 251] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 11. | Gaujoux S, Partelli S, Maire F, D’Onofrio M, Larroque B, Tamburrino D, Sauvanet A, Falconi M, Ruszniewski P. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98:4784-4789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 12. | Lee LC, Grant CS, Salomao DR, Fletcher JG, Takahashi N, Fidler JL, Levy MJ, Huebner M. Small, nonfunctioning, asymptomatic pancreatic neuroendocrine tumors (PNETs): role for nonoperative management. Surgery. 2012;152:965-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 13. | Falconi M, Eriksson B, Kaltsas G, Bartsch DK, Capdevila J, Caplin M, Kos-Kudla B, Kwekkeboom D, Rindi G, Klöppel G. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1097] [Cited by in RCA: 985] [Article Influence: 109.4] [Reference Citation Analysis (1)] |
| 14. | Regenet N, Carrere N, Boulanger G, de Calan L, Humeau M, Arnault V, Kraimps JL, Mathonnet M, Pessaux P, Donatini G. Is the 2-cm size cutoff relevant for small nonfunctioning pancreatic neuroendocrine tumors: A French multicenter study. Surgery. 2016;159:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Ridtitid W, Halawi H, DeWitt JM, Sherman S, LeBlanc J, McHenry L, Coté GA, Al-Haddad MA. Cystic pancreatic neuroendocrine tumors: outcomes of preoperative endosonography-guided fine needle aspiration, and recurrence during long-term follow-up. Endoscopy. 2015;47:617-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Larghi A, Capurso G, Carnuccio A, Ricci R, Alfieri S, Galasso D, Lugli F, Bianchi A, Panzuto F, De Marinis L. Ki-67 grading of nonfunctioning pancreatic neuroendocrine tumors on histologic samples obtained by EUS-guided fine-needle tissue acquisition: a prospective study. Gastrointest Endosc. 2012;76:570-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 17. | Farrell JM, Pang JC, Kim GE, Tabatabai ZL. Pancreatic neuroendocrine tumors: accurate grading with Ki-67 index on fine-needle aspiration specimens using the WHO 2010/ENETS criteria. Cancer Cytopathol. 2014;122:770-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | De Robertis R, D’Onofrio M, Zamboni G, Tinazzi Martini P, Gobbo S, Capelli P, Butturini G, Girelli R, Ortolani S, Cingarlini S. Pancreatic Neuroendocrine Neoplasms: Clinical Value of Diffusion-Weighted Imaging. Neuroendocrinology. 2016;103:758-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |