Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2802
Peer-review started: November 12, 2016
First decision: December 29, 2016
Revised: January 12, 2017
Accepted: February 17, 2017
Article in press: February 17, 2017
Published online: April 21, 2017
Processing time: 159 Days and 21.3 Hours
To determine incidence and clinical biomarkers of marked necroinflammation and fibrosis characteristics among chronic hepatitis B (CHB) patients with persistently normal alanine aminotransferase (PNALT).
Liver biopsy was performed on 115 CHB patients with PNALT. Necroinflammation and fibrosis were graded by the Knodell histologic activity index and the Ishak fibrosis score, respectively. Correlations between the available clinical parameters and necroinflammation and fibrosis were analysed.
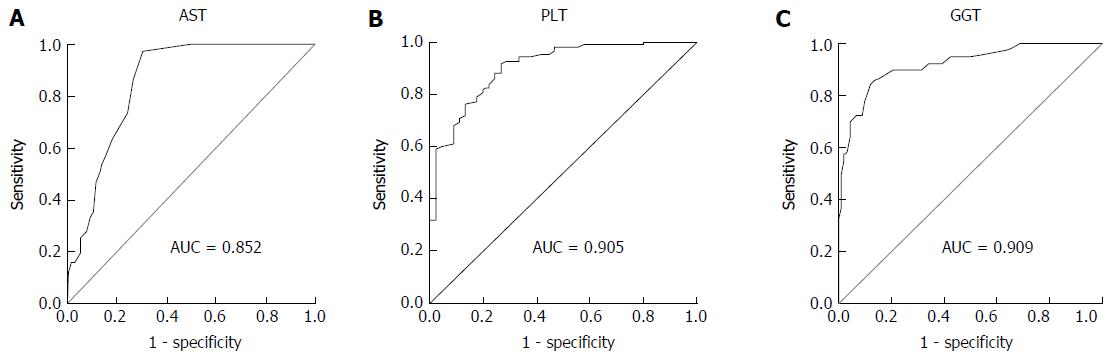
Marked necroinflammation (Knodell activity index ≥ 7) and fibrosis (Ishak fibrosis score ≥ 3) were found in 36.5% and 15.5% of CHB patients with PNALT, respectively. Following a univariate logistic regression analysis, multiple logistic regression analysis indicated that aspartate transaminase (AST) (AUROC = 0.852, cut-off value = 22.5 U/L) serves as an independent predictor of notable liver inflammation, while platelet (PLT) count (AUROC = 0.905, cut-off value = 171.5 ×109/mL) and gamma-glutamyl transpeptidase (GGT) (AUROC = 0.909, cut-off value = 21.5 U/L) level serve as independent predictors of notable liver fibrosis.
A considerable proportion of marked histological abnormalities existed in our cohort, who will benefit from optimal therapeutic strategies administered according to predictive indication by AST, PLT and GGT levels.
Core tip: Marked necroinflammation and fibrosis were present in the livers of chronic hepatitis B virus-infected Chinese patients with persistently normal alanine aminotransferase. Clinical parameters associated with liver injury were identified. The identified biomarkers can indicate patients with liver injury.
- Citation: Cheng JL, Wang XL, Yang SG, Zhao H, Wu JJ, Li LJ. Non-ALT biomarkers for markedly abnormal liver histology among Chinese persistently normal alanine aminotransferase-chronic hepatitis B patients. World J Gastroenterol 2017; 23(15): 2802-2810
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2802.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2802
Approximately 240 million people worldwide are chronically infected with the hepatitis B virus (HBV)[1,2]. Despite the availability of an effective HBV vaccine, 4.5 million new HBV infections occur each year[3]. Chronic HBV infection displays a broad spectrum of clinical manifestations, from inactive hepatitis B surface antigen (HBsAg) carrier status to chronic hepatitis B (CHB) infection with severe liver injury leading to cirrhosis, liver failure and hepatocellular carcinoma (HCC)[4].
Current antiviral therapy is effective in mitigating liver injury and blocking the progression of CHB to advanced stages of chronic liver disease. Current treatment guidelines recommend antiviral therapy for CHB patients with: (1) either serum alanine aminotransferase (ALT) levels twice the upper limit of normal (ULN) or liver histology showing grade (G) ≥ 2 (mild/spotty periportal inflammation; focal or unicellular necrosis) or stage (S) ≥ 2 (early periportal fibrosis or rare portal septa with intact architecture); and (2) HBV DNA levels of either ≥ 10000 IU/mL for patients who are negative for the hepatitis B e antigen (HBeAg) or ≥ 100000 IU/mL for HBeAg-positive patients[2,5,6]. Liver biopsy is also suggested for patients over 40 years of age with mildly elevated (1-2 × ULN) serum ALT levels. However, antiviral treatment is not recommended for patients with persistently normal alanine aminotransferase (PNALT) because they are categorized as having an absence of significant liver injury.
Elevated ALT is usually an indicator of liver injury and is an important reference for physicians making treatment decisions. However, some studies have demonstrated that ALT may not be sensitive enough to reflect hepatic necroinflammation under certain circumstances. Other studies found that 10% to 37% of HBsAg carriers with PNALT had significant necroinflammation and fibrosis, and 61.8% of HBeAg-negative inactive carriers had severe liver injury[7,8]; yet, it is a challenge to identify patients with PNALT who are experiencing liver injury and need antiviral treatment. Liver biopsy remains a preferred approach for confirming the presence and extent of hepatic injury before administration of antivirals, as advanced imaging techniques remain unable to accurately detect necroinflammation and early stages of fibrosis in the liver[9].
In this study, we investigated the frequency of hepatic necroinflammation and fibrosis among 115 Chinese CHB patients with PNALT who underwent liver biopsy. We analysed correlations between clinical parameters and marked histological changes in the liver and identified additional biomarkers, other than ALT, which may indicate notable hepatic necroinflammation and fibrosis.
Between January 1, 2010 and December 31, 2015, 115 CHB patients with PNALT were recruited and underwent liver biopsies at the First Affiliated Hospital, Medical College of Zhejiang University. All patients met the following inclusion criteria: (1) positivity for HBsAg for at least 6 mo; (2) ALT and aspartate transaminase (AST) testing every 3 mo showing normal levels for at least 1 year prior to liver biopsy; (3) HBV DNA level > 1000 IU/mL; and (4) naivety to antiviral treatment. Patients were excluded if they had significant alcohol consumption (30 g/d for males; 20 g/d for females), concomitant liver diseases or viral infections, including hepatitis C/D virus, human immunodeficiency virus dual-infection, auto-immune hepatitis, Wilson’s disease, biliary tract disease or cirrhosis. This study was approved by the Ethics Committee of Zhejiang University, and written informed consent was obtained from all participants.
Biochemical assays and viral parameters: Serum samples were obtained from all patients prior to liver biopsy. The serum biochemical tests included: ALT, AST, alkaline phosphatase (ALP), total bilirubin (TB), gamma-glutamyl transpeptidase (GGT), total bile acid (TBA), creatinine (Cr), albumin (ALB), globulin (GLB), albumin to globulin (A/G) ratio, white blood cell count (WBC), platelet (PLT) count, prothrombin time (PT) and thrombin time (TT). The ULN for ALT level was defined as 40 IU/L for males and 35 IU/L for females.
Serum HBsAg, anti-HBsAg, HBeAg, anti-HBeAg and hepatitis B core antibody (HBcAb) were tested by immunoassay (Abbott GmbH & Co. KG, Wiesbaden, Germany). Serum HBV DNA was measured by a quantitative fluorescence polymerase chain reaction (PCR) kit (Ai Kang Biological Technology Co. Ltd., Hangzhou, China).
Biopsy samples: Ultrasound-guided liver biopsies were obtained with 18G biopsy Magnum® needles (BARD Ltd., Tempe, AZ, United States). Samples of liver tissue that were at least 1.5 cm long or contained at least six portal areas were considered acceptable for inclusion in the study. Biopsy specimens were fixed and paraffin-embedded. Sections were stained with haematoxylin and eosin (HE) for morphological evaluation and Masson’s trichrome for assessment of fibrosis. The Knodell histologic activity index (HAI)[10] was used to grade necroinflammation from 0 to 18 points, with grade of 7 or greater indicating significant necroinflammation. The Ishak fibrosis score[11] was used to stage liver fibrosis from 0 to 6 points, and a score of 3 or higher (presence of bridging fibrosis or cirrhosis) reflected significant fibrosis. Two senior pathologists, who were blinded to the clinical data, independently read the sections, and joint discussions were used to resolve differences in scoring between the two pathologists.
SPSS (version 16.0; SPSS Inc., Chicago, IL, United States) was used to perform all statistical analyses. Categorical variables were analysed by Pearson’s χ2 test. Continuous variables were compared using independent t-tests for normally distributed data and Mann-Whitney U tests for skewed data. Simple and multiple logistic regression models were used to identify serum markers associated with marked liver necroinflammation and fibrosis. All significant factors identified by the univariate analysis were entered into the multivariate models for identifying predictors associated with marked alterations of liver histology. A P value less than 0.05 was considered statistically significant.
The baseline characteristics of 115 carriers with PNALT are shown in Table 1. The mean age was 39.7 years (range, 21-67 years), and 62 (54.2%) subjects were male. Among the 115 patients, 86 (55.5%) were HBeAg-positive and 69 (44.5%) were HBeAg-negative.
| All (n = 155) | HBeAg-positive (n = 86) | HBeAg-negative (n = 69) | |
| Age (yr) | 39.7 ± 9.9 | 42.9 ± 8.9 | 37.2 ± 9.9 |
| Male, n (%) | 84 (54.2) | 52 (60.9) | 34 (48.8) |
| HBV DNA (logIU/mL) | 5.8 ± 2.1 | 4.3 ± 1.4 | 6.9 ± 1.9 |
| WBC (109/mL) | 5.9 ± 3.1 | 5.9 ± 4.4 | 5.8 ± 1.5 |
| PLT (109/mL) | 190.08 ± 62.9 | 182.29 ± 57.7 | 196.3 ± 66.5 |
| ALT (U/L) | 26.0 ± 8.7 | 25.0 ± 9.2 | 26.8 ± 8.1 |
| AST (U/L) | 24.6 ± 6.6 | 24.1 ± 6.1 | 25.1 ± 7.0 |
| ALP (U/L) | 68.1 ± 20.1 | 67.1 ± 17.2 | 68.9 ± 22.2 |
| GGT (U/L) | 21.8 ± 14.6 | 21.1 ± 11.9 | 22.3 ± 16.5 |
| TB (g/L) | 13.6 ± 6.7 | 13.6 ± 7.5 | 13.6 ± 6.1 |
| PT (s) | 11.4 ± 1.1 | 11.4 ± 1.0 | 11.4 ± 1.4 |
| TT (s) | 19.1 ± 1.7 | 18.8 ± 1.8 | 19.3 ± 1.5 |
| A/G ratio | 1.7 ± 0.4 | 1.8 ± 0.4 | 1.7 ± 0.4 |
| Cr (μmol/L) | 70.2 ± 14.6 | 70.0 ± 15.2 | 70.4 ± 14.2 |
| TBA (μmol/L) | 8.7 ± 14.1 | 6.3 ± 6.0 | 10.7 ± 17.9 |
| GLB (g/L) | 27.2 ± 4.3 | 26.9 ± 4.1 | 27.5 ± 4.4 |
| ALB (g/L) | 45.6 ± 4.0 | 46.7 ± 4.0 | 44.5 ± 3.9 |
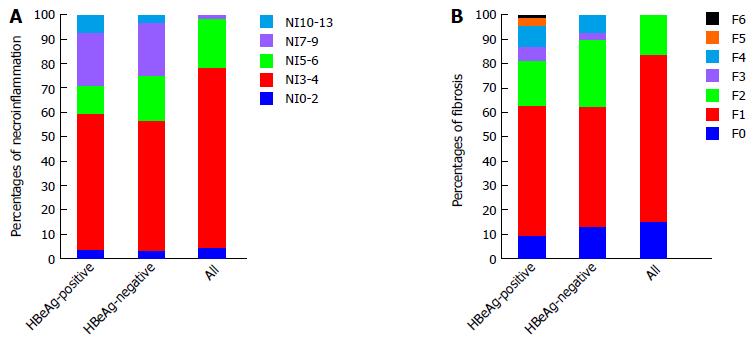
The frequency and score distributions for liver necroinflammation and fibrosis are shown in Figure 1. Marked necroinflammation (Knodell activity index ≥ 7) and fibrosis (Ishak fibrosis score ≥ 3) were observed in 36.5% (n = 42) of patients (of which 59.5% (n = 25) were HBeAg-positive, and 40.4% (n = 17) were HBeAg-negative) and 15.5% (n = 24) (of which 70.8% (n = 17) were HBeAg-positive, and 29.2% (n = 7) were HBeAg-negative) of subjects, respectively. Classic histological characteristics of necroinflammation and fibrosis are shown in Figure 2.
Distributions of marked liver abnormalities for those with lower (ALT ≤ 0.5 × ULN) and higher (0.5 × ULN < ALT < 1 × ULN) normal ALT levels are shown in Figure 3. There were no significant differences in liver necroinflammation and fibrosis between HBeAg-positive subgroup (P = 0.0264 and P = 0.094) and HBeAg-negative subgroup (P = 0.095 and P = 0.497 respectively); however, the HBeAg-positive subgroup tended to have more severe necroinflammation and fibrosis when compared to the HBeAg-negative group. And, the higher normal ALT levels displayed the same trend regardless of the state of the e antigen.
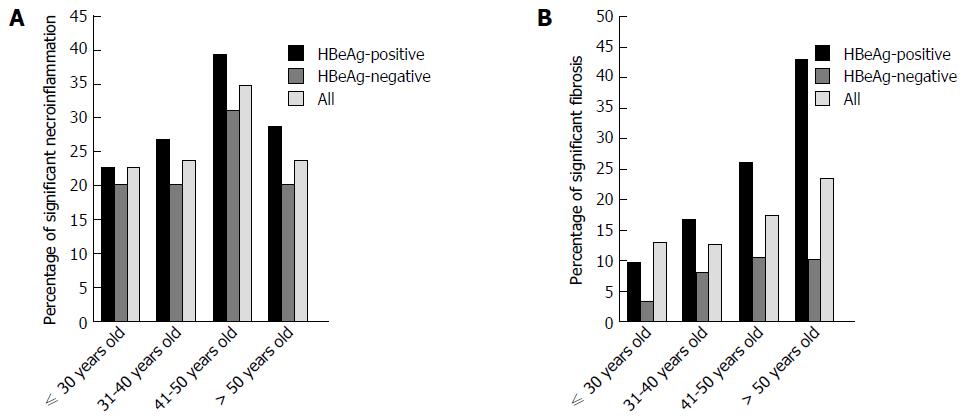
The percentage of biopsies with marked liver necroinflammation and fibrosis in patients among the different age ranges are shown in Figure 4. Percentage of liver necroinflammation increased from 22.6% in patients aged 30 years or younger to 34.6% in the 41-50 years group; then, the percentage decreased to 23.6% in patients older than 50 years. The highest prevalence of significant necroinflammation was detected in the 41-50 years group (n = 18, P = 0.713) (Figure 4A). The patients who were 41- to 50-years-old in the HBeAg-positive and HBeAg-negative subgroups showed a similar trend of significant necroinflammation (n = 9, 39.1%; n = 9, 31.0%, separately). The majority of subjects with significant fibrosis were 50-years-old (23.5%, P = 0.205), with a dramatic increase detected in the HBeAg-positive subgroup (42.9%) (Figure 4B). But, the percentage of significant fibrosis in the HBeAg-negative subgroup with 50 years old was similar with that of the 41-50 years group; however, there was again no statistical significance to the observed difference (P = 0.199; P = 0.707, separately).
Demographic and clinical characteristics of subjects with and without marked hepatic histological changes are presented in Table 2. Patients with significant liver necroinflammation had higher ALT and AST values (both, P < 0.01) and lower PLT values (P = 0.016) compared to patients without necroinflammation. Patients with marked liver fibrosis had higher AST, ALP, GGT and TBA values (P = 0.012, 0.004, 0.000 and 0.002, respectively) and lower PLT value (P = 0.001) compared to those without fibrosis.
| Significant necroinflammation (n = 42) | No significant necroinflammation (n = 113) | P value | Significant fibrosis (n = 24) | No significant fibrosis (n = 131) | P value | |
| Age (yr) | 40.0 ± 9.3 | 39.7 ± 10.1 | 0.854 | 41.3 ± 10.3 | 39.5 ± 9.8 | 0.416 |
| Male, n (%) | 13 | 79 | 0.417 | 20 | 23 | 0.505 |
| HBV DNA (logIU/mL) | 6.0 ± 2.0 | 5.7 ± 2.2 | 0.368 | 5.6 ± 2.0 | 5.8 ± 2.1 | 0.733 |
| WBC (109/mL) | 6.2 ± 5.7 | 5.7 ± 1.4 | 0.560 | 4.9 ± 1.2 | 6.0 ± 3.3 | 0.117 |
| PLT (109/mL) | 170.2 ± 58.4 | 197.5 ± 63.2 | 0.016 | 150.3 ± 51.1 | 197.4 ± 62.3 | 0.001 |
| ALT (U/L) | 29.6 ± 8.8 | 24.6 ± 8.3 | 0.001 | 29.7 ± 8.0 | 25.3 ± 8.6 | 0.116 |
| AST (U/L) | 27.5 ± 6.0 | 23.6 ± 6.0 | 0.004 | 27.8 ± 6.4 | 24.1 ± 6.6 | 0.012 |
| ALP (U/L) | 70.4 ± 25.0 | 67.2 ± 17.9 | 0.463 | 78.7 ± 29.4 | 66.1 ± 17.3 | 0.004 |
| GGT (U/L) | 25.8 ± 19.4 | 20.3 ± 12.1 | 0.423 | 33.9 ± 25.4 | 19.6 ± 10.3 | 0.000 |
| TB (g/L) | 14.1 ± 6.9 | 13.4 ± 6.7 | 0.538 | 16.0 ± 7.2 | 13.2 ± 6.6 | 0.057 |
| PT (s) | 11.6 ± 1.0 | 11.3 ± 1.2 | 0.100 | 11.7 ± 1.0 | 11.3 ± 1.2 | 0.152 |
| TT (s) | 19.4 ± 1.7 | 19.0 ± 1.6 | 0.235 | 19.2 ± 1.8 | 19.1 ± 1.6 | 0.776 |
| A/G ratio | 1.8 ± 0.4 | 1.7 ± 0.4 | 0.173 | 1.7 ± 0.3 | 1.8 ± 0.4 | 0.700 |
| Cr (μmol/L) | 66.7 ± 14.6 | 71.5 ± 14.4 | 0.070 | 71.8 ± 14.2 | 69.9 ± 14.7 | 0.557 |
| TBA (μmol/L) | 9.0 ± 8.1 | 8.7 ± 15.7 | 0.900 | 16.8 ± 30.5 | 7.3 ± 7.4 | 0.002 |
| GLB (g/L) | 26.5 ± 4.3 | 27.5 ± 4.2 | 0.183 | 27.3 ± 3.9 | 27.2 ± 4.3 | 0.915 |
| ALB (g/L) | 45.1 ± 3.4 | 45.8 ± 4.2 | 0.335 | 44.2 ± 3.5 | 45.9 ± 4.1 | 0.058 |
Clinical parameters that may function as predictors for marked liver necroinflammation and fibrosis were identified by univariate and multivariate analyses (Table 3). Univariate analysis showed that PLT (P = 0.030), ALT (P = 0.009) and AST (P = 0.002) were independently associated with marked liver inflammation. Multiple logistic regression analysis with stepwise forward selection identified AST (P = 0.007 < 0.05) as a possible independent predictor for marked liver inflammation (OR = 1.682, 95%CI: 0.791-0.913). The area under receiver operating characteristic curve (AUROC) for AST was 0.852, a 22.5 U/L AST cut-off value for predicting moderate necroinflammation (Knodell activity index HAI ≥ 7) had 86.7% sensitivity and 82.5% specificity (Figure 5A). PLT (P = 0.003), AST (P = 0.036), ALP (P = 0.006), GGT (P = 0.007) and TBA (P = 0.048) were independently associated with marked liver fibrosis by univariate logistic regression analysis, and multiple logistic regression analysis showed PLT (P = 0.008, OR = 0.688, 95%CI: 0.854-0.955) and GGT (P = 0.036, OR = 1.453, 95%CI: 0.856-0.963) levels as possible independent predictors for marked liver fibrosis. The AUROCs for PLT and GGT were 0.905 and 0.909; and, when 171.5× 109/mL for PLT and 21.5 U/L for GGT were used as the cut-off values for predicting moderate fibrosis (Ishak fibrosis score ≥ 3), there were 81.8% and 80.0% sensitivity and 86.7% & 82.5% specificity, respectively (Figure 5B and C).
| Significant necroinflammation | Significant fibrosis | |||||||
| Univariate | Multivariate | Univariate | Multivariate | |||||
| OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 0.994 (0.949, 1.042) | 0.812 | 1.003 (0.968, 1.040) | 0.120 | ||||
| Male | 1.351 (0.530, 3.440) | 0.529 | 1.346 (0.656, 2.760) | 0.418 | ||||
| HBV-DNA (logIU/mL) | 1.249 (0.978, 1.595) | 0.075 | 1.078 (0.910, 1.276) | 0.385 | ||||
| WBC (109/mL) | 0.907 (0.648, 1.269) | 0.569 | 0.611 (0.359, 1.039) | 0.069 | ||||
| PLT (109/mL) | 0.990 (0.980, 0.999) | 0.03 | 0.692 (0.646, 0.709) | 0.003 | 0.688 (0.641, 0.723) | 0.008 | ||
| ALT (U/L) | 1.081 (1.020, 1.147) | 0.009 | 1.059 (0.985,1.139) | 0.121 | ||||
| AST (U/L) | 1.590 (1.502, 1.651) | 0.0002 | 1.682 (1.613, 1.704) | 0.007 | 1.091 (1.006, 1.185) | 0.036 | ||
| ALP (U/L) | 1.018 (0.994, 1.042) | 0.139 | 1.051 (1.015, 1.089) | 0.006 | ||||
| GGT (U/L) | 1.021 (0.993, 1.050) | 0.151 | 1.524 (1.491, 1.587) | 0.007 | 1.453 (1.406, 1.511) | 0.036 | ||
| TB (g/L) | 0.988 (0.921, 1.059) | 0.723 | 1.025 (0.945, 1.112) | 0.547 | ||||
| PT (s) | 1.683 (0.941, 3.009) | 0.079 | 2.022 (1.012, 4.042) | 0.083 | ||||
| TT (s) | 1.235 (0.945, 1.615) | 0.122 | 1.294 (0.948, 1.768) | 0.105 | ||||
| A/G ratio | 2.053 (0.570, 7.393) | 0.271 | 0.580 (0.099, 3.394) | 0.546 | ||||
| Cr (μmol/L) | 0.974 (0.941, 1.009) | 0.143 | 1.000 (0.957, 1.046) | 0.997 | ||||
| TBA (μmol/L) | 1.063 (0.998, 1.131) | 0.057 | 1.065 (1.001, 1.133) | 0.048 | ||||
| GLB (g/L) | 0.953 (0.862, 1.053) | 0.345 | 1.001 (0.877, 1.143) | 0.986 | ||||
| ALB (g/L) | 0.931 (0.836, 1.036) | 0.191 | 0.923 (0.805, 1.058) | 0.250 | ||||
In this study, we performed liver biopsies on 115 chronically infected HBV patients with PNALT and found that 36.5% and 15.5% of them had marked liver necroinflammation and fibrosis, respectively. Thus, nearly one-third of these patients experienced liver injury despite PNALT. Tan et al[12] reported only 9.8% necroinflammation and 12.1% fibrosis in Chinese CHB patients whose ALT levels were persistently normal. However, our findings were consistent with another previous study, in which 37% of CHB patients with PNALT levels were found to have marked necroinflammation and fibrosis in the livers[13]. Yet other studies have further suggested that CHB patients with normal ALT levels have an increased risk of long-term cirrhotic complications and HCC[14,15]. Published studies suggest that ALT may not be an effective marker for hepatic injury in selecting patients for antiviral therapy, since serum ALT levels may fail to indicate liver injury under certain circumstances.
The American Association for the Study of Liver Diseases notes that the ULN ALT range increases from childhood through adulthood, especially in males, and that the upper limit is about 10% higher in men aged 40 years compared to those at 25[2]. Some scholars have questioned whether the current upper limit of ALT should be lowered. Prati et al[16] suggested lowering it to 30 U/L for men and 19 U/L for women. An Asian study based on 1105 healthy individuals also concluded the ULN of ALT level should be lowered to 33 U/L for men and 25 U/L for women[17]. In addition, patients with ALT above 1.5 × ULN (defined as 53 U/L for males and 36 U/L for females in that study) were already at increased risk for cirrhotic complications[14]. Thus, patients with ALT at the ULN will likely experience liver injury.
A number of studies have shown no significant differences in marked liver necroinflammation and fibrosis among patients with up to twice the ULN of ALT[18,19], suggesting that ALT is sometimes not sensitive enough to indicate the extent of liver injury. Our results suggest that a majority of patients aged 41-50 had marked liver necroinflammation and fibrosis. A likely explanation for age-associated increase in liver injury is that liver injury in patients with PNALT occurs on a small scale that may not cause ALT elevation, even though the accumulation of liver injury over decades can lead to significant alterations in liver histology[20]. However, in our study, prevalence of liver damage appeared to decline in those 50 and older.
No difference was noted in the percentages of liver necroinflammation and fibrosis between HBeAg-positive and -negative subgroups (P > 0.05). Similar percentages of fibrosis in these groups suggest that fibrosis observed in the HBeAg-negative patients was likely carried over from the HBeAg-positive phase. The similar percentages of necroinflammation in these two groups were a surprise, suggesting a portion of HBeAg-negative patients were experiencing the liver injury as reported in HBeAg-negative patients who had pre-core stop mutations accompanied by high level of HBV replication and elevated liver injury. Data from published studies have failed to yield consensus on this issue. For instance, Chao et al[21] reviewed 9 studies (n = 830) and found that Asian CHB patients with ALT levels ≤ 40 U/L had significant fibrosis, irrespective of HBeAg status. Another study found that significant histological changes in the liver were rare in HBeAg-negative patients with PNALT levels[22].
We also analysed correlations of biochemical and virological parameters with liver necroinflammation and fibrosis. It appeared that a higher AST level may predict significant necroinflammation. This correlation was in agreement with the report that AST level can accurately reflect the extent of liver necroinflammation in HBeAg-positive CHB patients[23].
In this study, lower PLT count and elevated GGT were independently associated with marked fibrosis (P = 0.008 and 0.036, respectively). These findings were consistent with previous studies in which decreased PLT counts were accompanied by liver fibrosis among CHB patients[24-26], due largely to decreased production of thrombopoietin by the reduced number of functional hepatocytes[27]. As reported, serum GGT levels can be an independent predictor for liver fibrosis[28]. Our findings were consistent with a previous study that showed both PLT count and GGT correlated with significant fibrosis[29].
There were a few limitations in our study. First, this was a retrospective cross-sectional study that introduced selection bias. Second, the size of our cohort was small; however, CHB patients with PNALT do not routinely undergo liver biopsy, and some patients were reluctant to accept such an invasive procedure. Thus, the 115 patients in our study should be an appropriate size in this regard. We plan to screen for possible prognosis markers from the serum and tissues of the PNALT-CHB patients with molecular and proteomic tools, and then to combine these findings with the clinical parameters to achieve better prediction of prognosis.
In summary, we found that nearly one-third of CHB patients with PNALT had significant alterations in their liver histology, including prominent necroinflammation and fibrosis. We further found that patients with lower normal ALT level and HBeAg negativity were more likely to have liver injury, and that most patients with significant pathological changes in their livers were 41-50 years old. Higher AST may help indicate liver injury. Our results imply: (1) the current ULN of ALT is too high to sensitively indicate liver injury if which occurs on a small scale; (2) CHB patients with PNALT should be screened for liver injury, and higher than 22.5 IU/L and 21.5 IU/L of AST and GGT, and lower than 171.5 × 109/mL of PLT can be used as an additional reference for selection of patients for liver biopsy to confirm liver injury; and (3) if patients with PNALT do have severe liver injury confirmed by liver biopsy, they should be given appropriate therapy to prevent further progression of liver disease.
Chronic hepatitis B (CHB) patients with persistently normal alanine aminotransferase (PNALT) are rarely given antiviral treatment because they are believed to have minimal or no liver injury. However, some studies demonstrate that there are significant liver necroinflammation and fibrosis among those patients with PNALT, and the alanine aminotransferase (ALT) utility is limited in detecting liver injury in this category of patients.
The research hotspot is to determine the incidences of liver necroinflammation and fibrosis, and correlate them with non-ALT clinical parameters among a cohort of Chinese CHB patients with PNALT.
This study found that the liver morphological changes are more accurately reflected with Knodell necroinflammation index and Ishak fibrosis score, comparing the traditional G/S scores.
The current treatment guidelines do not recommend antiviral therapy for CHB patients with PNALT. This study suggests that a portion of these patients need liver biopsy to verify presence or absence of significant liver injury or, alternatively, non-ALT markers can be used to detect the significant liver injury.
CHB patients with PNALT represent a specific group of CHB patients who persistently show normal ALT level during long clinical course.
The data presented in the report are very suggestive of a new “reformulation” of liver injury in CHB patients. The paper deals with an interesting approach to CHB patients and liver damage.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boscá L, Romanelli RG S- Editor: Ma YJ L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Sorrell MF, Belongia EA, Costa J, Gareen IF, Grem JL, Inadomi JM, Kern ER, McHugh JA, Petersen GM, Rein MF. National Institutes of Health Consensus Development Conference Statement: management of hepatitis B. Ann Intern Med. 2009;150:104-110. [PubMed] |
| 2. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 3. | Chan HL, Jia J. Chronic hepatitis B in Asia-new insights from the past decade. J Gastroenterol Hepatol. 2011;26 Suppl 1:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | McMahon BJ. Natural history of chronic hepatitis B. Clin Liver Dis. 2010;14:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 5. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S; Chronic Hepatitis B Guideline Working Party of the Asian-Pacific Association for the Study of the Liver. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 6. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 7. | Kumar M, Sarin SK, Hissar S, Pande C, Sakhuja P, Sharma BC, Chauhan R, Bose S. Virologic and histologic features of chronic hepatitis B virus-infected asymptomatic patients with persistently normal ALT. Gastroenterology. 2008;134:1376-1384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 320] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Lin CL, Liao LY, Liu CJ, Yu MW, Chen PJ, Lai MY, Chen DS, Kao JH. Hepatitis B viral factors in HBeAg-negative carriers with persistently normal serum alanine aminotransferase levels. Hepatology. 2007;45:1193-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Fung J, Lai CL, Seto WK, Yuen MF. The use of transient elastography in the management of chronic hepatitis B. Hepatol Int. 2011;5:868-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Knodell RG, Ishak KG, Black WC, Chen TS, Craig R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1:431-435. [PubMed] |
| 11. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] |
| 12. | Tan Y, Ye Y, Zhou X, Chen L, Wen D. Age as a predictor of significant fibrosis features in HBeAg-negative chronic hepatitis B virus infection with persistently normal alanine aminotransferase. PLoS One. 2015;10:e0123452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Lai M, Hyatt BJ, Nasser I, Curry M, Afdhal NH. The clinical significance of persistently normal ALT in chronic hepatitis B infection. J Hepatol. 2007;47:760-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 263] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Yuen MF, Yuan HJ, Wong DK, Yuen JC, Wong WM, Chan AO, Wong BC, Lai KC, Lai CL. Prognostic determinants for chronic hepatitis B in Asians: therapeutic implications. Gut. 2005;54:1610-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 292] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 15. | Kumada T, Toyoda H, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, Kanamori A, Atsumi H, Takagi M, Arakawa T. Incidence of hepatocellular carcinoma in patients with chronic hepatitis B virus infection who have normal alanine aminotransferase values. J Med Virol. 2010;82:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002;137:1-10. [PubMed] |
| 17. | Lee JK, Shim JH, Lee HC, Lee SH, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Estimation of the healthy upper limits for serum alanine aminotransferase in Asian populations with normal liver histology. Hepatology. 2010;51:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 18. | Seto WK, Lai CL, Ip PP, Fung J, Wong DK, Yuen JC, Hung IF, Yuen MF. A large population histology study showing the lack of association between ALT elevation and significant fibrosis in chronic hepatitis B. PLoS One. 2012;7:e32622. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Wang H, Xue L, Yan R, Zhou Y, Wang MS, Cheng MJ. Comparison of histologic characteristics of Chinese chronic hepatitis B patients with persistently normal or mildly elevated ALT. PLoS One. 2013;8:e80585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 590] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 21. | Chao DT, Lim JK, Ayoub WS, Nguyen LH, Nguyen MH. Systematic review with meta-analysis: the proportion of chronic hepatitis B patients with normal alanine transaminase ≤ 40 IU/L and significant hepatic fibrosis. Aliment Pharmacol Ther. 2014;39:349-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Papatheodoridis GV, Manolakopoulos S, Liaw YF, Lok A. Follow-up and indications for liver biopsy in HBeAg-negative chronic hepatitis B virus infection with persistently normal ALT: a systematic review. J Hepatol. 2012;57:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Cheong JY, Kim DJ, Hwang SG, Yang JM, Kim YB, Park YN, Cho SW. Serum markers for necroinflammatory activity in patients with chronic viral hepatitis and normal or mildly elevated aminotransferase levels. Liver Int. 2011;31:1352-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Wu IC, Lai CL, Han SH, Han KH, Gordon SC, Chao YC, Tan CK, Sievert W, Tanwandee T, Xu D. Efficacy of entecavir in chronic hepatitis B patients with mildly elevated alanine aminotransferase and biopsy-proven histological damage. Hepatology. 2010;51:1185-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Adinolfi LE, Giordano MG, Andreana A, Tripodi MF, Utili R, Cesaro G, Ragone E, Durante Mangoni E, Ruggiero G. Hepatic fibrosis plays a central role in the pathogenesis of thrombocytopenia in patients with chronic viral hepatitis. Br J Haematol. 2001;113:590-595. [PubMed] |
| 26. | Kawasaki T, Takeshita A, Souda K, Kobayashi Y, Kikuyama M, Suzuki F, Kageyama F, Sasada Y, Shimizu E, Murohisa G. Serum thrombopoietin levels in patients with chronic hepatitis and liver cirrhosis. Am J Gastroenterol. 1999;94:1918-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Nagamine T, Ohtuka T, Takehara K, Arai T, Takagi H, Mori M. Thrombocytopenia associated with hepatitis C viral infection. J Hepatol. 1996;24:135-140. [PubMed] |
| 28. | Giannini E, Ceppa P, Botta F, Fasoli A, Romagnoli P, Cresta E, Venturino V, Risso D, Celle G, Testa R. Steatosis and bile duct damage in chronic hepatitis C: distribution and relationships in a group of Northern Italian patients. Liver. 1999;19:432-437. [PubMed] |
| 29. | Fung J, Lai CL, Fong DY, Yuen JC, Wong DK, Yuen MF. Correlation of liver biochemistry with liver stiffness in chronic hepatitis B and development of a predictive model for liver fibrosis. Liver Int. 2008;28:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |