Published online Apr 28, 2017. doi: 10.3748/wjg.v23.i16.2995
Peer-review started: December 26, 2016
First decision: January 19, 2017
Revised: January 31, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 28, 2017
Processing time: 124 Days and 4.3 Hours
To examine treatment decisions of gastroenterologists regarding the choice of prescribing 5-aminosalycilates (5ASA) with corticosteroids (CS) versus corticosteroids alone for patients with active ulcerative colitis (UC).
A cross-sectional questionnaire exploring physicians’ attitude toward 5ASA + CS combination therapy vs CS alone was developed and validated. The questionnaire was distributed to gastroenterology experts in twelve countries in five continents. Respondents’ agreement with stated treatment choices were assessed by standardized Likert scale. Background professional characteristics of respondents were analyzed for correlation with responses.
Six hundred and sixty-four questionnaires were distributed and 349 received (52.6% response rate). Of 340 eligible respondents, 221 (65%) would continue 5ASA in a patient hospitalized for intravenous CS treatment due to a moderate-severe UC flare, while 108 (32%) would stop the 5ASA (P < 0.001), and 11 (3%) are undecided. Similarly, 62% would continue 5ASA in an out-patient starting oral CS. However, only 140/340 (41%) would proactively start 5ASA in a hospitalized patient not receiving 5ASA before admission. Most (94%) physicians consider the safety profile of 5ASA as very good. Only 52% consider them inexpensive, 35% perceive them to be expensive and 12% are undecided. On multi-variable analysis, less years of practice and perception of a plausible additive mechanistic effect of 5ASA + CS were positively associated with the decision to continue 5ASA with CS.
Despite the absence of data supporting its benefit, most gastroenterologists endorse combination of 5ASA + CS for patients with active moderate-to-severe UC. Randomized controlled trials are needed to assess if 5ASA confer any benefit for these patients.
Core tip: Patients with moderate-severe active ulcerative colitis are often treated with corticosteroids. Whether 5-aminosalycilates (5ASA) offer any benefit when combined with corticosteroids for these patients has not been explored. This global survey among expert gastroenterology physicians in 12 countries shows division of opinion regarding this treatment choice, but demonstrates that the majority of doctors administer corticosteroids with continued 5ASA, despite the absence of evidence supporting this combination. If this “crowd wisdom” is correct and the addition of 5ASA offers even small added benefit for these severely sick patients, this could comprise an important simple tool for improving outcomes in acute ulcerative colitis. The results of this survey call for the need for a controlled clinical trial to examine this treatment choice.
- Citation: Ben-Horin S, Andrews JM, Katsanos KH, Rieder F, Steinwurz F, Karmiris K, Cheon JH, Moran GW, Cesarini M, Stone CD, Schwartz D, Protic M, Roblin X, Roda G, Chen MH, Har-Noy O, Bernstein CN. Combination of corticosteroids and 5-aminosalicylates or corticosteroids alone for patients with moderate-severe active ulcerative colitis: A global survey of physicians' practice. World J Gastroenterol 2017; 23(16): 2995-3002
- URL: https://www.wjgnet.com/1007-9327/full/v23/i16/2995.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i16.2995
The role of corticosteroids (CS) in the treatment of moderate-to-severe exacerbation of ulcerative colitis (UC) is well established[1-3]. Many of these patients present on existing therapy with 5-aminosalycilates (5ASA), which are efficacious in mild-moderate UC. However, there are no data investigating whether the continuation of 5ASA agents in combination with CS in patients with a moderate-to-severe UC flare offers any additional benefit compared to treatment with CS alone. Arguably, 5ASA might act in concert with CS to exert an additional therapeutic benefit. Conversely, if 5ASA are of no benefit in this situation, then patients can be spared from an unnecessary drug and its unjustified expense.
Therefore, the goal of the present study was to explore the real-life practice of gastrointestinal (GI) and inflammatory bowel disease (IBD) experts with respect to prescribing 5ASA to patients with moderate-severe UC needing CS treatment. A secondary aim was to investigate factors associated with physicians’ therapeutic choices.
This was a cross-sectional survey of GI physicians in 12 countries in five continents: Australia, Brazil, Canada, China, France, Greece, Israel, Italy, Korea, Serbia, United Kingdom and United States. GI fellows were excluded, but GI specialists were eligible regardless of whether they work in a hospital or exclusively at an out-patient setting. The questionnaire was distributed by sub-investigators to respondents directly or through an additional contact (one-stage “Snow-balling” technique). We deliberately elected to distribute questionnaires directly, as this technique was previously found to result in a higher response rate[4], as opposed to low response rates often experienced by surveys conducted through mailing lists of professional societies’ members or through survey websites[5,6]. The study was approved by the Sheba Medical Center Institutional Review Board.
A prior search of the literature did not identify an existing questionnaire exploring the research question. Therefore, an English-language questionnaire was constructed, using a balanced positive and negative-phrased question structure (Reverse phrasing) to reduce response-bias (supplementary Table 1). A standardized Likert scale ranging between1-5 was used to assess the different degrees of agreement to a presented therapeutic strategy. Data about the respondent physicians’ professional background were also obtained. The questionnaire was approved by all co-investigators and was additionally examined by three external IBD experts for content validity, to ascertain whether the content of the questionnaire was appropriate and relevant to the study purpose. The questionnaire was distributed to physicians in its English format to avoid variations caused by the process of translation/back-translation into several different languages. Comprehensibility was validated by six GI physicians (two native English speakers and four non-native speakers). Further, a question inquiring about ease of English comprehensibility was inserted, and responses from individual physicians who graded comprehensibility of the questionnaire as less than “easy” or “very easy” were a-priori excluded. Additionally, a pre-planned sensitivity analysis was performed including only respondents who practice medicine in English speaking countries (United States, Canada, United Kingdom and Australia).
| Parameter | n (%) | |
| University hospital | 256 (78.8) | |
| Practice setting | Regional/district hospital | 33 (10.1) |
| Outpatient clinic | 36 (11.1) | |
| Less than 25% | 183 (55.3) | |
| IBD in clinic | 25% or more (IBD expert) | 148 (46.7) |
| Years of practice, median (IQR, range) | 10.5 (5-20, 0.5-60) | |
| Country of practice | Australia | 31 (9.1) |
| Brazil | 22 (6.5) | |
| Canada | 40 (11.8) | |
| China | 38 (11.2) | |
| France | 9 (2.6) | |
| Greece | 46 (13.5) | |
| Israel | 33 (9.7) | |
| Italy | 19 (5.8) | |
| South Korea | 20 (5.9) | |
| Serbia | 10 (2.9) | |
| United Kingdom | 19 (5.8) | |
| United States | 53 (15.6) |
Descriptive statistics were used to detail distribution of responses among the five possible grades of agreement for each question. When comparative analyses were performed, scales 1 and 2 (“Strongly agree” and “tend to agree”) were grouped together as responses endorsing the presented therapeutic decision, while a Likert response of 4 (“tend to disagree”) and 5 (“strongly disagree”) were grouped as not endorsing the proposed therapy decision. Responses of 3 (“undecided”) were separately analyzed. Sample size was determined by taking into account five variables, of which four had multiple sub items. For the resulting 15 variables, and using a subject to item ratio of 1:15, at least 225 subjects were needed to reduce the risk of over-fitting the logistic regression model[6]. Because of the global nature of this survey and absence of data on the total world number of GI specialists, power calculation for total sample size could not be performed, rendering the results exploratory. Comparisons of proportions were performed by Z test when single population was considered or by Fisher exact test when comparing proportions between two populations (e.g., responses of IBD experts versus non-experts). In the absence of definitive definition of what constitutes an IBD expert and in line with previous studies[4], respondents with ≥ 25% of their patients being IBD patients were pragmatically defined as IBD experts.
Multi-variable analysis was performed using backward logistic regression for all variables of interest. All statistics were performed using MedCalc statistical software (Marieke, Denmark). P value of < 0.05 was considered significant.
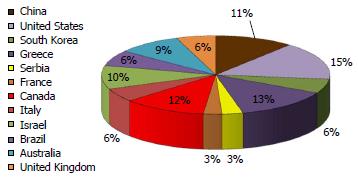
Overall, 664 questionnaires were distributed in 12 countries in five continents. Of these, 349 completed questionnaires were received, yielding a response rate of 52.6%. Nine questionnaires were excluded: Two were received from GI trainees and seven from respondents indicating only fair comprehension of the questionnaire (four from Italy, two from China and one from the United States). Thus, a total of 340 returned questionnaires were eligible for analysis. The distribution of the country of origin among eligible respondents is shown in Figure 1. The background professional characteristics of the respondents are shown in Table 1.
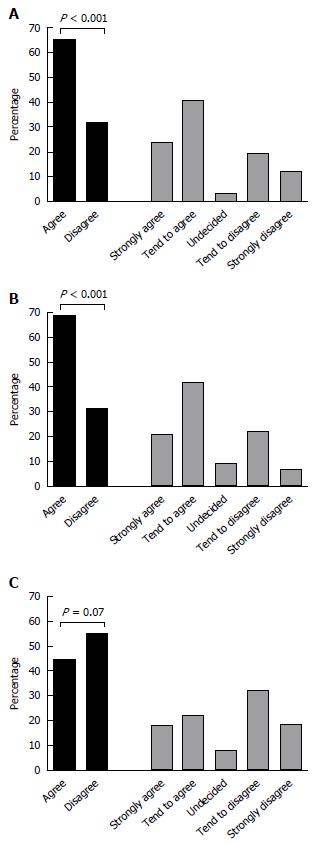
Overall, 221 (65%) of the respondents would strongly agree or tend to agree to continue 5ASA in a patient hospitalized for intravenous CS treatment due to a moderate-severe flare of UC, compared with 108 (32%) who would stop the 5ASA (P < 0.001), and 11 (3%) who were undecided. This comparison is shown in Figure 2A.
Similarly, when starting oral CS for a non-hospitalized outpatient with a mild-moderate UC flare despite optimized 5ASA treatment, the majority (212/340, 62%) of physicians would continue 5ASA, whereas 97/340 (28.5%) physicians would stop 5ASA and administer CS mono-therapy (P < 0.001; Figure 2B). In contrast, only 140/340 of the physicians would start 5ASA concurrently with CS in a patient hospitalized with UC flare who was not already on 5ASA prior to admission, compared to 172/340 who opted to treat such patients with CS mono-therapy (P = 0.07; Figure 2C).
When inquired about English language comprehensibility of the questionnaire, 284/347 (82.7%) respondents considered it to be very easy, 55 (15.8%) as easy, and seven (2%) as fairly understandable. None of the respondents rated the questionnaire as difficult or as very difficult to comprehend. As specified above, the seven respondents with only “fair” understanding of the language were excluded from further analysis. Nevertheless, to further validate the responses, a sensitivity analysis was performed including only the 143/340 physicians practicing in English-speaking countries (Australia, Canada, United States and United Kingdom). Similar to the results in the entire cohort, 64.5% of the English-speaking physicians endorsed continuing 5ASA in a hospitalized steroid-treated patient with moderate-severe UC, and 73% would continue 5ASA in an out-patient starting CS for nonresponsive mild-moderate UC flare.
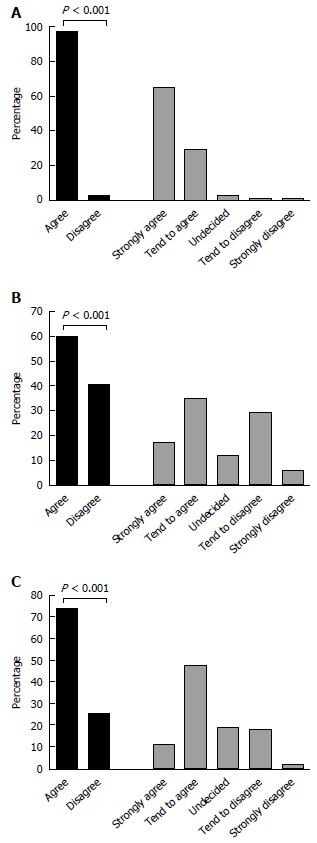
To explore if physicians’ perceived costs or safety of 5ASA played any role in the therapeutic choice between combination 5ASA + CS or corticosteroid alone, respondents were also queried about their opinion towards these topics. Overall, the vast majority (94%) of physicians perceive 5ASA as having very good safety profile (Figure 3A). However, opinions were more split with respect to whether 5ASA are expensive, or not (Figure 3B); yet, there was no difference in the preference for combination 5ASA + CS versus CS alone between physicians who perceived 5ASA as non-expensive to those who considered them expensive (OR = 1.45, 95%CI: 0.88-2.3, P = 0.14). There was a trend for physicians who agreed that 5ASA drugs have very good safety profile to be more likely to prefer combination 5ASA + CS, compared to physicians who disagreed to the statement of 5ASA safety (OR = 4.2, 95%CI: 1.02-17.1, P = 0.045), but this analysis was limited by the small number of doctors perceiving 5ASA as not very safe (n = 9, 3% of the respondents). In contrast, physicians who professed a belief that mechanistically 5ASA may still exert some biologic effect when combined with CS, were significantly more likely to prescribe this combination compared to those not believing in this additive or synergistic mechanism (OR = 7.3, 95%CI: 4-13.3, P < 0.001).
Along with perceptions on cost and safety of 5ASA, several background professional characteristics of respondents were investigated for their correlation with the decision to administer combination CS + 5ASA or CS alone. On uni-variable analysis, there was a non-significant trend for IBD experts (n = 183) to be less likely to endorse combination 5ASA + CS compared to GI doctors who are not IBD experts (n = 148, OR = 0.63, 95%CI: 0.39-1.03, P = 0.07). GI doctors working in an out-patient clinic setting (n = 32) were more likely to recommend combination 5ASA + CS to hospitalized UC patients compared to physicians with hospital based positions (n = 281, OR = 3.7, 95%CI: 1.3-11.1, P = 0.01). Physicians who recommended combination therapy had been practicing as a GI expert for a median of 10 years (IQR25-75 5-18) compared to median of 13 years (IQR 6-21.5) for physicians recommending monotherapy with CS alone (P = 0.03). On multi-variable analysis, however, perception of a plausible synergistic/additive mechanism of action for 5ASA with corticosteroids and less years of experience as GI expert were the only two variables that had a positive independent association with the decision to administer combination therapy (Table 2).
| Variable | Rate | Unadjusted odds ratio for prescribing combination 5ASA + CS | 95%CI | P value | Adjusted odds ratio for prescribing combination 5ASA + CS | 95%CI | P value |
| Believe 5ASA are very safe | 94% | 4.20 | 1.02-17.1 | 0.045 | 2.9 | 0.4-22 | 0.3 |
| Believe 5ASA are not expensive | 53% | 1.45 | 0.88-2.3 | 0.140 | 1.3 | 0.7-2.6 | 0.4 |
| Believe 5ASA mode-of-action may synergize with CS | 60% | 7.30 | 4-13.3 | 0.001 | 7.9 | 3.8-17 | < 0.001 |
| IBD expert | 55% | 0.63 | 0.39-1.03 | 0.070 | 0.6 | 0.3-1.2 | 0.14 |
| Work in out-patient clinic setting | 11% | 3.70 | 1.3-11.1 | 0.010 | 2.7 | 0.6-12 | 0.2 |
| Years of practice (odds ratio/yr) | - | - | - | - | 0.97 | 0.94-0.99 | 0.04 |
This study shows widely divergent practices among GI physicians and IBD experts with respect to prescribing 5ASA in combination with CS versus prescribing CS alone for patients with active UC. Nevertheless, the majority of experts elected to continue 5ASA in this scenario, despite the absence of evidence for a benefit of this combination. It is possible that some physicians who opt against administering 5ASA to acute UC patients are wary of bio-availability of orally administered drugs in this situation, but this question was not investigated in the present study. Many of the physicians professed a belief that a biologic additive or synergistic effect of 5ASA and CS is plausible and also perceive the safety profile of 5ASA as very good, presumably leading them to use concurrent 5ASA + CS. However, this is an unproven combination with associated costs, and 5ASA safety has not been specifically scrutinized in moderate-severe patients. Moreover, 5ASA have not been approved for moderate-severe active UC by any regulatory agency. Thus, evidence to support or refute the benefit of 5ASA in combination with CS should be sought to enable rational and evidence-based therapeutic decisions for these patients. Indirect evidence from studies of moderate-severe active UC treated with infliximab has not indicated a difference in response rate between sub-groups of patients treated or not with concomitant 5ASA[7]. However, data on concurrent 5ASA treatment in clinical trials of CS is sparse and even head-to-head comparisons of maximal mesalamine dosing with CS are lacking[8]. In retrospective cohorts not addressing this question directly, sub-groups of CS-treated patients with or without 5ASA fared similarly[9-11]. In an analysis of patients with CS-induced remission included in three clinical trials, patients who continued mesalamine after CS tapering had higher rates of maintained remission compared to patients on placebo maintenance[12]. Notwithstanding, this comparison may not be extra-polated to the dilemma of adding 5ASA to CS during the induction of remission treatment phase itself. In the only study to date directly addressing this question, there was statistically significant benefit in univariable analysis for reduced complications and need for salvage therapy among the 156 patients treated with combination 5ASA + CS compared to CS alone (n = 63)[13]. However, the benefit was not demonstrable on multi-variable analysis, raising the possibility of additional confounders playing a role in the observed results. In the absence of solid data to support or reject the use of 5ASA in combination with corticosteroids, controlled prospective data are imperative, and one such randomized controlled clinical trial has been launched[14].
Several limitations of the present study should be acknowledged. Respondents were approached directly by the sub-investigators, thereby making it impossible to definitely exclude a selection bias-by-acquaintance. However, post-hoc analyses showed that respondents approached by any single investigator still had very divergent response pattern (data not shown), arguing against a homogenous group of “think-alike” experts. Moreover, the present methodology allowed for a high response rate of > 50%. In contrast, the alternative survey methodology whereby physicians are picked from a professional society member list or similar non-personal method, notoriously yields low response rate of 10%-40%[5,6]. Such a low response rate may result in a different selection bias, whereby participating respondents are a minority of the targeted general population and not representative of it. Notably, as evidence is lacking, physician opinions as obtained here are empiric-based, and does not imply that the approach of the majority is necessarily the best therapeutic strategy. Another limitation of this concise questionnaire is that it did not include some additional items that may influence physicians’ choices, such as local availability of salvage therapies.
In conclusion, this global survey shows that GI and IBD experts have diverse 5ASA prescription practices for patients with moderate-severe active UC treated with CS. Nonetheless, despite the absence of data, the majority of experts endorse the use of combination 5ASA + CS for these patients. Possible adverse events - albeit rare - as well as medication costs should be borne in mind when making this decision. Conversely, experts who do not add 5ASA may be missing out on a relatively safe and possibly effective additive therapy. Thus, data from prospective clinical trials are direly needed for rational evidence-based decision in this common clinical scenario.
The authors wish to thank Prof. Yan Chen, from The Second Affiliated Hospital of Zhejiang University School of Medicine, Hangzhou, China, and Prof. Min Zhi, from The Sixth Affiliated hospital of Sun Yat-Sen University, Guangzhou, China for their valuable help with study logistics and questionnaires distribution. The authors also wish to thank Prof. Benjamin Avida, Dr. Henit Yanai and Prof Dan Turner from Israel, for their help with questionnaire validation.
Patients with moderate-severe active ulcerative colitis are often treated with corticosteroids. Whether 5-aminosalycilates (5ASA) offer any benefit when combined with corticosteroids for these patients has not been explored. Moreover, it is unknown how do physicians treating acute ulcerative colitis (UC) patients make this therapy choice. If the addition of 5ASA offers even a small added benefit for this severely sick patients and saves a minority of them from the need for salvage therapy or urgent surgery, this could comprise an important simple tool for improving outcomes of these severely ill patients. Conversely, if this combination is no more efficacious then corticosteroids alone, then patients can be spared from a futile additional drug with its associated costs. Thus, this global survey explored the approach of expert gastroenterology physicians in 14 countries to combining 5ASA with corticosteroids to gain preliminary insight into real-life practice in this scenario and investigate factors driving this management decision.
Although patients with moderate-severe UC are commonly treated with corticosteroids, roughly a third of them will not respond and require salvage therapy with infliximab or with cyclosporine and/or urgent colectomy. With these limited therapeutic options, investigations into factors that may increase rate of response to mainstay corticosteroid treatment are direly needed. This survey aims to map gastrointestinal (GI) experts’ strategy with respect to one such possible intervention, namely the addition of 5ASA to corticosteroids.
This is the first study to assess the practice of GI experts with respect to 5ASA usage during acute moderate-severe UC. It shows for the first time that despite the absence of evidence supporting this strategy, most physicians administer combination 5ASA with corticosteroids rather than corticosteroids alone. It also provides novel data about factors driving physicians’ decisions in this scenario, specifically showing this decision to be driven by mechanistic considerations unrelated to costs and safety of medication.
This study highlights an often overlooked clinical dilemma - whether to co-treat patients with moderate severe UC with combination 5ASA + corticosteroids or administer corticosteroids monotherapy. As such, it provides preliminary data and framework on which a controlled clinical trial can be based. It will also likely to make physicians more cognizant of this clinical decision, spurring further research into this - and other interventions - as possible avenues to increase the response rate to conventional non-costly and safe therapies in active UC.
Although this is a well written article and involves a lot of work.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lakatos PL, Popp C, Villafranca CM S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J. 1955;2:1041-1048. [PubMed] |
| 2. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 3. | Robertson DJ, Imperiale TF. Stool Testing for Colorectal Cancer Screening. Gastroenterology. 2015;149:1286-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Yanai H, Nguyen GC, Yun L, Lebwohl O, Navaneethan U, Stone CD, Ghazi L, Moayyedi P, Brooks J, Bernstein CN. Practice of gastroenterologists in treating flaring inflammatory bowel disease patients with clostridium difficile: antibiotics alone or combined antibiotics/immunomodulators? Inflamm Bowel Dis. 2011;17:1540-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 5. | Klag T, Stange EF, Wehkamp J. Management of Crohn’s disease - are guidelines transferred to clinical practice? United European Gastroenterol J. 2015;3:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Spiegel BM, Ho W, Esrailian E, Targan S, Higgins PD, Siegel CA, Dubinsky M, Melmed GY. Controversies in ulcerative colitis: a survey comparing decision making of experts versus community gastroenterologists. Clin Gastroenterol Hepatol. 2009;7:168-174, 174.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462-2476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2744] [Cited by in RCA: 2885] [Article Influence: 144.3] [Reference Citation Analysis (2)] |
| 8. | Zeuzem S, Gane E, Liaw YF, Lim SG, DiBisceglie A, Buti M, Chutaputti A, Rasenack J, Hou J, O’Brien C. Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. J Hepatol. 2009;51:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Llaó J, Naves JE, Ruiz-Cerulla A, Marín L, Mañosa M, Rodríguez-Alonso L, Cabré E, Garcia-Planella E, Guardiola J, Domènech E. Intravenous corticosteroids in moderately active ulcerative colitis refractory to oral corticosteroids. J Crohns Colitis. 2014;8:1523-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Jeon HH, Lee HJ, Jang HW, Yoon JY, Jung YS, Park SJ, Hong SP, Kim TI, Kim WH, Cheon JH. Clinical outcomes and predictive factors in oral corticosteroid-refractory active ulcerative colitis. World J Gastroenterol. 2013;19:265-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Molnár T, Farkas K, Nyári T, Szepes Z, Nagy F, Wittmann T. Response to first intravenous steroid therapy determines the subsequent risk of colectomy in ulcerative colitis patients. J Gastrointestin Liver Dis. 2011;20:359-363. [PubMed] |
| 12. | Lichtenstein GR, Gordon GL, Zakko S, Murthy U, Sedghi S, Pruitt R, Barrett AC, Bortey E, Paterson C, Forbes WP. Long-Term Benefit of Mesalamine Granules for Patients Who Achieved Corticosteroid-Induced Ulcerative Colitis Remission. Dig Dis Sci. 2016;61:221-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Har-Noy O, Kim B, Haiat R, Engel T, Ungar B, Eliakim R, Kim WH, Cheon JH, Ben-Horin S. Combination of Corticosteroids with 5-Aminosalicylic Acids Compared to Corticosteroids Alone for hospitalized active Ulcerative Colitis patients. IMAJ. 2016;18:613-618. |
| 14. | Corticosteroids 5-aminosalicylic Acid Compared to corticosteroids in the Treatment of Moderate-severe Ulcerative Colitis. Identifier: NCT01941589. Available from: http://www.ClinicalTrials.gov. |