Published online Apr 28, 2017. doi: 10.3748/wjg.v23.i16.2948
Peer-review started: January 8, 2017
First decision: January 19, 2017
Revised: February 1, 2017
Accepted: March 30, 2017
Article in press: March 30, 2017
Published online: April 28, 2017
Processing time: 112 Days and 20.3 Hours
To investigate the association between a recent gastrointestinal (GI) endoscopy and the subsequent risk of pyogenic liver abscess (PLA).
We designed a nested case control study. Using the Taiwan National Health Insurance Research Database, 2135 patients with a first diagnosis of PLA were identified from 1998 to 2011. Another 10675 patients without PLA matched by age and sex were selected as reference controls. We identified and compared the possible risk factors for PLA and GI endoscopies performed before the index date (when PLA was diagnosed) between the two cohorts. Multivariate analysis was conducted to examine the risk of PLA within the 90 d after the GI endoscopies.
Patients with a history of diabetes [adjusted odds ratio (aOR) = 4.92, 95%CI: 1.78-13.61], end-stage renal disease (aOR = 3.98, 95%CI: 1.45-10.91), biliary tract infection (aOR = 2.68, 95%CI: 2.11-3.40), liver cirrhosis (aOR = 2.19, 95%CI: 1.39-3.46), GI malignancies (aOR = 5.68, 95%CI: 4.23-7.64), appendicitis (aOR = 3.16, 95%CI: 2.27-4.41), diverticulitis (aOR = 1.64, 95%CI: 1.01-2.64), and recent endoscopic retrograde cholangiopancreatography (aOR = 27.04, 95%CI: 11.65-62.72) were significantly associated with an increased risk of PLA. After adjusting for the above risk factors and the frequency of outpatient department visits and abdominal ultrasounds during 90 d before the index date, an upper GI panendoscopy (aOR = 2.75, 95%CI: 2.05-3.69) but not a lower GI endoscopy (aOR = 1.07, 95%CI: 0.62-1.86) was significantly associated with PLA.
An upper GI panendoscopy performed before 90 d may increase the risk of PLA.
Core tip: A pyogenic liver abscess (PLA) is a potential lethal disease with known pathogeneses, including biliary tract infection and portal venous bacterial spreading. Gastrointestinal (GI) endoscopies are common procedures that sometimes have complications of mucosa trauma, local infection, and bacteremia. The relationship between GI endoscopy and subsequent PLA has not yet been documented. This large nested case-control study has shown a significant association between a recent upper GI panendoscopy and increased risk of PLA, though a lower GI endoscopy and the invasive procedure itself of a GI endoscopy did not increase the risk of PLA. Furthermore, patients with diabetes mellitus, end-stage renal disease, liver cirrhosis, biliary tract infection, and GI malignancies could also have a higher risk of PLA. In summary, clinical physician should not ignore the risk of development of PLA after patients receiving an upper GI panendoscopy, especially in those with diabetes mellitus, end-stage renal disease, liver cirrhosis, biliary tract infection, and GI malignancies.
- Citation: Tsai MJ, Lu CL, Huang YC, Liu CH, Huang WT, Cheng KY, Chen SCC. Recent upper gastrointestinal panendoscopy increases the risk of pyogenic liver abscess. World J Gastroenterol 2017; 23(16): 2948-2956
- URL: https://www.wjgnet.com/1007-9327/full/v23/i16/2948.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i16.2948
A pyogenic liver abscess (PLA) is the most common type of visceral abscess and is a potentially life-threatening disease with distinct incidence rates worldwide[1-3]. In western countries, the annual PLA incidence rate is around 1-2.3 per 100000[2-4] with an overall mortality of around 10%[2,4,5]. In Taiwan, the annual incidence has increased steadily from 11.2 per 100000 in 1996 to 17.6 per 100000 in 2004[1].
The direct ascending spread of bacteria from the biliary tract or a hematologic spread of bacteria from organs of the portal systems due to gastrointestinal lesions with mucosa defects or a compromised mucosa barrier[5,6], is the well-known pathogenesis. Therefore, the documented risk factors for PLA include diabetes mellitus (DM)[1,7], end-stage renal disease (ESRD)[8,9], biliary tract infection (BTI)[10], liver cirrhosis[11,12], colorectal cancer[13-17], hepatobiliary tract cancer[18,19], and endoscopic retrograde cholangiopancreatography (ERCP)-related biliary tract procedures[20-23]. In addition, serial case reports have also demonstrated that acute appendicitis or diverticulitis may result in PLA through bacterial spreading from portal systems[24-29].
Gastrointestinal (GI) endoscopies are common procedures for the diagnosis and treatment of GI diseases. Although the risk of adverse events associated with these procedures is low, complications still occasionally happen under extensive usage. Common complications of GI endoscopy include perforation, hemorrhage, and infection. According to the literature, the mean rate of bacteremia after ERCP ranges from 6.4% to 18%[21]. These bacteremia episodes may evolve into clinical complications, including cholangitis, cholecystitis, PLA, pancreatic pseudocyst infection and even sepsis, at a rate ranging from 0.04% to 8.6%[20-23]. Hence, prophylaxis antibiotics are recommended for patients who receive ERCP with a bile duct obstruction and without adequate biliary drainage[20]. Transient bacteremia after a diagnostic upper GI (UGI) panendoscopy and colonoscopy has also been reported to occur at a mean rate of 4.4%[20,30,31]. Therefore, the development of PLA after a GI endoscopy through the hemorrhagic spread of bacteria is possible[20,24,31-33].
Until now, no large-scale study has been conducted to investigate the relationship between GI endoscopy and the subsequent risk of PLA. The aim of this study was to determine whether patients who had undergone a recent GI endoscopy had an increased risk of PLA compared to those who had not undergone GI endoscopy, based on a nationwide population-based database in Taiwan.
This study was based on claims data from the Taiwan National Health Insurance (NHI) program, which was initiated in 1995 to provide affordable healthcare for all residents in Taiwan. There are currently more than 23 million enrollees in the program, representing over 99% of Taiwan’s entire population. For research purposes, claims data have been updated annually in the National Health Insurance Research Database (NHIRD) by the National Health Research Institute (NHRI). The NHRI released sets of sampling files, called the Longitudinal Health Insurance Database (LHID), for the year 2005 (LHID 2005). The NHRI randomly sampled 1000000 beneficiaries in 2005 from the entire population of NHI beneficiaries. All registrations and claims data for these 1000000 beneficiaries from 1996 to 2011 were included in the LHID 2005. The primary data source of this study was retrieved from the LHID 2005. In this cohort dataset, the original identification number of each patient was scrambled to protect patient privacy, thus patients’ informed consent was not needed. The study was approved by the Institutional Review Board of the Ditmanson Medical Foundation Chia-Yi Christian Hospital (CYCH-IRB 104034).
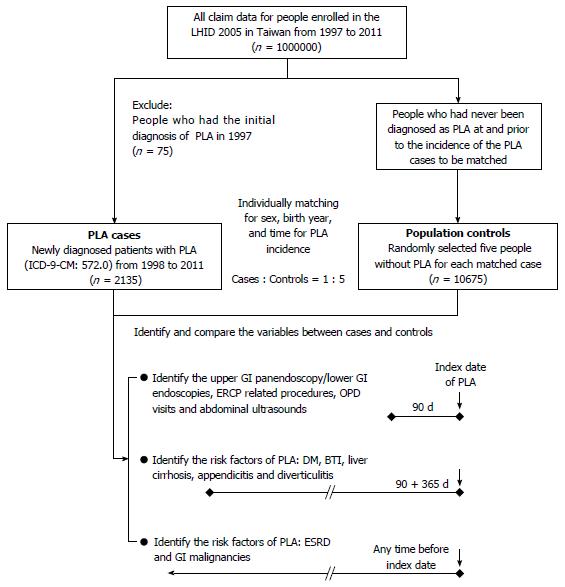
The present study was designed as a nested case-control study. We used LHID 2005 to identify patients with first-diagnosed PLA (International Classification of Disease, 9th Revision, Clinical Modification [ICD-9-CM], code 572.0) from either outpatient or inpatient medical records from 1998 to 2011. The diagnosis date of PLA served as the index date. For each patient with PLA, five matched controls were selected from the remaining patients in the database using an incidence density sampling method[34]. The controls were randomly selected from people who had a matched birth year and sex, and who had not been diagnosed with PLA from 1997 to the PLA occurrence date (index date) of their matched cases (Figure 1).
To evaluate the association between a recent digestive endoscopy and PLA, we reviewed inpatient and outpatient medical records of cases and controls in the 90 d period before the index date (Figure 1). Digestive endoscopy was determined by specific NHI order codes: UGI panendoscopy, 28016C; lower gastrointestinal (LGI) endoscopy; 28017C (colonoscopy); 28011C (rectoscopy); and 28013C (sigmoidoscopy). Endoscopy with or without invasive procedures, like biopsy, polypectomy, hemostasis and foreign body removal, were also determined by specific codes: 28030C (endoscopic biopsy); 47043B (endoscopic hemostasis); 47074C (panendoscopic polypectomy); 47083C (UGI foreign body removal); 28031C (colonoscopic or enteroscopic biopsy); 49014C (colonoscopic polypectomy); 49023C (rectoscopic hemostasis); 49025C (colonoscopy with removal of a foreign body); and 49026C (endoscopic hemostasis for colon bleeding). Because PLA had already been demonstrated as a complication of ERCP[20-23], we identified, controlled, and excluded ERCP-related procedures, including ERCP (33024B), endoscopic retrograde pancreas drainage (33033B), endoscopic retrograde biliary drainage (56020B), endoscopic papillotomy with stone extraction (56033B), endoscopic sphincterotomy (56031B), and endoscopic nasobiliary drainage (56021B), according to the specific codes. Moreover, to control other unidentified causes, usage dependence bias and confounding factors that may promote to a diagnosis of PLA, we also controlled for the frequency of outpatient department (OPD) visits and abdominal ultrasound examinations (19001C and 19009C) during the 90 d period before the index date in a multivariate analysis. Since the bureau of NHI regularly checked the claims dataset to ensure the validity of all procedure codes that patients received before reimbursement, we believe that the above endoscopy procedure records should be reliable. There was also a possibility that the patients who received endoscopic procedures were not recorded in the NHI database. For example, patients who received self-paid endoscopic procedures were not included. However, the NHI program covers near 100% of Taiwan’s entire population. The ratio of these self-paid endoscopic procedures was extremely low and can almost be neglected.
In addition to demographic variables, documented risk factors for PLA, including diabetes mellitus (ICD-9-CM code 250), BTI (ICD-9-CM codes 574.0-574.4, 575.0, 575.1, 575.10-575.12, and 576.1-576.3), liver cirrhosis (ICD-9-CM codes 571.2, 571.5, and 571.6), appendicitis (ICD-9-CM codes 540.0, 540.1, 541, and 542) and diverticulitis (ICD-9-CM codes 562.10-562.13), were also identified during the 15 mo (90 + 365 d) before the index date (Figure 1). Moreover, patients with ESRD or GI malignancies that were registered in the Critical Illness Database of NHIRD before the index date (not limited to the 15-month duration before the index date), were also identified (Figure 1). Malignancies of the GI tract included malignancies of colon (ICD-9-CM codes 153.0-153.9), rectosigmoid junction (154.0), rectum (154.1), anus (154.2, 154.3, and 154.8), liver (155.0 and 155.2), intrahepatic bile duct (155.1), gallbladder (156.0), extrahepatic bile ducts (156.1, 156.2, 156.8, and 156.9), stomach (151.0-151.9), small intestine (152.0-152.3, 152.8, and 152.9), and pancreas (157.0-157.4, 157.8, and 157.9).
Frequencies of demographic and clinical characteristics were described and compared between cases and controls using χ2 test. To evaluate the net effect of a recent UGI panendoscopy or LGI endoscopy on PLA risk, a multivariate analysis adjustment for the well documented or possible risk factors for PLA was conducted. The frequency of OPD visits and the frequency of abdominal ultrasound examinations were also adjusted to prevent a usage dependency bias for patients who had other risk and confounding factors of PLA which would lead to a higher frequency of OPD visits and ultrasound examinations. A conditional logistic regression model was performed to estimate the adjusted odds ratio (OR) and its 95% confidence interval (CI). Significance was set at P < 0.05. All statistical analyses were performed using SAS (version 9.3, SAS Institute Inc., Cary, NC, United States). In addition to the analysis of a recent 90 d GI endoscopy and PLA risk, we also conducted another sensitivity analysis of a recent 180 d and 1 year (365 d) GI endoscopy by the aforementioned methods to validate the association between GI endoscopy and PLA risk.
During 1998 to 2011, 2135 PLA cases and 10675 matched controls were obtained from LHID 2005. The annual PLA incidence in Taiwan during this period was 15.25 cases per 100000. The mean age was 58.9 ± 16.5 year and 60% were males. PLA mostly happened in those aged 45-64 year (41.4%). The leading comorbidities of patients with PLA were BTI (11.7%), diabetes (9.7%), and liver cirrhosis (6.8%) (Table 1). Patients with PLA were more likely to have the following comorbidities than patients without PLA: diabetes (P < 0.001), ESRD (P < 0.001), BTI (P < 0.001), liver cirrhosis (P < 0.001), malignancies of the GI tract (P < 0.001), appendicitis (P < 0.001), and diverticulitis (P < 0.001) (Table 1).
| Demographic and clinical characteristics | Cases(n = 2135) | Controls(n = 10675) | Total(n = 12810) | P value |
| Age, yr | ||||
| < 20 | 31 (1.5) | 164 (1.5) | 195 | 0.991 |
| 20-44 | 391 (18.3) | 1952 (18.3) | 2343 | |
| 45-64 | 883 (41.4) | 4423 (41.4) | 5306 | |
| 65-74 | 454 (21.3) | 2229 (20.9) | 2683 | |
| 75+ | 376 (17.6) | 1907 (17.9) | 2283 | |
| mean ± SD | 58.8 ± 16.5 | 58.8 ± 16.5 | 58.8 ± 16.5 | 0.991 |
| Gender | ||||
| Female | 854 (40.0) | 4270 (40.0) | 5124 | 1.000 |
| Male | 1281 (60.0) | 6405 (60.0) | 7686 | |
| Diabetes | ||||
| With | 206 (9.7) | 253 (2.4) | 459 | < 0.001 |
| Without | 1929 (90.4) | 10422 (97.6) | 12351 | |
| ESRD | ||||
| With | 46 (2.2) | 73 (0.7) | 119 | < 0.001 |
| Without | 2089 (97.9) | 10602 (99.3) | 12691 | |
| BTI | ||||
| With | 249 (11.7) | 99 (0.9) | 348 | < 0.001 |
| Without | 1886 (88.3) | 10576 (99.1) | 12462 | |
| Liver cirrhosis | ||||
| With | 146 (6.8) | 97 (0.9) | 243 | < 0.001 |
| Without | 1989 (93.2) | 10578 (99.1) | 12567 | |
| GI malignancy | ||||
| With | 62 (2.9) | 80 (0.8) | 142 | < 0.001 |
| Without | 2073 (97.1) | 10595 (99.3) | 12668 | |
| Appendicitis | ||||
| With | 10 (0.47) | 9 (0.08) | 19 | < 0.001 |
| Without | 2125 (99.53) | 10666 (99.92) | 12791 | |
| Diverticulitis | ||||
| With | 12 (0.56) | 11 (0.1) | 23 | < 0.001 |
| Without | 2123 (99.44) | 10664 (99.9) |
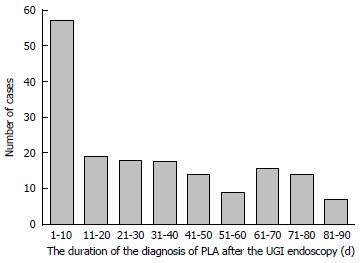
During the 90 d period before the index date, patients with PLA had a significantly higher rate of having undergone an UGI panendoscopy (P < 0.001), LGI endoscopy (P < 0.001), and ERCP-related procedures (P < 0.001) than patients without PLA. Fifty seven (33.1%) PLA cases occurred within the first 10 d after an endoscopic examination (Figure 2), with a median of 25.5 d (interquartile range, 7-53 d). Moreover, the frequency of OPD visits (P < 0.001) and frequency of abdominal ultrasound examinations (P < 0.001) during the 90 d period before the index date were also significantly higher in patients with PLA (Table 2).
| Demographic and clinical characteristics | Cases(n = 2135) | Controls(n = 10675) | Total(n = 12810) | P value |
| Frequency of OPD visits | ||||
| < 1 | 119 (5.6) | 2781 (26.1) | 2900 | < 0.001 |
| 1-2 | 243 (11.4) | 1989 (18.6) | 2232 | |
| 3-7 | 774 (36.3) | 3403 (31.9) | 4177 | |
| ≥ 8 | 999 (46.8) | 2502 (23.4) | 3501 | |
| Frequency of abdominal ultrasound | ||||
| 0 | 1692 (79.3) | 10329 (96.8) | 12021 | < 0.001 |
| 1 | 358 (16.8) | 321 (3.0) | 679 | |
| ≥ 2 | 85 (4.0) | 25 (0.2) | 110 | |
| Abdominal ultrasound | ||||
| with | 443 (20.8) | 346 (3.2) | 789 | < 0.001 |
| without | 1692 (79.3) | 10329 (96.8) | 12021 | |
| ERCP-related procedures | ||||
| with | 107 (5.0) | 9 (0.1) | 116 | < 0.001 |
| without | 2028 (95.0) | 10666 (99.9) | 12694 | |
| Upper GI panendoscopy | ||||
| with | 172 (8.1) | 131 (1.2) | 303 | < 0.001 |
| without | 1963 (91.9) | 10544 (98.8) | 12507 | |
| Lower GI endoscopy | ||||
| with | 36 (1.7) | 53 (0.5) | 89 | < 0.001 |
| without | 2099 (98.3) | 10622 (99.5) | 12721 | |
| Upper or lower GI endoscopy | ||||
| with | 189 (8.9) | 171 (1.6) | 360 | < 0.001 |
| without | 1946 (91.2) | 10504 (98.4) | 12450 | |
| Upper GI panendoscopy | ||||
| upper GI only1 | 150 (7.0) | 130 (1.2) | 280 | < 0.001 |
| upper GI with ERCP-related procedures | 22 (1.0) | 1 (0.0) | 23 | |
| ERCP without upper GI | 85 (4.0) | 8 (0.1) | 93 | |
| None2 | 1878 (88.0) | 10536 (98.7) | 12414 | |
| Lower GI endoscopy | ||||
| lower GI only1 | 34 (1.6) | 53 (0.5) | 87 | < 0.001 |
| lower GI with ERCP-related procedures | 2 (0.1) | 0 (0.0) | 2 | |
| ERCP without lower GI | 105 (4.9) | 9 (0.1) | 114 | |
| None2 | 1994 (93.4) | 10613 (99.4) | 12607 | |
| Upper or lower GI endoscopy | ||||
| upper or lower GI only1 | 166 (7.8) | 170 (1.6) | 336 | < 0.001 |
| upper or lower GI with ERCP-related procedures | 23 (1.1) | 1 (0.0) | 24 | |
| ERCP without upper or lower GI | 84 (3.9) | 8 (0.1) | 92 | |
| None2 | 1862 (87.2) | 10496 (98.3) | 12450 | |
The net effect of a recent UGI panendoscopy and LGI endoscopy and PLA risk was evaluated by a multivariate analysis after adjusting for risk factors for PLA, recent ERCP-related procedures, and frequency of OPD visits and abdominal ultrasounds (Table 3). The results showed that patients who had undergone a recent UGI panendoscopy had significantly higher odds of having PLA than patients who had not undergone an UGI panendoscopy (OR = 2.75, 95%CI: 2.05-3.69, P < 0.001). The number needed to harm (NNH) for UGI panendoscopy in PLA was 15 patients (95%CI: 12-17). However, there was no significant difference between patients with or without a LGI endoscopy (OR = 1.07, 95%CI: 0.62-1.86, P = 0.803). Because a few cases who received an UGI panendoscopy or LGI endoscopy also received ERCP-related procedures during the preceding 90 d (Table 2), we also used another multivariate analysis model to calculate the OR of only UGI panendoscopy or LGI endoscopy (i.e., patients with concurrent ERCP-related procedures were excluded). The results still showed that an UGI panendoscopy was significantly associated with PLA (OR = 2.70, 95%CI: 2.01-3.63, P < 0.001; NNH = 17, 95%CI: 14-21) (Supplementary Table 1). Another sensitivity analysis conducted for a recent 180 d and 1 year GI endoscopy, and risk of PLA, had similar results. The OR for PLA risk was higher during the previous 90 d (OR = 2.75) than the 180 d (OR = 2.33, P < 0.001) and 1 year UGI panendoscopy (OR = 1.75, P < 0.001).
| aOR | 95%CI | P value | ||
| Diabetes | With vs without | 4.92 | 1.78-13.61 | 0.002 |
| ESRD | With vs without | 3.98 | 1.45-10.91 | 0.007 |
| BTI | With vs without | 2.68 | 2.11-3.40 | < 0.001 |
| Liver cirrhosis | With vs without | 2.19 | 1.39-3.46 | < 0.001 |
| GI malignancy | With vs without | 5.68 | 4.23-7.64 | < 0.001 |
| Appendicitis | With vs without | 3.16 | 2.27-4.41 | < 0.001 |
| Diverticulitis | With vs without | 1.64 | 1.01-2.64 | 0.044 |
| Frequency of OPD visits | 1-2 vs < 1 | 2.65 | 2.10-3.34 | < 0.001 |
| 3-7 vs < 1 | 4.75 | 3.86-5.85 | < 0.001 | |
| 8+ vs < 1 | 6.72 | 5.43-8.32 | < 0.001 | |
| Frequency of Ultrasound | ≥ 2 vs 0 | 3.26 | 2.69-3.95 | < 0.001 |
| 1 vs 0 | 5.69 | 3.35-9.66 | < 0.001 | |
| ERCP-related procedures | With vs without | 27.04 | 11.65-62.72 | < 0.001 |
| UGI panendoscopy | With vs without | 2.75 | 2.05-3.69 | < 0.001 |
| LGI Endoscopy | With vs without | 1.07 | 0.62-1.86 | 0.803 |
Diabetes (OR = 4.92, 95%CI: 1.78-13.61, P = 0.002), ESRD (OR = 3.98, 95%CI: 1.45-10.91, P = 0.007), BTI (OR = 2.68, 95%CI: 2.11-3.40, P < 0.001), liver cirrhosis (OR = 2.19, 95%CI: 1.39-3.46, P < 0.001), history of GI tract malignancies (OR = 5.68, 95%CI: 4.23-7.64, P < 0.001), and ERCP-related procedures (OR = 27.04, 95%CI: 11.65-62.72, P < 0.001), which have been documented previously as PLA risk factors, were also significantly associated with PLA in this study. Additionally, patients with a history of appendicitis (OR = 3.16, 95%CI: 2.27-4.41, P < 0.001) or diverticulitis (OR = 1.64, 95%CI: 1.01-2.64, P = 0.044) also had significantly higher odds of having PLA than patients without appendicitis or diverticulitis, but the case numbers of these two illnesses were small (Table 3).
We further analyzed the association between the risk of PLA and the invasiveness of UGI or LGI tract endoscopy. The results showed no significant association with PLA risk on whether the invasive procedures were performed or not in both an UGI panendoscopy (OR = 0.91, 95%CI: 0.47-1.77) and LGI endoscopy (OR = 0.97, 95%CI: 0.32-2.96) (Table 4).
| Cases (n = 2135) | Controls (n = 10675) | Total | aOR1 | 95%CI | P value | |
| Upper GI panendoscopy | ||||||
| Without invasive procedure2 | 130 (6.09) | 98 (0.92) | 228 | Ref. | ||
| With invasive procedure | 42 (1.97) | 33 (0.31) | 75 | 0.91 | 0.47-1.77 | 0.787 |
| None3 | 1963 (91.94) | 10544 (98.77) | 12507 | 0.35 | 0.26-0.49 | < 0.001 |
| Lower GI endoscopy | ||||||
| Without invasive procedure2 | 24 (1.12) | 38 (0.36) | 62 | Ref. | ||
| With invasive procedure | 12 (0.56) | 15 (0.14) | 27 | 0.97 | 0.32-2.96 | 0.958 |
| None3 | 2099 (98.31) | 10622 (99.5) | 12721 | 0.66 | 0.35-1.23 | 0.193 |
| Upper or Lower GI endoscopy | ||||||
| Without invasive procedure2 | 138 (6.46) | 124 (1.16) | 262 | Ref. | ||
| With invasive procedure | 51 (2.39) | 47 (0.44) | 98 | 0.87 | 0.49-1.54 | 0.624 |
| None3 | 1946 (91.15) | 10504 (98.4) | 12450 | 0.40 | 0.29-0.54 | < 0.001 |
This large nested case-control study has shown a significant association between a recent UGI panendoscopy and increased risk of PLA (OR = 2.75, 95%CI: 2.05-3.69, NNH = 15). However, a LGI endoscopy and the invasive procedure itself of a GI endoscopy did not seem to increase the risk of PLA.
Bacterial translocation of GI microbial flora into the bloodstream may occur during an endoscopy due to mucosal injury. Although the risk of infection in remote tissues (i.e., infective endocarditis) caused by endoscopy-related transient bacteremia is extremely low, local infection may occur in which a typically sterile space or tissue is breached and contaminated by an endoscopic accessory[20,21]. Once the local infection progresses, it may develop into portal venous bacteremia and lead to PLA.
Previous studies have shown that bacteremia occurred mostly within 30 min after GI endoscopy[21]. In the present study, we found that 33.1% of PLA occurred within 10 d after an UGI panendoscopy. In addition, UGI panendoscopy in the previous 90 d had higher OR for PLA than the previous 180 d and 1 year panendoscopy. As the PLA occurred after UGI panendoscopy and the OR subsided with time, a causal relationship between them is very likely. Though the mean duration that PLA occurred after an UGI panendoscopy was 32 d (median 25.5 d), the shortest duration of finding the PLA after panendoscopy was only 1 d. Therefore, the possibility of a PLA coincidentally exists when receiving panendoscopy could not be ignored, because some patients with early PLA may also present upper GI symptoms which lead them to receive an UGI panendoscopy. The reasonable incubation time of developing PLA after panendoscopy is an interesting and important issue and deserves further study.
Our study also demonstrated that an UGI panendoscopy was significantly associated with a subsequent risk of PLA, but a LGI endoscopy was not. The distance between the liver and the examined organs may be the reason for this finding. For example, the colon is further away from the portal venous and lymphatic circulations to the liver than the esophagus, stomach, and duodenum, therefore a LGI endoscopy might have a lower probability of occurrence of PLA. Moreover, a complicated mesenteric lymphatic defense system in the LGI tract may also decrease the probability of portal venous bacteremia induced by a LGI endoscopy. In addition, high intraluminal air pressure due to air inflation during a duodenum examination in an UGI panendoscopy may also increase the additional risk of retrograde bacterial translocation from the biliary tract and increase the probability of PLA. All of the above factors may explain our findings that an UGI panendoscopy was significantly associated with a subsequent higher risk of PLA than a LGI endoscopy.
In theory, invasive procedures including biopsy, polypectomy, hemostasis, or foreign body removal will result in direct mucosal damage. Presumably, the risk of bacterial translocation into local tissue or circulation will increase due to the endogenous microbial flora gaining a portal of entry. Previous studies have shown that an interventional endoscopy may cause a higher incidence of bacteremia[21,35]. Hence, prophylaxis antibiotics were suggested for some high risk procedures, especially in patients with liver cirrhosis and acute GI bleeding or valvular heart disease[35,36]. However, in this study we found no significant difference in the risk of PLA between a GI endoscopy with and without invasive procedures. One explanation is that an UGI panendoscopy itself is invasive enough to result in an increased risk of PLA. Another possibility may be that some patients receiving invasive procedures were prescribed with prophylactic antibiotics or had concurrent antibiotics usage. Thus, it prevented the development of PLA, since the use of antibiotics has been recommended for invasive procedures with a high risk of bacteremia[20,21,37].
PLA secondary to inflammatory diseases of the GI tract, like acute appendicitis or diverticulitis, have been described in serial case reports through the mechanism of portal bacteremia[25-29], but scarcely studies have verified their association. Recently, one study demonstrated appendectomy correlates with increased risk of PLA[38]. In our study, we identified patients with a diagnosis of acute appendicitis or diverticulitis during the 15 mo before having PLA. Although the ratio and sample size of a history of acute appendicitis or diverticulitis in patients with PLA were small [0.47% (n = 10) and 0.56% (n = 12), respectively, Table 1], both were significantly associated with an increased risk of PLA (OR = 3.16, 95%CI: 2.27-4.41 in acute appendicitis; and OR = 1.64, 95%CI: 1.01-2.64 in diverticulitis; Table 3). This finding should remind us that PLA may develop after having appendicitis or diverticulitis.
Because the dataset of this study has covered over a decade of cases, we also take the change of endoscopy procedure into consideration. The most-related points are the infection controls for the procedures, which has meant the carrying of lower infectious complications and it has highlighted the high level and up-to-date GI endoscopy disinfection procedures in Taiwan[39,40]. The etiology for the development of PLA after GI endoscopy can be iatrogenic and the positive bacterial culture rate from these GI instruments could be high[40]. Hence, manual washing, automated endoscope washer reprocessing and adequate drying/storage after rinsing with regular surveillance culture were recently advised to diminish iatrogenic infectious complications[40].
The use of a representative, nationwide, population-based sample to investigate the risk factors of PLA increases the validity of our results. This large sample size granted us the statistical power to detect differences between the study groups. Nevertheless, there are several limitations in the present study. First, similar to other studies that used administrative data, unmeasured confounders may exist in our study. Second, the use of prophylactic antibiotics for patients who underwent invasive endoscopy was not considered in the analysis, which might confine the interpretation of the antibiotics on reducing the risk of PLA. Third, because the study was based on an administrate claims dataset, the diagnosis of PLA was defined by ICD-9-CM rather than detail clinical information, coding error is possible. Further validation may be needed. However, the NHI regularly checked the claims dataset to ensure the validity before reimbursement, we believe the ratio of coding error is extremely low[41]. In addition, the NHIRD has been extensively used in research, the same definition of PLA has been published in peer-reviewed journals previously[7-9,42]. Finally, a lack of microbiologic data limited the analysis of the association among causative pathogens, site of endoscopy, and PLA. Further study with a larger sample size that adjusts for the influence of concurrent antibiotic use may be needed to verify these findings.
In conclusion, a higher risk of PLA was found in patients who had recently undergone an UGI panendoscopy, especially within the first 10 d after panendoscopy. Clinical physician should not ignore the risk of development of PLA after patients receiving an UGI panendoscopy, especially in those with diabetes mellitus, ESRD, liver cirrhosis, BTI, and GI malignancies.
A pyogenic liver abscess (PLA) is a potential lethal disease with known pathogeneses, including biliary tract infection (BTI) and portal venous bacterial spreading. Gastrointestinal (GI) endoscopies are common procedures that sometimes have complications of mucosa trauma, local infection, and bacteremia. The relationship between GI endoscopy and subsequent PLA has not been documented.
This study investigates the association between a recent GI endoscopy and the subsequent risk of PLA.
A higher risk of PLA was found in patients who had recently undergone an upper GI panendoscopy, especially within the first 10 d after panendoscopy. Furthermore, patients with diabetes mellitus, end-stage renal disease, liver cirrhosis, BTI, and GI malignancies also could have a much higher risk of PLA.
These findings remind clinical physician that PLA may occur in those high-risk patients when they undergo an upper GI panendoscopy.
This manuscript provides the updated evidence to the readers. The topic is an important one and deserves a practical value.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cerwenka HR, Joseph Lo Z, Liao KF, Lee HC, Poovorawan K S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Tsai FC, Huang YT, Chang LY, Wang JT. Pyogenic liver abscess as endemic disease, Taiwan. Emerg Infect Dis. 2008;14:1592-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 2. | Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol. 2004;2:1032-1038. [PubMed] |
| 3. | Jepsen P, Vilstrup H, Schønheyder HC, Sørensen HT. A nationwide study of the incidence and 30-day mortality rate of pyogenic liver abscess in Denmark, 1977-2002. Aliment Pharmacol Ther. 2005;21:1185-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Mohsen AH, Green ST, Read RC, McKendrick MW. Liver abscess in adults: ten years experience in a UK centre. QJM. 2002;95:797-802. [PubMed] |
| 5. | Seeto RK, Rockey DC. Pyogenic liver abscess. Changes in etiology, management, and outcome. Medicine (Baltimore). 1996;75:99-113. [PubMed] |
| 6. | Rahimian J, Wilson T, Oram V, Holzman RS. Pyogenic liver abscess: recent trends in etiology and mortality. Clin Infect Dis. 2004;39:1654-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 394] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Foo NP, Chen KT, Lin HJ, Guo HR. Characteristics of pyogenic liver abscess patients with and without diabetes mellitus. Am J Gastroenterol. 2010;105:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 8. | Hong CS, Chung KM, Huang PC, Wang JJ, Yang CM, Chu CC, Chio CC, Chang FL, Chien CC. Epidemiology and mortality of liver abscess in end-stage renal disease dialysis patients: Taiwan national cohort study. PLoS One. 2014;9:e88078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Tsai LW, Chao PW, Ou SM, Chen YT, Shih CJ, Li SY, Chen TW, Chen TJ, Liu CT. Pyogenic liver abscess in end-stage renal disease patients: a nationwide longitudinal study. Hemodial Int. 2015;19:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Cheng HC, Chang WL, Chen WY, Kao AW, Chuang CH, Sheu BS. Long-term outcome of pyogenic liver abscess: factors related with abscess recurrence. J Clin Gastroenterol. 2008;42:1110-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Mølle I, Thulstrup AM, Vilstrup H, Sørensen HT. Increased risk and case fatality rate of pyogenic liver abscess in patients with liver cirrhosis: a nationwide study in Denmark. Gut. 2001;48:260-263. [PubMed] |
| 12. | Mølle I, Thulstrup AM, Jepsen P, Sørensen HT, Vilstrup H. Liver cirrhosis is risk factor for pyogenic liver abscesses. BMJ. 2001;323:52-53. [PubMed] |
| 13. | Qu K, Liu C, Wang ZX, Tian F, Wei JC, Tai MH, Zhou L, Meng FD, Wang RT, Xu XS. Pyogenic liver abscesses associated with nonmetastatic colorectal cancers: an increasing problem in Eastern Asia. World J Gastroenterol. 2012;18:2948-2955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Jeong SW, Jang JY, Lee TH, Kim HG, Hong SW, Park SH, Kim SG, Cheon YK, Kim YS, Cho YD. Cryptogenic pyogenic liver abscess as the herald of colon cancer. J Gastroenterol Hepatol. 2012;27:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | Jang DK, Jeong SH, Lee SH, Lee M, Jang ES, Kim JW, Hwang JH, Ryu JK, Kim YT, Lee YJ. Computed tomographic colonography is valuable for post-treatment evaluation and screening of hidden colorectal cancer in patients with cryptogenic pyogenic liver abscess. Digestion. 2014;89:175-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Koo HC, Kim YS, Kim SG, Tae JW, Ko BM, Lee TI, Jeong SW, Jang JY, Kim HS, Lee SH. Should colonoscopy be performed in patients with cryptogenic liver abscess? Clin Res Hepatol Gastroenterol. 2013;37:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Zakout R, Santos JM, Ferreira C, Victorino RM. Colonoscopy for ‘cryptogenic’ pyogenic liver abscess? Colorectal Dis. 2010;12:71-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Law ST, Li KK. Is hepatic neoplasm-related pyogenic liver abscess a distinct clinical entity? World J Gastroenterol. 2012;18:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Lin YT, Liu CJ, Chen TJ, Chen TL, Yeh YC, Wu HS, Tseng CP, Wang FD, Tzeng CH, Fung CP. Pyogenic liver abscess as the initial manifestation of underlying hepatocellular carcinoma. Am J Med. 2011;124:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | ASGE Standards of Practice Committee, Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, Muthusamy VR, Pasha SF, Saltzman JR, Shaukat A, Wang A, Cash BD. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (2)] |
| 21. | Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57:546-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 22. | Katsinelos P, Dimiropoulos S, Katsiba D, Arvaniti M, Tsolkas P, Galanis I, Papaziogas B, Limenopoulos V, Baltajiannis S, Vasilladis I. Pseudomonas aeruginosa liver abscesses after diagnostic endoscopic retrograde cholangiography in two patients with sphincter of Oddi dysfunction type 2. Surg Endosc. 2002;16:1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Davion T, Braillon A, Delamarre J, Delcenserie R, Joly JP, Capron JP. Pseudomonas aeruginosa liver abscesses following endoscopic retrograde cholangiography. Report of a case without biliary tract disease. Dig Dis Sci. 1987;32:1044-1046. [PubMed] |
| 24. | Bonenfant F, Rousseau E, Farand P. Streptococcus anginosus pyogenic liver abscess following a screening colonoscopy. Can J Infect Dis Med Microbiol. 2013;24:e45-e46. [PubMed] |
| 25. | Zerem E, Sušić A. Multiple pyogenic liver abscesses formed after appendectomy: the role of percutaneous drainage in a critically ill patient. Acta Med Acad. 2012;41:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Murarka S, Pranav F, Dandavate V. Pyogenic liver abscess secondary to disseminated streptococcus anginosus from sigmoid diverticulitis. J Glob Infect Dis. 2011;3:79-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Nasir AA, Adeniran JO, Abdur-Rahman LO, Abdulkadir AY, Inikori AK, Taiwo JO. Pyogenic liver abscess in children: is ruptured appendix still relevant as cause? Case report. Niger Postgrad Med J. 2009;16:176-178. [PubMed] |
| 28. | Belkahla N, Maamouri N, Ouerghi H, Cheikh I, Nouira K, Khalfallah T, Ben Mami N. Phlegmonous appendicitis revealed by pyogenic liver abscesses. Tunis Med. 2007;85:596-599. [PubMed] |
| 29. | Robert M, Paparel P, Arnal E, Voiglio E, Caillot JL. [Liver abscesses secondary to sigmoid diverticulitis: report of four cases]. Rev Med Interne. 2004;25:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | ASGE Standards of Practice Committee, Ben-Menachem T, Decker GA, Early DS, Evans J, Fanelli RD, Fisher DA, Fisher L, Fukami N, Hwang JH, Ikenberry SO, Jain R, Jue TL, Khan KM, Krinsky ML, Malpas PM, Maple JT, Sharaf RN, Dominitz JA, Cash BD. Adverse events of upper GI endoscopy. Gastrointest Endosc. 2012;76:707-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (2)] |
| 31. | ASGE Standards of Practice Committee, Fisher DA, Maple JT, Ben-Menachem T, Cash BD, Decker GA, Early DS, Evans JA, Fanelli RD, Fukami N, Hwang JH, Jain R, Jue TL, Khan KM, Malpas PM, Sharaf RN, Shergill AK, Dominitz JA. Complications of colonoscopy. Gastrointest Endosc. 2011;74:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 230] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 32. | Ekert P, Bernard B, Congy F, Langlois P, Pelletier S, Devy C, Valla D. [Liver abscess after endoscopic sclerotherapy of hemorrhagic stomach ulcer]. Gastroenterol Clin Biol. 1993;17:397. [PubMed] |
| 33. | Farrell RJ, Krige JE, Bornman PC, Terblanche J. Liver abscess after treatment for bleeding duodenal ulcer. Lancet. 1993;341:1025. [PubMed] |
| 34. | Greenland S, Thomas DC. On the need for the rare disease assumption in case-control studies. Am J Epidemiol. 1982;116:547-553. [PubMed] |
| 35. | Rey JR, Axon A, Budzynska A, Kruse A, Nowak A. Guidelines of the European Society of Gastrointestinal Endoscopy (E.S.G.E.) antibiotic prophylaxis for gastrointestinal endoscopy. European Society of Gastrointestinal Endoscopy. Endoscopy. 1998;30:318-324. [PubMed] |
| 36. | Hirota WK, Petersen K, Baron TH, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Waring JP, Fanelli RD, Wheeler-Harbough J. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58:475-482. [PubMed] |
| 37. | Chavez-Tapia NC, Barrientos-Gutierrez T, Tellez-Avila F, Soares-Weiser K, Mendez-Sanchez N, Gluud C, Uribe M. Meta-analysis: antibiotic prophylaxis for cirrhotic patients with upper gastrointestinal bleeding - an updated Cochrane review. Aliment Pharmacol Ther. 2011;34:509-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 184] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 38. | Liao KF, Lai SW, Lin CL, Chien SH. Appendectomy correlates with increased risk of pyogenic liver abscess: A population-based cohort study in Taiwan. Medicine (Baltimore). 2016;95:e4015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Sheu BS, Wu CY, Wu MS, Chiu CT, Lin CC, Hsu PI, Cheng HC, Lee TY, Wang HP, Lin JT. Consensus on control of risky nonvariceal upper gastrointestinal bleeding in Taiwan with National Health Insurance. Biomed Res Int. 2014;2014:563707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 40. | Chiu KW, Lu LS, Chiou SS. High-level disinfection of gastrointestinal endoscope reprocessing. World J Exp Med. 2015;5:33-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 41. | Cheng CL, Lee CH, Chen PS, Li YH, Lin SJ, Yang YH. Validation of acute myocardial infarction cases in the national health insurance research database in taiwan. J Epidemiol. 2014;24:500-507. [PubMed] |
| 42. | Lin YT, Liu CJ, Chen TJ, Fung CP. Long-term mortality of patients with septic ocular or central nervous system complications from pyogenic liver abscess: a population-based study. PLoS One. 2012;7:e33978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |