Published online May 7, 2017. doi: 10.3748/wjg.v23.i17.3053
Peer-review started: December 16, 2016
First decision: December 29, 2016
Revised: January 17, 2017
Accepted: March 20, 2017
Article in press: March 20, 2017
Published online: May 7, 2017
Processing time: 143 Days and 21.7 Hours
To investigate the role of CXC chemokine receptor (CXCR)-7 and CXCL12 in lymph node and liver metastasis of gastric carcinoma.
In 160 cases of gastric cancer, the expression of CXCR7 and CXCL12 in tumor and matched tumor-adjacent non-cancer tissues, in the lymph nodes around the stomach and in the liver was detected using immunohistochemistry to analyze the relationship between CXCR7/CXCL12 expression and clinicopathological features and to determine whether CXCR7 and CXCL12 constitute a biological axis to promote lymph node and liver metastasis of gastric cancer. Furthermore, the CXCR7 gene was silenced and overexpressed in human gastric cancer SGC-7901 cells, and cell proliferation, migration and invasiveness were measured by the MTT, wound healing and Transwell assays, respectively.
CXCR7 expression was up-regulated in gastric cancer tissues (P = 0.011). CXCR7/CXCL12 expression was significantly related to high tumor stage and lymph node (r = 0.338, P = 0.000) and liver metastasis (r = 0.629, P = 0.000). The expression of CXCL12 in lymph node and liver metastasis was higher than that in primary gastric cancer tissues (χ2 = 6.669, P = 0.010; χ2 = 25379, P = 0.000), and the expression of CXCL12 in lymph node and liver metastasis of gastric cancer was consistent with the positive expression of CXCR7 in primary gastric cancer (r = 0.338, P = 0.000; r = 0.629, P = 0.000). Overexpression of the CXCR7 gene promoted cell proliferation, migration and invasion. Silencing of the CXCR7 gene suppressed SGC-7901 cell proliferation, migration and invasion. Human gastric cancer cell lines expressed CXCR7 and showed vigorous proliferation and migratory responses to CXCL12.
The CXCR7/CXCL12 axis is involved in lymph node and liver metastasis of gastric cancer. CXCR7 is considered a potential therapeutic target for the treatment of gastric cancer.
Core tip: The CXC chemokine receptor (CXCR)-7/CXCL12 axis could play an important role in metastasis of certain cancers. However, little is known about the effect of CXCR7/CXCL12 on the process of gastric cancer. This study investigated the role of CXCL12 and CXCR7 in lymph node and liver metastasis of gastric carcinoma. We found that the CXCR7/CXCL12 axis was involved in the lymph node and liver metastasis of gastric cancer. Overexpression of the CXCR7 gene promoted cell proliferation, migration and invasion. Silencing of the CXCR7 gene suppressed these processes. CXCR7 was considered a potential therapeutic target for the treatment of gastric cancer.
- Citation: Xin Q, Zhang N, Yu HB, Zhang Q, Cui YF, Zhang CS, Ma Z, Yang Y, Liu W. CXCR7/CXCL12 axis is involved in lymph node and liver metastasis of gastric carcinoma. World J Gastroenterol 2017; 23(17): 3053-3065
- URL: https://www.wjgnet.com/1007-9327/full/v23/i17/3053.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i17.3053
Gastric carcinoma is a disease with a high death rate, making it the second most common cause of cancer death worldwide, following lung cancer. The high mortality of gastric cancer is due to metastasis, and the most common metastatic site is the lymph nodes, followed by the liver, indicating an urgent need for new diagnostic markers and treatment approaches[1,2].
In recent years, chemokines and their receptors have been found to be expressed on cancer cells and may mediate cancer progression and metastasis. Malignant cells can express chemokine receptors and respond to chemokine gradients, which may be related to the growth and spread of cancer. Stromal cell-derived factor 1 (SDF-1) is a very important chemotactic factor that stimulates proliferation, dissociation, migration, and invasion in a wide variety of tumor cells, including gastric cancer[3-5]. For many years, CXCR4 was believed to be the only receptor for CXCL12. However, several recent reports have provided evidence that CXCR7 (RDC-1) is an identified chemokine receptor that shares the same ligand (CXCL12) as CXCR4. CXCL12 binds to CXCR7 with greater affinity than CXCR4 (Kd = 0.4 nmol/L vs 3.6 nmol/L)[2]. In humans, CXCR7 is expressed in embryonic neuronal and heart tissue, some hematopoietic cells, and activated endothelium[6,7], but on few other normal cell types. Moreover, CXCR7 is expressed in various cancers, including breast cancer[8], lung cancer[9], and glioma[10], and was shown to promote the growth and metastasis of various tumor models[9,10]. The main ligand for CXCR7 is CXCL12, which binds to CXCR7 with high affinity, but CXCR7 may also bind the alternative ligand CXCL11 with low affinity.
Although CXCR7 is expressed by many different tumors, studies of CXCR7 expression in gastric cancer are few in number. Zhi et al[11] and Ma et al[12] have reported that CXCR7 transcripts have been detected in gastric cancer cells, including MGC803, SGC7901 and BGC823 cells, and Lee et al[5] reported that CXCR7 was differentially expressed in gastric adenocarcinoma tissues. However, most of the studies concerning CXCL12 and CXCR7 have been conducted in vitro, and the definitive pathophysiological functions of the CXCR7/CXCL12 axis in human gastric cancer require further research.
In this study, we investigated the expression of CXCR7 and CXCL12 in gastric tissues, normal gastric mucosa, lymph nodes and liver tissues. Using a combination of overexpression and RNA interference approaches, we precisely interrogated the role of CXCR7 in gastric cancer cell growth, migration and invasion in vitro.
The study included surgically resected specimens from 160 patients (72 men and 88 women, aged 65.7 ± 11.4 years) with gastric cancer. All of the patients underwent gastrectomy at the Tianjin Medical University Cancer Institute and Hospital. Non-tumoral gastric tissues were obtained at least 5 cm from the tumor at the same time. The cases were almost evenly divided between the two major types of gastric cancer: intestinal (120 patients) and diffuse (40 patients). These two types are defined by the Lauren’s histological classification[13]. According to the Union for International Cancer Control tumornodemetastasis (TNM) classification[14], cancers were classified as pT1 + T2 (n = 66) and pT3 + pT4 (n = 94), with positive nodal involvement in 96 cases (all confirmed by histopathological examination) and 30 cases having liver metastasis at the time of gastrectomy (confirmed by either histopathological examination or computed tomography). The lymph nodes around the stomach did not have metastasis in 64 cases. Twenty-nine liver tissues with no metastasis came from resected specimens of non-neoplastic diseases, and 29 liver metastasis tissues were from patients with intestinal-type gastric cancer (after the imaging diagnosis of liver metastasis of gastric cancer, one of the 30 patients refused to undergo fine-needle aspiration). Patients enrolled in the study had not received any chemo- or radiotherapy before diagnosis. Routine chemotherapy had been given to the patients with an advanced-stage disease after operation, but no radiation treatment was performed in any of patients included in our study. Patients were excluded if they had previously been exposed to any targeted therapy, chemotherapy, radiotherapy, or intervention therapy for gastric cancer.
The human recombinant CXCL12 and the mouse anti-human CXCR7 monoclonal antibody were obtained from Dako Company. CXCR7-specific siRNA and CXCR7 overexpressing vector were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). The CCK-8 reagent kit was purchased from Sigma (United States). Total RNA extraction kits (RNAfast200) were purchased from Fastagen Biotechnology (Shanghai); reverse transcription kits were purchased from TaKaRa (Japan). PCR primers were synthesized by Shanghai Bioengineering & Technology Services. Millicell small chambers were purchased from Millipore (United States); Matrigel and MTS kits were purchased from BD Biosciences (United States). The PCR amplification apparatus was produced by Gene Company; all of the primers used in RT-PCR were designed and synthesized by Beijing Aoke.
Human gastric cancer SGC-7901 cells were maintained under standard conditions (37 °C and 5% CO2) in tissue culture flasks and were grown in minimum essential medium supplemented with 10% fetal bovine serum (FBS). Cells in the logarithmic growth phase were used in all experiments.
Immunohistochemical staining was performed using the Ultra Tek HRP and anti-CXCR7 antibody according to the manufacturer’s instructions. In brief, sections were prepared from gastric cancer tissue blocks, mounted on charged slides with APES (Sigma), and fixed for 1-2 h at 60 °C before staining. Next, the sections were deparaffinized in xylene and rehydrated in graded alcohol solutions. After antigen retrieval by heating (95 °C) in citrate buffer (pH 6) for 15 min, endogenous peroxidase was blocked by the treatment of sections with 3% hydrogen peroxidase for 10 min. After blocking with 2% bovine serum albumin (BSA) for 10 min, the slides were incubated with anti-CXCR7 antibody (1:200) diluted in antibody diluents (S3022; Dako) overnight in humid chamber at 4 °C. The slides were washed and then incubated with the anti-mouse biotinylated secondary antibody for 20 min, followed by incubation with HRP-conjugated streptavidin for 20 min. The slides were washed and treated with 3,3’-diaminobenzidine (DAB) chromogen for 5 min and counterstained with Mayer’s hematoxylin, followed by mounting.
The cell membrane or cytoplasm showed light yellow or brown yellow staining, and five randomly selected high-magnification (× 400) fields were assessed using the following scoring scales. The range of positive cells was scored as follows: 0, no positive cells; 1, 1%-29%; 2, 30%-59%; 3, ≥ 60% staining. The staining intensity was scored as: 0, negative; 1, mild yellow staining; 2, moderate brown staining; or 3, dark brown staining. According to the product of the range of positive cells and staining intensity score, (-) negative expression referred to a score < 2 and (+) positive expression referred to a score ≥ 2.
Human gastric cancer SGC-7901 cells were cultured in RPMI supplemented with 10% FBS, under the conditions of 37 °C, 5% CO2, and saturated humidity. The human CXCR7 sequence was digested out from the pcDNA 3.1 + plasmid (kindly provided by ChemoCentryx, Mountain View, CA, United States). CXCR7 siRNA (sc-35421) was purchased from Santa Cruz Biotechnology. Transfections were performed using Lipofectamine 2000 according to the manufacturer’s instructions. The samples were treated with EndoFectinTM, and the CXCR7 overexpression cell line and CXCR7 knockdown cell line were established. The following groups were included: group A: blank control group (Control), blank vector group (Vector), and CXCR7 overexpression group; group B: blank control group (Control), blank vector group (Vector), and CXCR7 knockdown group.
Cells were cultured to reach 70%-80% confluence in six-well plates. Total RNA was extracted from the cells using the Ultrapure RNA Kit (CWbio Co., Ltd, Cat. #CW0581) according to the manufacturer’s instructions, and the gene expression was measured. The reaction temperature was 94 °C. After 5 min of denaturation, 40 cycles of amplification were performed using the ABI 7500 real time PCR system; each cycle consisted of predenaturation at 95 °C for 10 min, denaturation at 95 °C for 15 s and extension at 60 °C for 60 s. The reaction was activated at 72 °C for 10 min, and then terminated at 4 °C. GAPDH was used as an internal control. Chemi Image 5500 automated electrophoresis gel image analyzer was used to determine the relative mean gray values (A) of the target product and β-actin internal control; the expression index (I) of the target product mRNA was calculated using the formula: I = Aproduct/A β-actin.
Cells were cultured to reach 70%-80% confluence in six-well plates, and then the cells were digested and collected. Whole protein was obtained using RIPA lysis buffer and centrifuged at 12000 g for 10 min. The total protein concentration was measured by the bicinchoninic acid method. Next, 100 mg of protein lysates were separated by 12% SDS-PAGE. The proteins were transferred to PVDF membranes and the membranes were blocked with 5% skimmed milk with PBST containing 0.05% Tween 20 at room temperature for 2 h. The primary antibody (CXCR7, 1:200) was added and incubated at room temperature for 2 h. The membrane was washed with PBST three times and then incubated with the secondary antibody for 1 h. The immunoreactive bands were washed and observed. α-Actin was used as an internal control.
Following intervention, the cells were digested with trypsin, prepared into 5 × 103 cells/well suspensions with serum-free medium, and then inoculated into a 96-well culture plate at 200 μL per well, followed by the addition of 20 μL of CCK-8 solution and 100 ng/mL CXCL12. The plate was returned to a 5% CO2 incubator at 37 °C with saturation humidity for 0 h, 48 h, or 96 h. Finally, the plate was placed in an enzyme-linked immunoassay analyzer, and the absorbance value (OD) of each well was measured at 450 nm. Each group had six wells; the cell proliferation values of each group were calculated.
The cell migration assay was conducted as previously described. After incubation for 6 h, the growth medium was changed to basal medium with CXCL12 (100 ng/mL). Twenty-four hours later, the wounds were observed using bright-field microscopy, and multiple images were taken at areas flanking the intersections of the wound and marker lines at the start and end of the experiment. The gap distance of the wound was measured at three different sites using Photoshop software, and the data were normalized to the average of the control. Graphs were plotted against the percentage of the migration distance the cells moved before and after treatment.
Cell invasion in response to CXCL12 was assayed in the Biocoat Matrigel invasion chamber (Becton Dickinson, United States) using an 8-μm porosity polycarbonate filter membrane that was coated with Matrigel. The Transwell chamber was pre-cooled at 4 °C. Next, the upper chamber was evenly laid with 20 mL of Matrigel and was incubated at 37 °C for 3 h. Approximately 3 × 105 cells were added into the upper chamber, and 600 mL of medium with 0.1% BSA was added into the lower chamber with fibronectin (50 mg/mL). Next, the cells were cultured at 37 °C for 24 h. The cells were fixed on the upper layer of the membrane by formalin. The number of invasive cells was determined by counting the hematoxylin-stained cells. For quantification, the cells were counted under a microscope in five fields (up, down, median, left, and right. × 200).
SPSS17.0 software was used for data processing. Measurement data are expressed as the mean ± SD and were compared using analysis of variance. Pair-wise comparisons between groups were performed using the Student-Newman-Keuls method. The above hypothesis test was two-sided; associations between expression levels in gastric cancer and clinicopathological features were determined using a χ2-test. The variables considered for the univariate analysis consisted of patient-related and tumor-related variables. Pearson correlation analysis was used for correlation analysis. P < 0.05 was considered to be statistically significant.
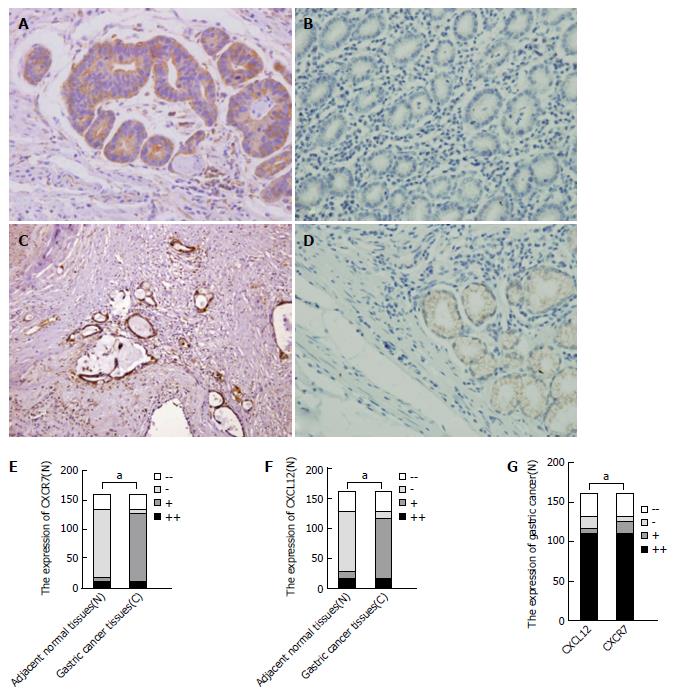
To ascertain whether the expression of CXCL12 and CXCR7 proteins is elevated in gastric cancer tissues, we first evaluated CXCL12 and CXCR7 expression by immunohistochemical analyses in tumor tissues and normal gastric tissues. In cancer tissues, CXCR7 was highly expressed (Figure 1A), with a positive expression rate of 78.75% (126/160). CXCL12 was also highly expressed in gastric cancer (Figure 1C), with an expression rate of 68.13% (109/160). But in normal gastric tissue, CXCL12 and CXCR7 were expressed at a very low level (Figure 1B and D). CXCL12 and CXCR7 expression was significantly higher in cancer tissues than in normal tissues (P = 0.011, P = 0.011) (Figure 1E and F). The expression of CXCL12 and CXCR7 was correlative in gastric cancer tissue (P = 0.000, Figure 1G). We also observed CXCR7 staining in inflammatory cells and some parts of mesenchymal tissue. CXCR7 was detected in tumor-associated blood vessels in nearly all specimens of gastric cancer, but not in blood vessels from nonmalignant tissues. In gastric carcinoma, CXCR7 expression in the gastric cancer cells within the lumen of lymph and blood vessels was strongly positive, and stronger than the expression intensity in gastric cancer tissue itself.
In gastric carcinoma, the expression of CXCL12 and CXCR7 was related to tumor size, depth of invasion, Lauren’s classification of the tumor, lymph node metastasis, and clinical stage. We did not observe any other association between CXCR7 expression and other clinical findings such as age, gender, differentiation (Table 1). Analysis of CXCL12+CXCR7+, CXCL12+CXCR7-/ CXCL12-CXCR7+, and CXCL12-CXCR7- gastric carcinoma tissues showed that CXCRL12+CXCR7+ gastric cancer cells were more prone to lymph node and liver metastasis, and they were positively correlated with tumor size, depth of invasion and clinical stage, suggesting that CXCL12+CXCR7+ cells are more likely to grow and metastasize in gastric cancer (Table 2).
| Factor | n | CXCR12 expression | CXCR7 expression | ||||||
| - | + | χ2 | P value | - | + | χ2 | P value | ||
| Gender | 0.008 | 0.930 | 1.643 | 0.200 | |||||
| Male | 72 | 20 | 52 | 12 | 60 | ||||
| Female | 88 | 25 | 63 | 22 | 66 | ||||
| Age (yr) | 0.040 | 0.841 | 2.497 | 0.114 | |||||
| < 56 | 66 | 18 | 48 | 10 | 56 | ||||
| ≥ 56 | 94 | 27 | 67 | 24 | 70 | ||||
| Tumor diameter (cm) | 5.006 | 0.025 | 5.694 | 0.017 | |||||
| < 3.5 | 70 | 26 | 44 | 21 | 49 | ||||
| ≥ 3.5 | 90 | 9 | 25 | 13 | 77 | ||||
| Lauren’s classification | 5.452 | 0.020 | 4.034 | 0.045 | |||||
| Diffuse type | 40 | 17 | 23 | 13 | 27 | ||||
| Intestinal type | 120 | 28 | 92 | 21 | 99 | ||||
| Differentiation | 4.718 | 0.030 | 0.107 | 0.803 | |||||
| Well-moderately | 78 | 23 | 55 | 13 | 65 | ||||
| Poorly | 42 | 5 | 37 | 8 | 34 | ||||
| Depth of invasion | 7.057 | 0.008 | 5.502 | 0.019 | |||||
| T1 + T2 | 66 | 26 | 40 | 20 | 46 | ||||
| T3 + T4 | 94 | 19 | 75 | 14 | 80 | ||||
| Clinical stages | 3.944 | 0.047 | 5.125 | 0.024 | |||||
| I + II | 69 | 25 | 44 | 22 | 54 | ||||
| III + IV | 91 | 20 | 71 | 12 | 72 | ||||
| Lymph node metastasis | 10.435 | 0.001 | 10.980 | 0.001 | |||||
| + | 96 | 18 | 78 | 12 | 84 | ||||
| - | 64 | 27 | 37 | 22 | 42 | ||||
| Liver metastasis | 3.606 | 0.044 | 4.402 | 0.027 | |||||
| + | 29 | 3 | 26 | 1 | 28 | ||||
| - | 91 | 25 | 66 | 18 | 73 | ||||
| Factor | n | CXCR12+CXCR7+ | CXCL12+CXCR7-/CXCL12-CXCR7+ | CXCR12-CXCR7- | r | P value |
| Diameter (cm) | 0.205 | 0.009 | ||||
| < 3.5 | 70 | 41 | 11 | 18 | ||
| ≥ 3.5 | 90 | 69 | 11 | 10 | ||
| Depth of invasion | 0.240 | 0.002 | ||||
| T1 + T2 | 66 | 37 | 11 | 18 | ||
| T3 + T4 | 94 | 73 | 11 | 10 | ||
| Clinical stages | 0.200 | 0.001 | ||||
| I + II | 69 | 41 | 9 | 19 | ||
| III + IV | 91 | 69 | 13 | 9 | ||
| Lymph node metastasis | 0.310 | 0.000 | ||||
| - | 64 | 34 | 11 | 19 | ||
| + | 96 | 86 | 11 | 9 | ||
| Liver metastasis | 0.227 | 0.016 | ||||
| - | 91 | 60 | 16 | 15 | ||
| + | 29 | 26 | 2 | 1 |
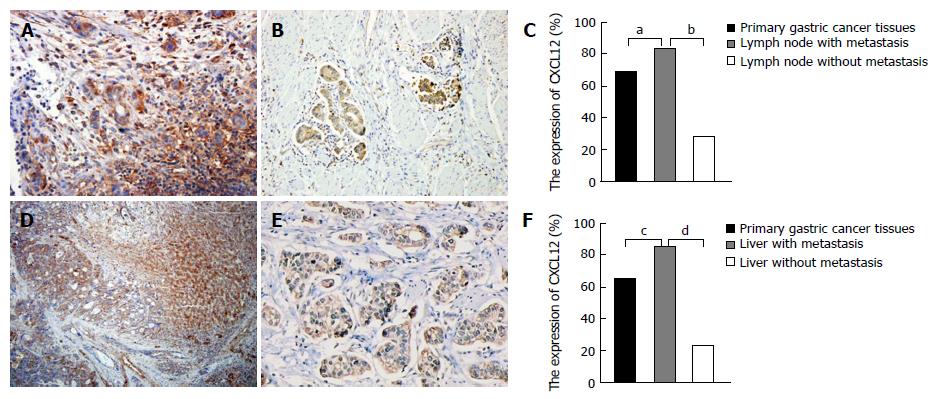
Among the 160 lymph nodes that we collected, 96 had cancer metastasis and the remaining nodes were normal. The expression of CXCL12 was also significantly higher in the lymph nodes with metastasis than in the lymph nodes without (χ2 = 49.313, P = 0.000). And the expression of CXCL12 in lymph node metastasis was higher than in primary gastric cancer tissues (χ2 = 6.669, P = 0.010 )(Figure 2A-C). In metastatic lymph nodes, the expression of CXCL12 was also expressed in the peripheral inflammatory cells.
Among the 58 livers that we collected, 29 livers had cancer metastasis and the remaining livers were normal. The expression of CXCL12 was also significantly higher in the livers with metastasis than in their normal counterparts (χ2 = 5.317, P = 0.021). And the expression of CXCL12 in liver metastasis of gastric cancer cells was higher than that in primary type of gastric cancer (χ2 = 25.379, P = 0.000) (Figure 2D-F). The expression of CXCL12 was also found in the normal liver tissues around the liver metastasis.
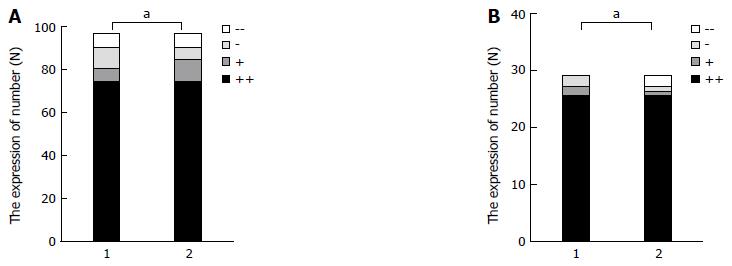
Pearson correlation analysis showed that the positive expression of CXCL12 in lymph node and liver metastasis of gastric cancer was correlated with the positive expression of CXCR7 in gastric cancer (r = 0.338, P = 0.000; r = 0.629, P = 0.000) (Figure 3A and B).
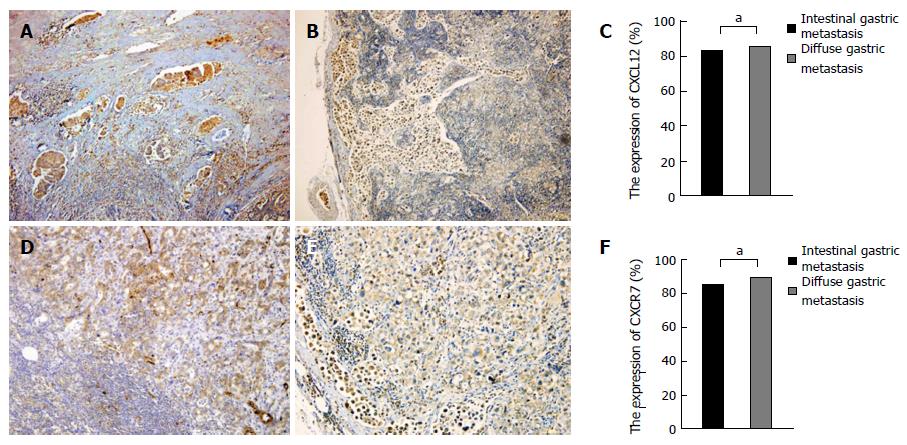
Based on the above experimental results, it is shown that CXCL12/CXCR7 can promote lymph node and liver metastasis of gastric cancer. Our previous work found that CXCL12/CXCR7 biological axis can promote lymph node and liver metastasis of intestinal type gastric cancer by immunohistochemistry, so we studied the difference in CXCL12/CXCR7 expression between lymph node metastasis with intestinal type gastric cancer and that with diffuse type gastric cancer. Statistical analysis showed that there was no statistical difference in the expression of CXCL12/CXCR7 in these two groups (χ2 = 0.042, P = 0.837;χ2 = 0.265, P = 0.606) (Figure 4A and B).
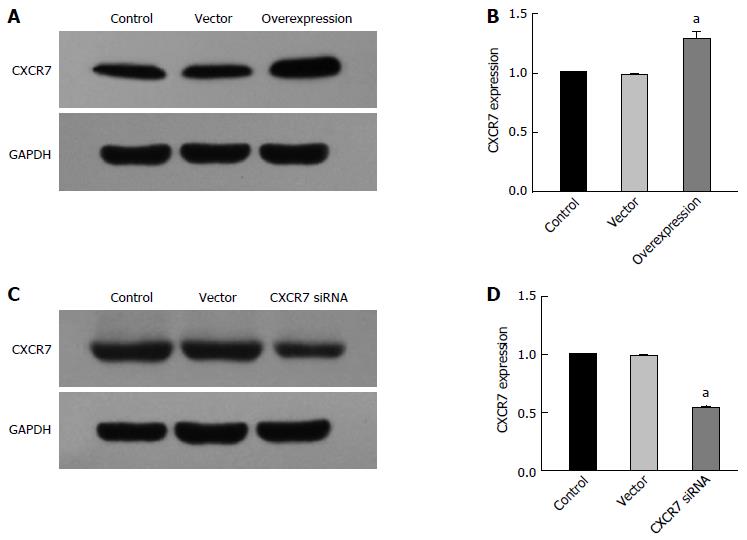
In order to study the potential role of CXCR7 in SGC-7901 cells, we established the CXCR7 overexpressing vector and CXCR7 siRNA vector and then scrambled the overexpression and siRNA vector used to transfect SGC-7901 cells. Two groups were built: group A: control group (Control), blank vector group (Vector), and CXCR7 overexpression group; group B: control group (Control), blank vector group (Vector), and CXCR7 knockdown group. The result was tested using RT-PCR and Western blot. As shown in Figure 5A, CXCR7 mRNA level was increased in CXCR7 overexpressing cells, compared with the control group (P = 0.000) and the blank vector group (P = 0.000). In contrast, CXCR7 mRNA level was reduced in CXCR7 siRNA transfected cells (P = 0.000, P = 0.000) (Figure 5C). Like RT-PCR results, in CXCR7 overexpressing cells the expression level of CXCR7 protein was increased, but the expression level of CXCR7 protein were reduced in CXCR7 siRNA transfected cells (Figure 5B and D). These results demonstrated that the expression of CXCR7 was specifically up-regulated/silenced in SGC-7901 cells.
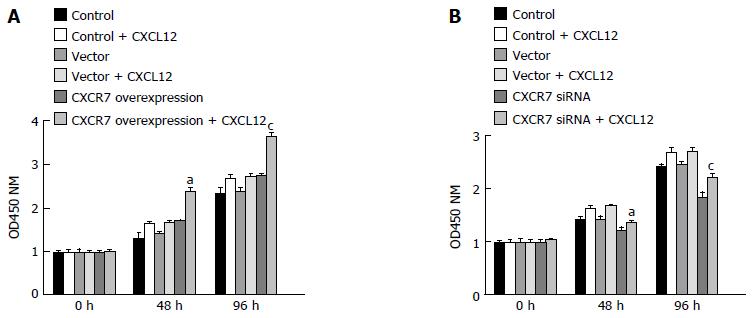
CCK-8 experiments showed that cells overexpressing CXCR7 proliferated more rapidly than control cells, whereas cells with depleted CXCR7 grew more slowly than control cells (Figure 6). Moreover, the CXCR7-transfected SGC-7901 cells showed a substantial increase in cell number in the presence of CXCL12 (100 ng/mL), compared with non-treated ones; but proliferation was not increased following CXCL12 stimulation in the cells transfected with the blank vector. These results indicated that CXCL12 engagement to CXCR7 can induce proliferation.
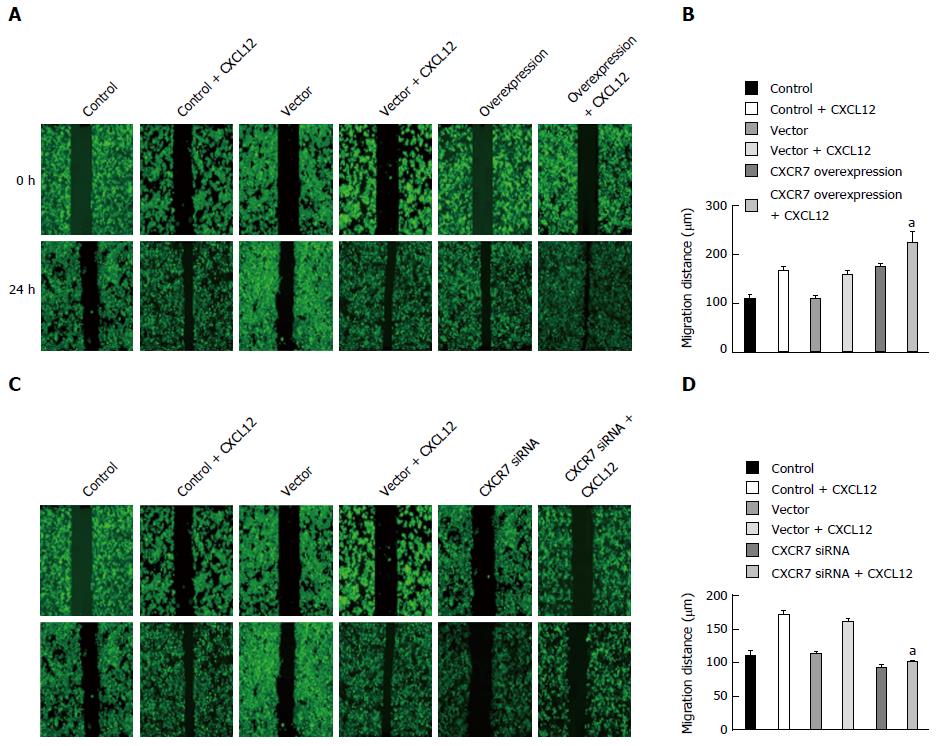
Besides enhanced growth advantage of tumor cells, increased cell invasion is a determinant hallmark of metastatic tumor cells. Thus, we sought to explore the influence of CXCR7 on cell migration. Wound healing assay showed that ectopic overexpression of CXCR7 in SGC7901 cells significantly enhanced cell migration induced by CXCL12 compared with the Control cells and Vector cells (Figure 7A, P = 0.011); in contrast, the reverse effects were observed when CXCR7 was silenced in SGC7901 cells (Figure 7B, P = 0.004).
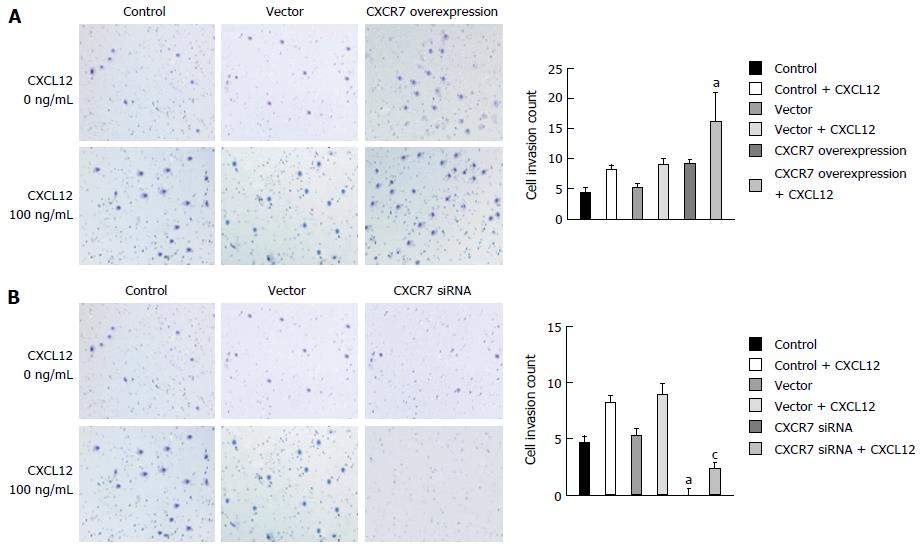
Invasion is also one of the important steps in tumor metastasis. The CXCR7/CXCL12 interaction was reported to regulate invasive and metastatic behavior of several tumors. We measured the effect of CXCR7 overexpression and silencing on the invasion ability of SGC-7901 cells in vitro by Transwell assay. As shown in Figure 8A and B, SGC-7901 cells spontaneously invaded through artificial basement membrane in the absence of CXCL12. In addition, we found that CXCL12 induced a significant increase of cancer cell invasion through Matrigel, and CXCR7 overexpressing cells displayed increased invasive ability compared with the Control cells and Vector cells. Cells with CXCR7 knockdown displayed decreased invasive ability compared with the control cells and Vector cells (Figure 8). Taken together, these findings indicate that CXCR7 overexpression could potently enhance the invasive ability of SGC-7901 cells induced by CXCL12 and that silencing of CXCR7 inhibits the invasive behavior of the cells.
Chemokine and chemokine receptor pairs have been identified to play pivotal roles in cancer initiation and progression. CXCL12 and its receptors (CXCR4 and CXCR7) are important members. They are implicated in several aspects, including the migration, adhesion, proliferation and survival of tumor cells, and the formation of tumor-associated vessels and invasion[15]. CXCR7, initially named receptor Dog cDNA1/RDC1, is a second receptor for CXCL12, and it can bind chemokines CXCL11/ITAC and macrophage migratory inhibitory factor (MIF)[16,17]. Due to the mutation at the “DRAILIV” motif[18], CXCR7 cannot activate G proteins that are associated with typical chemokine receptors. CXCR7 has been proposed as a “decoy” receptor, mainly functioning to shape the CXCL12 gradient for the guidance of cell migration in different models[19,20]. By contrast, other studies provided evidence that CXCR7 can activate downstream signal transduction molecules and cytokine production, promote the survival of tumor cells by preventing apoptosis, and impact cell adhesion and invasion through complex signaling processes[9,10,21].
To the best of our knowledge, this study is the first demonstrating that CXCR7 was widely expressed in human gastric cancer tissue by immunohistochemistry and that the expression of CXCR7 was increased compared with that in normal gastric tissues. CXCR7 was present on tumor-associated blood vessels but not on the normal vasculature. Immunohistochemical staining of human breast and lung cancer tissue also revealed extensive CXCR7 expression on tumor-associated blood vessels and cancer cells[9]. Additionally, in hepatocellular carcinoma, the suppression of CXCR7 expression by RNA interference impairs in vitro cellular VEGF secretion and angiogenesis[22]. Thus, CXCR7 may contribute to gastric cancer development by regulating angiogenesis; in human hepatocellular carcinoma cells, the overexpression of CXCR7 induces the angiogenic capacity via the AKT signaling pathway[23]. In gastric cancer cells, CXCR7 is highly expressed, but CXCR7 is poorly expressed in normal gastric cells. This result suggests that CXCR7 might play a role in gastric tumorigenesis. In our in vitro studies, we found that the proliferation of gastric cancer cells was significantly increased in the presence of CXCR7 overexpression and showed significant profile responses to CXCL12. Importantly, silencing CXCR7 can effectively suppress this proliferative capacity of gastric cancer cells. This suggested that CXCR7/CXCL12 can promote the proliferation of gastric cancer cells, and the anti-proliferative effect of the down-regulation of CXCR7 may be an important factor in the growth mechanism. Based on our experimental results, we believe that CXCR7/CXCL12 can promote the growth of gastric cancer cells. These findings are similar to observations in hepatocellular and breast carcinoma models[8,24]. However, in thyroid cancer K1 cells and TFF3-dependent activation of cells, the overexpression of CXCR7 had no effect on cell proliferation[25,26]. Different studies have described that in different tumor cell types, depending on the differentiation status and environment, CXCR7 may play a different role. In prostate cancer, IL-8-regulated CXCR7 stimulates EGFR signaling to promote prostate cancer growth, and the growth-promoting activity does not require its ligands[27]. However, in hepatocellular carcinoma, CXCR7 activates the ERK pathway to mediate cell proliferation. The mechanisms by which CXCR7 participates in the growth of gastric cancer cells warrant further investigation, and the specific mechanism will be further studied in our future work. Additionally, we found that the expression of CXCR7 in the cancer cells in metastatic vascular lumen was higher than that in gastric carcinoma tissues, suggesting that CXCR7 may have a role in the metastasis of gastric cancer.
Our clinicopathological study revealed that CXCR7 expression was significantly positive in gastric cancer with a high tumor stage and lymph node and liver metastases. The larger the tumor diameter and infiltration depth, the stronger the expression of CXCR7. Regarding the different clinical stages, the expression level of CXCR7 in stage III + IV was significantly higher than that in stage I + II gastric cancer. These results suggested that the expression of CXCR7 might be involved in the invasion and metastasis of gastric cancer cells, and CXCR7-positive gastric cancer may have a strong migratory potential. CXCL12+CXCR7+ gastric cancer tissues are more prone to lymph node and liver metastases compared to CXCL12+CXCR7-/CXCL12-CXCR7+ and CXCL12-CXCR7- gastric cancer tissues. A tumor diameter greater than 3.5 cm, depth T3 + T4, and stage III + IV showed stronger CXCL12+CXCR7+ expression in gastric cancer. Our results from the in vitro experiments appeared to support this hypothesis. In this study, we also found that CXCR7, by binding to CXCL12, can promote invasion in SGC7901 cells, and CXCR7/CXCL12 can promote gastric cancer cell invasion, confirming the results of a previous study in other parts of tissues and cells. Additionally, in prostate cancer, CXCR7 potentially promoted invasion through its downstream targets CD44 and cadherin-11[28].
It has been shown that higher levels of CXCL12 in target organs such as the liver or lymph nodes attract and recruit cancer cells, which subsequently form liver or lymph node metastases[29]. In gastric cancer, the CXCR4/CXCL12 signaling axis played an important role in the process of lymph node metastasis and liver metastasis[15,30]. Additionally, CXCR4-expressing gastric carcinoma cells are preferentially attracted to the peritoneal cavity, where its ligand, SDF-1, is produced abundantly by peritoneal mesothelial cells[31]. Unlike CXCR4, however, CXCR7 fails to signal through Gαi proteins and does not facilitate cell migration in response to CXCL12; it appears to act as a CXCL12 scavenger controlling the chemokine levels in favor of CXCR4-mediated chemotaxis[32]. Additionally, some reports have found that CXCR7 could not mediate cancer cell migration[33,34]. However, a significant increase in CXCL12 was found in lymph nodes and livers with cancer cell metastasis compared with that in normal lymph nodes and livers, and the positive expression of CXCL12 in lymph node and liver metastasis of gastric cancer was consistent with the positive expression of CXCR7 in gastric cancer. This confirmed that cancer cells with highly expressed CXCR7 can migrate towards a CXCL12 gradient established in specific target organs. Additionally, the overexpression of CXCR7 in SGC7901 cells significantly enhanced cell migration by binding to CXCL12 in vitro. By contrast, the reverse effects were observed when CXCR7 was silenced in SGC7901 cells. Our studies strongly supported that the CXCR7/CXCL12 signaling axis could promote migration in gastric cancer. CXCR7, which cannot signal directly through G protein-linked pathways, can nevertheless affect cellular signaling networks by forming a heteromeric complex with CXCR4. The CXCR4-CXCR7 heterodimer complex recruits â-arrestin, resulting in the preferential activation of â-arrestin-linked signaling pathways[35]. In late neural progenitor cells (NPCs), CXCR7 mediates migration to CXCL12 in the absence of CXCR4 through extracellular signal-regulated kinases (ERK) 1/2[36] but TFF3 induced cell migration independently from the ERK1/2 signaling pathway[26].
In conclusion, the results in this study indicate that CXCR7 was highly expressed in gastric cancer tissue and was increased with tumor clinical stage. The CXCR7/CXCL12 signaling axis appears to be involved in the lymph node and liver metastasis of gastric cancer. Based on these results, specific therapies with chemokine receptor antagonists could be helpful in the treatment of patients with gastric cancer metastasis.
Gastric cancer is one of the most common malignant tumors. Most deaths from gastric cancer are caused by treatment failure due to metastasis, of which lymph node and liver metastases are the most common cause. Therefore, inhibition of gastric cancer metastasis is thought to be an important therapeutic strategy. However, the molecular mechanisms involved in this process have not been fully elucidated.
Many studies have shown that CXC chemokine receptor-7 (CXCR7) was expressed in many types of cancer cells. The CXCR7/and stromal cell-derived factor-12 (CXCL12) axis plays a major role in survival, proliferation, migration and adhesion of many kinds of tumor cells. However, little is known about the effect of CXCL12/CXCR7 on the process of gastric cancer. Thus, the definitive pathophysiological function of the CXCR7/CXCL12 axis in lymph node and liver metastasis of gastric cancer needs further research.
Little is known about the effect of CXCL12/CXCR7 on the process of gastric cancer. This study found that CXCR7 is expressed in gastric cancer and CXCR7/CXCL12 axis is involved in lymph node and liver metastasis of gastric cancer. Furthermore, this in vitro study suggested that silencing of the CXCR7 gene suppressed proliferation, migration and invasion of gastric cancer cells.
By understanding how the CXCR7/CXCL12 axis is involved in lymph node and liver metastasis of gastric cancer, this study could represent a future strategy for therapeutic intervention in patients with lymph node and liver metastasis of gastric cancer.
Chemokines are a family of small heparin-binding and secretory proteins, and through interactions with their corresponding receptors, they can control and activate many types of cells. According to the position of the four conserved cysteine residues in the amino acid sequence, they are classified into four groups: CXC, CX3C, CC and C. CXCL12 is a member of the CXC subfamily. For a long time, CXCR4 was thought to be the only receptor for CXCL12. However, several recent reports have provided evidence that CXCR7 is another receptor of CXCL12. CXCL12 binds to CXCR7 with greater affinity than CXCR4.
The authors reported that the CXCR7/CXCL12 axis could play an important role in metastasis of gastric carcinoma. They concluded that the CXCR7/CXCL12 axis was involved in the lymph node and liver metastasis of gastric cancer. This study was interesting and excellent, and this article was well written.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aoyagi K, Kouraklis G S- Editor: Yu J L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Abe P, Mueller W, Schütz D, MacKay F, Thelen M, Zhang P, Stumm R. CXCR7 prevents excessive CXCL12-mediated downregulation of CXCR4 in migrating cortical interneurons. Development. 2014;141:1857-1863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996-7007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 601] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | He H, Wang C, Shen Z, Fang Y, Wang X, Chen W, Liu F, Qin X, Sun Y. Upregulated expression of C-X-C chemokine receptor 4 is an independent prognostic predictor for patients with gastric cancer. PLoS One. 2013;8:e71864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Ishigami S, Natsugoe S, Okumura H, Matsumoto M, Nakajo A, Uenosono Y, Arigami T, Uchikado Y, Setoyama T, Arima H. Clinical implication of CXCL12 expression in gastric cancer. Ann Surg Oncol. 2007;14:3154-3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Lee HJ, Song IC, Yun HJ, Jo DY, Kim S. CXC chemokines and chemokine receptors in gastric cancer: from basic findings towards therapeutic targeting. World J Gastroenterol. 2014;20:1681-1693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 71] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Tiveron MC, Boutin C, Daou P, Moepps B, Cremer H. Expression and function of CXCR7 in the mouse forebrain. J Neuroimmunol. 2010; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Yu S, Crawford D, Tsuchihashi T, Behrens TW, Srivastava D. The chemokine receptor CXCR7 functions to regulate cardiac valve remodeling. Dev Dyn. 2011;240:384-393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Wani N, Nasser MW, Ahirwar DK, Zhao H, Miao Z, Shilo K, Ganju RK. C-X-C motif chemokine 12/C-X-C chemokine receptor type 7 signaling regulates breast cancer growth and metastasis by modulating the tumor microenvironment. Breast Cancer Res. 2014;16:R54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci USA. 2007;104:15735-15740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 10. | Hattermann K, Held-Feindt J, Lucius R, Müerköster SS, Penfold ME, Schall TJ, Mentlein R. The chemokine receptor CXCR7 is highly expressed in human glioma cells and mediates antiapoptotic effects. Cancer Res. 2010;70:3299-3308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 11. | Zhi Y, Chen J, Zhang S, Chang X, Ma J, Dai D. Down-regulation of CXCL12 by DNA hypermethylation and its involvement in gastric cancer metastatic progression. Dig Dis Sci. 2012;57:650-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Ma DM, Luo DX, Zhang J. SDF-1/CXCR7 axis regulates the proliferation, invasion, adhesion, and angiogenesis of gastric cancer cells. World J Surg Oncol. 2016;14:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [PubMed] |
| 14. | Sobin LH. TNM, sixth edition: new developments in general concepts and rules. Semin Surg Oncol. 2003;21:19-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 163] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | Zhao BC, Wang ZJ, Mao WZ, Ma HC, Han JG, Zhao B, Xu HM. CXCR4/SDF-1 axis is involved in lymph node metastasis of gastric carcinoma. World J Gastroenterol. 2011;17:2389-2396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 54] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 16. | Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201-2213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 941] [Cited by in RCA: 1027] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 17. | Tarnowski M, Grymula K, Liu R, Tarnowska J, Drukala J, Ratajczak J, Mitchell RA, Ratajczak MZ, Kucia M. Macrophage migration inhibitory factor is secreted by rhabdomyosarcoma cells, modulates tumor metastasis by binding to CXCR4 and CXCR7 receptors and inhibits recruitment of cancer-associated fibroblasts. Mol Cancer Res. 2010;8:1328-1343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Thelen M, Thelen S. CXCR7, CXCR4 and CXCL12: an eccentric trio? J Neuroimmunol. 2008;198:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Venkiteswaran G, Lewellis SW, Wang J, Reynolds E, Nicholson C, Knaut H. Generation and dynamics of an endogenous, self-generated signaling gradient across a migrating tissue. Cell. 2013;155:674-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Mahabaleshwar H, Boldajipour B, Raz E. Killing the messenger: The role of CXCR7 in regulating primordial germ cell migration. Cell Adh Migr. 2008;2:69-70. [PubMed] |
| 21. | Lin L, Han MM, Wang F, Xu LL, Yu HX, Yang PY. CXCR7 stimulates MAPK signaling to regulate hepatocellular carcinoma progression. Cell Death Dis. 2014;5:e1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Chen Y, Teng F, Wang G, Nie Z. Overexpression of CXCR7 induces angiogenic capacity of human hepatocellular carcinoma cells via the AKT signaling pathway. Oncol Rep. 2016;36:2275-2281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 24. | Xue TC, Chen RX, Han D, Chen J, Xue Q, Gao DM, Sun RX, Tang ZY, Ye SL. Down-regulation of CXCR7 inhibits the growth and lung metastasis of human hepatocellular carcinoma cells with highly metastatic potential. Exp Ther Med. 2012;3:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Zhu X, Bai Q, Lu Y, Lu Y, Zhu L, Zhou X, Wu L. Expression and function of CXCL12/CXCR4/CXCR7 in thyroid cancer. Int J Oncol. 2016;48:2321-2329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Dieckow J, Brandt W, Hattermann K, Schob S, Schulze U, Mentlein R, Ackermann P, Sel S, Paulsen FP. CXCR4 and CXCR7 Mediate TFF3-Induced Cell Migration Independently From the ERK1/2 Signaling Pathway. Invest Ophthalmol Vis Sci. 2016;57:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 27. | Singh RK, Lokeshwar BL. The IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling to promote prostate cancer growth. Cancer Res. 2011;71:3268-3277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283-4294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 367] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 29. | Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3911] [Cited by in RCA: 3965] [Article Influence: 165.2] [Reference Citation Analysis (0)] |
| 30. | Iwasa S, Yanagawa T, Fan J, Katoh R. Expression of CXCR4 and its ligand SDF-1 in intestinal-type gastric cancer is associated with lymph node and liver metastasis. Anticancer Res. 2009;29:4751-4758. [PubMed] |
| 31. | Yasumoto K, Koizumi K, Kawashima A, Saitoh Y, Arita Y, Shinohara K, Minami T, Nakayama T, Sakurai H, Takahashi Y. Role of the CXCL12/CXCR4 axis in peritoneal carcinomatosis of gastric cancer. Cancer Res. 2006;66:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 32. | Hoffmann F, Müller W, Schütz D, Penfold ME, Wong YH, Schulz S, Stumm R. Rapid uptake and degradation of CXCL12 depend on CXCR7 carboxyl-terminal serine/threonine residues. J Biol Chem. 2012;287:28362-28377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Choi YH, Burdick MD, Strieter BA, Mehrad B, Strieter RM. CXCR4, but not CXCR7, discriminates metastatic behavior in non-small cell lung cancer cells. Mol Cancer Res. 2014;12:38-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 34. | Hernandez L, Magalhaes MA, Coniglio SJ, Condeelis JS, Segall JE. Opposing roles of CXCR4 and CXCR7 in breast cancer metastasis. Breast Cancer Res. 2011;13:R128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 35. | Décaillot FM, Kazmi MA, Lin Y, Ray-Saha S, Sakmar TP, Sachdev P. CXCR7/CXCR4 heterodimer constitutively recruits beta-arrestin to enhance cell migration. J Biol Chem. 2011;286:32188-32197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 36. | Chen Q, Zhang M, Li Y, Xu D, Wang Y, Song A, Zhu B, Huang Y, Zheng JC. CXCR7 Mediates Neural Progenitor Cells Migration to CXCL12 Independent of CXCR4. Stem Cells. 2015;33:2574-2585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |