Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2763
Peer-review started: November 8, 2016
First decision: December 19, 2016
Revised: January 8, 2017
Accepted: March 6, 2017
Article in press: March 6, 2017
Published online: April 21, 2017
Processing time: 163 Days and 7.7 Hours
To assess the impact of underlying liver disease etiology on the presenting features and outcomes in a large cohort of patients with hepatocellular carcinoma (HCC).
A prospective database of all patients with HCC was established from 1998 to March 2012. One thousand and seventy-eight patients were categorized into three groups, based on the etiology of their liver disease: hepatitis B virus (HBV), hepatitis C virus (HCV) and non-viral liver disease (NVLD). Overall survival was determined by Kaplan Meier analysis to time of death or last follow-up.
HCC patients with HCV (85%) were more likely to be diagnosed as part of a surveillance program, compared to HBV or NVLD (both 71%) (P < 0.001). Patients with NVLD were more likely to receive best supportive care (29%) compared to those with HBV (21%) or HCV (20%) (P < 0.02). Twelve percent of NVLD and 13% of HBV patients underwent liver transplantation compared to 21% of HCV patients (P = 0.001). Median survival from presentation was lowest in NVLD (1.7 years) when compared to HBV (2.8 years) and HCV (2.6 years) (P < 0.05). In multivariate analysis, independent predictors of survival included Child Turcotte Pugh score, size of dominant lesion, absence of vascular invasion, and management with surgical resection or liver transplantation. Patient age and the etiology of the underlying liver disease were not independent predictors of survival
Patients with NVLD and HCC were less likely to be enrolled in a HCC surveillance program and are less likely to have curative therapies such as liver resection and transplantation after diagnosis with HCC, when compared to patients with Hepatitis B and Hepatitis C.
Core tip: In this prospective study of 1078 patients, we examined the relationship between etiology of liver disease with clinical presentation and outcome, following a diagnosis of hepatocellular carcinoma. Our results are clinically useful and relevant for gastroenterologists worldwide. In our diverse and multi-ethnic cohort, patients diagnosed with hepatocellular carcinoma (HCC), who have a background of non-viral liver disease are: (1) Less likely to be participating in an HCC surveillance program; (2) Have more severe liver disease at diagnosis; (3) Have a greater tumor burden at diagnosis; and (4) Less likely to have curative therapies for HCC like liver transplantation or liver resection.
- Citation: Mohsen W, Rodov M, Prakoso E, Charlton B, Bowen DG, Koorey DJ, Shackel NA, McCaughan GW, Strasser SI. Patients with non-viral liver disease have a greater tumor burden and less curative treatment options when diagnosed with hepatocellular carcinoma. World J Gastroenterol 2017; 23(15): 2763-2770
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2763.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2763
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer deaths worldwide, with nearly 700000 deaths attributed to HCC each year. While the burden of HCC is highest in Asia and Africa, its incidence is rising in the developed world, in countries such as the United Kingdom, France, the United States and Australia[1,2]. The majority of HCC in both developed and developing countries is attributable to viral hepatitis. Hepatitis B virus (HBV) and hepatitis C virus (HCV) are becoming more prevalent in developed countries, due to migration from high prevalence areas and transmission in past decades from injecting drug use[1]. Subjects with HCV and HBV related cirrhosis are at particular risk of HCC. The annual incidence rates of HCC in patients with HCV and HBV are 1%-9% and 0.5%-6% respectively[3,4].
Increasing rates of obesity and diabetes in the developed world have contributed to the rise of nonalcoholic fatty liver disease (NAFLD). Some patients with NAFLD, particularly those with nonalcoholic steatohepatitis (NASH), progress to cirrhosis and subsequently to HCC[5-8]. The association between alcoholic cirrhosis and HCC is well established. A recent study showed that alcoholic cirrhosis was the major risk factor in 29% of patients diagnosed with HCC at the Mayo Clinic[5]. The mechanisms by which alcoholic liver disease and NAFLD contribute to the development of HCC are thought to be similar[9,10]. These are largely dependent on the presence of liver cirrhosis as a prerequisite for the development of HCC[11,12].The annual incidence of HCC among patients with alcoholic and NASH cirrhosis has been reported as 2%-8%[1,2,11].
HCC is associated with a high morbidity and mortality rate, particularly among those with cirrhosis. The five-year survival rate is 14%-18%[13,14]. Survival with HCC relates to both the degree of underlying liver impairment and tumor stage at diagnosis[5]. Despite limited data from randomized clinical trials[15] early detection offers the best chance for curative treatment for patients with HCC[16-18].
HCC risk factors have been clearly established and surveillance of those at risk is recommended. The American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) recommend an ultrasound of the liver every 6 mo, for patients with cirrhosis due to underlying viral and non-viral liver disease (NVLD)[10]. Routine surveillance of patients with HBV and HCV cirrhosis has been well adopted by gastrointestinal subspecialists however screening of patients with non-viral liver cirrhosis, particularly due to alcohol and NASH appears to be less consistent[16-19]. The aim of this study was to assess the impact of liver disease etiology on the presenting features and outcomes of HCC.
A prospective database of all patients with HCC was established in 1998 at the Royal Prince Alfred Hospital in Sydney, Australia. This is the only liver transplant centre in the state of NSW. This database was censored for this retrospective study in March 2012. Analysis was undertaken of all patients (n = 1078) in this database and patients were categorized into three groups, based on the etiology of their liver disease: HBV, HCV or NVLD. The clinical database and this study were approved by the SLHD Ethics Review Committee (RPAH Zone). Informed consent was obtained.
A diagnosis of HBV infection required the presence of hepatitis B surface antigen (HBsAg). Infection with HCV was confirmed with anti-HCV antibodies and/or HCV PCR. Alcoholic liver disease was diagnosed on the basis of a compatible history of sustained heavy alcohol intake greater than 40 g/d coupled with the absence of viral markers and other causes of liver disease. This diagnosis was assisted with a liver biopsy, if one was available. The diagnosis of NAFLD was made following the exclusion of viral hepatitis and other causes of liver disease in a patient with known fatty liver disease or metabolic risk factors such as obesity and diabetes.
Cirrhosis was established in patients either by liver biopsy and/or the basis of results of clinical, laboratory and imaging studies. A diagnosis of HCC was based on positive histopathology on liver biopsy, fine needle aspiration or surgical resection and/or dynamic hepatic imaging demonstrating the presence of one or more liver lesions with enhancement on arterial phase and washout on the venous/delayed phase[20]. The size and number of lesions and presence of vascular invasion were determined by multidisciplinary review of imaging (CT or MRI) and radiology reports.
Inclusion criteria included age greater than 18 years of age and a diagnosis of HCC confirmed on dynamic imaging (as described above) and/or supported by histopathology from a fine needle biopsy or surgical resection. Exclusion criteria included: (1) HBV and HCV co infection; and (2) Age less than 18 years of age.
Data were extracted from the computerized data based, electronic medical records, and clinic and hospital files. Variables included: patient demographics (age, sex, ethnicity) etiology of underlying liver disease, presence of cirrhosis, alpha- fetoprotein (AFP) level, Child Turcotte Pugh (CTP) score and Model for End Stage Liver Disease (MELD) score at presentation with HCC. MELD and CTP score were calculated based on the collection of blood results including albumin, creatinine, International normalized ratio (INR) and bilirubin. Clinic letters were used to determine if subjects had encephalopathy or ascites. Tumor-related variables included the number of lesions and size of the dominant lesion, extent of disease and the presence or absence of vascular invasion and/or extra hepatic spread. Liver clinic letters and hospital discharge summaries were used to determine if the patient was diagnosed as a result of screening.
Continuous variables were screened for normality using the Kolgmogorov-Smirnov and Shapiro-Wilk tests of normality. Non-parametric tests were used to analyse these variables with the Kruskal-Wallis test used to test for overall significance and Mann-Whitney U Tests used for planned pair wise comparisons. The independent variable was cause of liver disease (HBV, HCV, or NVLD). Cross tabulations of the categorical and ordinal variables (ethnicity, vascular invasion, metastases, CTP score, and size of lesion) broken down by cause of liver disease (HBV, HCV, or NVLD) were produced and χ2 tests of independence were performed to establish whether proportions were independent of cause of liver disease. To test for specific differences in proportions between the causes of liver disease for the variables of interest, Z-tests were computed with the Bonferroni correction adjustment used for all pair wise comparisons within a row for the comparison of percentages. Survival analysis was performed using the Kaplan-Meier estimator. The variable used was number of years from first presentation to death (uncensored cases) or last contact (censored cases). Analysis was performed with pair wise comparisons of survival between the different causes of liver disease (HBV, HCV, or NVLD).
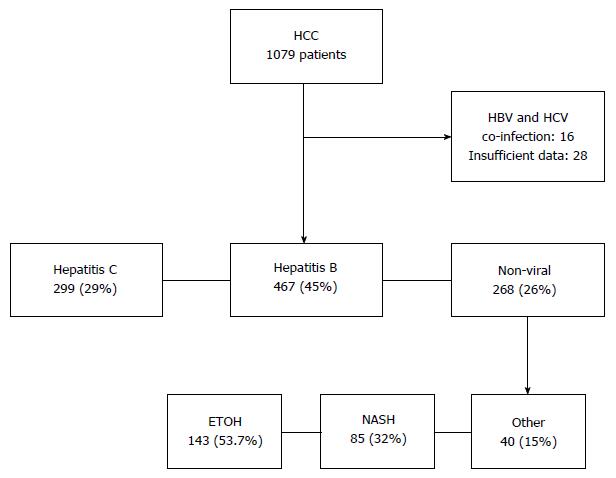
Of the 1078 patients included in the database, 28 patients (2.6%) with incomplete data and 16 patients (1.5%) with HBV and HCV co-infection were excluded. Of the remaining 1034 patients analysed in this study, liver disease was due to HCV in 467 (45%), HBV in 299 (29%) and NVLD in 268 (26%) patients. Alcoholic liver disease contributed to the majority of the NVLD cohort with 144 patients (54%), followed by NAFLD in 85 (32%) (Figure 1). Causes of liver disease in the remainder, included Hereditary Hemochromatosis (7%), Primary Biliary Cirrhosis (3%), Primary Sclerosing Cholangitis (2%), Autoimmune Hepatitis (1%), Alpha-1 antitrypsin deficiency (1%) and Congenital Hepatic Fibrosis (0.4%) (Table 1).
| HBV | HCV | Non-viral | |
| No patients | 467 (29) | 299 (45) | 268 (26) |
| Etiology of non-viral liver disease | |||
| Alcoholic liver disease | 144 (53.7) | ||
| NAFLD | 85 (31.7) | ||
| Haemochromatosis | 18 (6.7) | ||
| Primary sclerosing cholangitis | 6 (2.3) | ||
| Primary biliary cirrhosis | 8 (3) | ||
| Alpha-1 antitrypsin deficiency | 3 (1.1) | ||
| Congenital hepatic fibrosis | 1 (0.4) | ||
| Autoimmune hepatitis | 3 (1.1) |
When diagnosed with HCC, patients with a background of NVLD were older (median 64 years), compared with those with HBV (57 years) or HCV (55 years) (P < 0.001). The majority of patients diagnosed with HCC were male but gender did not differ between the three study groups. Patients with NVLD and HCV patients were more likely to be Caucasian, whereas the HBV cohort were predominantly of Asian ethnicity (P < 0.001) (Table 2).
| HBV | HCV | Non-viral | P value | |
| Male (%) | 84.3 | 79.2 | 80.6 | < 0.250 |
| Ethnicity (%) | ||||
| Caucasian | 16.4 | 73.4 | 87.3 | < 0.001 |
| Asian | 75.8 | 18.2 | 9.0 | < 0.001 |
| Middle Eastern | 3.7 | 7.1 | 2.2 | < 0.001 |
| Polynesian | 2.3 | 0.6 | 0.0 | < 0.001 |
| Aboriginal | 0.3 | 0.2 | 1.1 | < 0.001 |
| African | 1.3 | 0.4 | 0.4 | < 0.001 |
| Median age (95%CI) | 57 (55-59) | 55 (54-56) | 64 (63-65) | < 0.001 |
| Screening program (%) | 71 | 84 | 71 | < 0.001 |
| Median AFP (95%CI) | 60 (40-98) | 21 (16-28) | 9 (6-19) | < 0.001 |
HCC was more likely to be diagnosed within a screening/surveillance program in patients with HCV (84.1%). Seventy-one percent of patients with HBV or NVLD were participating in a screening program, prior to diagnosis with HCC (P < 0.001) (Table 2).
In those newly diagnosed with HCC, patients with NVLD and HCV presented with more severe underlying liver disease, compared to those with HBV. This was demonstrated by MELD scores and CTP classification (Table 3). The median MELD scores of the NVLD and HCV groups were 8.78 (95%CI: 8.1-10.2) and 8.2 (95%CI: 7.6-8.8) respectively. The median MELD for the HBV groups was 6.42 (95%CI: 5.8-7.3). This was statistically significant when comparing the NVLD and HCV groups to the HBV cohort (P < 0.001). A higher proportion of patients with HCV (43.4%) and NVL (45.4%) had hepatic decompensation (CTP B or C) at presentation, compared to those with HBV (18.6%) (P < 0.001). Of patients with HCV, 2.6% did not have cirrhosis, whereas 12.8% of those with NVLD and 22.2% of those with HBV did not have cirrhosis (P < 0.001).
| HBV (%) | HCV (%) | Non-viral (%) | P value | |
| Child-Pugh score | ||||
| A | 58.6 | 53.9 | 41.9 | < 0.001 |
| B | 13.7 | 29.0 | 28.3 | < 0.001 |
| C | 4.9 | 14.4 | 17.1 | < 0.001 |
| Non-cirrhotic | 22.8 | 2.6 | 12.8 | < 0.001 |
| Median MELD score | 6.4 | 8.2 | 8.8 | < 0.001 |
| Cirrhosis | 77.8 | 97.4 | 87.2 | < 0.001 |
Patients with a background of NVLD and HBV had a greater tumor burden at presentation, compared to patients with a background of HCV (Table 4). Size of dominant lesion, proportion with liver involvement over 50% and evidence of macro vascular invasion were all more common in those with NVLD and HBV, whereas patients with HCV were more likely to have lesions under 5 cm. The median AFP was higher in the HBV patients than in those with HCV or NVLD (P < 0.001).
| HBV (%) | HCV (%) | Non-viral (%) | P value | |
| Size of dominant lesion | ||||
| < 1 cm | 1.3 | 2.8 | 2.6 | < 0.001 |
| 1-3 cm | 34.4 | 46.5 | 32.1 | < 0.001 |
| 3-5 cm | 22.4 | 23.8 | 20.5 | < 0.001 |
| > 5 cm | 32.0 | 18.0 | 34.0 | < 0.001 |
| Unknown | 10.0 | 9.2 | 10.4 | < 0.001 |
| Tumor extent > 50% | 12.4 | 4.2 | 12.5 | < 0.001 |
| Vascular invasion | 10.4 | 6.7 | 12.3 | < 0.020 |
Patients with HCC in the setting of NVLD were more likely to receive best supportive care and less likely to receive curative treatments such as resection and transplantation, compared to patients with HCV and HBV (Table 5). Nearly a third of patients with NVLD were unsuitable for active management at presentation (P < 0.02). A greater proportion of patients with HCC on a background of HCV (21.4%) underwent liver transplantation, compared to patients with NVLD (12.3%) and HBV (13.4%) (P < 0.002). Twenty-four point four percent of patients with HBV-associated HCC underwent surgical resection as primary management, a significantly higher rate that in those with HCV (8.8%) or NVLD (13.1%) (P < 0.001). All three groups were equally as likely to have TACE as primary treatment (39.2%-45.4%). Ablative therapies including percutaneous ethanol injection, radiofrequency ablation and microwave ablation, were more common as primary treatment in HCV-associated HCC (P = 0.011).
| Treatment | Hepatitis B (%) | Hepatitis C (%) | Non-viral (%) | P value |
| TACE | 44.5 | 45.4 | 39.2 | 0.099 |
| Ablation | 11.1 | 18.4 | 10.8 | 0.011 |
| Resection | 24.4 | 8.8 | 13.1 | < 0.001 |
| Transplant | 13.4 | 21.4 | 12.3 | 0.001 |
| Sorafenib | 5 | 4.1 | 4.1 | 0.098 |
| Supportive care | 21 | 20 | 29 | < 0.02 |
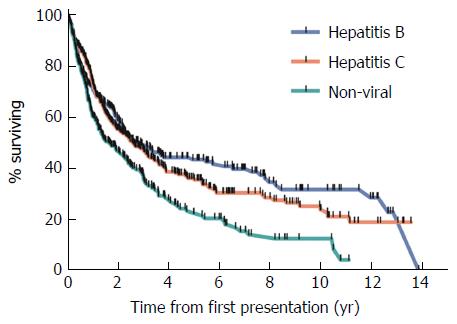
Overall survival was lowest in patients with NVLD with a median survival of 1.7 years (Figure 2). Overall survival was 2.8 and 2.6 years in HBV and HCV patients respectively (P < 0.05). Survival at 1, 2 and 5 years was lower in those with NVLD, compared to HCV and HBV patients (P < 0.05). Mortality in all groups was mainly related to tumor progression and/or liver failure.
In multivariate analysis, independent predictors of survival included CTP score, size of dominant lesion, absence of vascular invasion, and management with surgical resection or liver transplantation (Table 6).
| Variable | Reference | HR | 95%CI for HR | P value |
| Hepatitis B | Non-viral | 0.900 | 0.674-1.200 | 0.472 |
| Hepatitis C | Non-viral | 1.086 | 0.841-1.401 | 0.527 |
| Age at presentation | Years | 1.009 | 1.000-1.018 | 0.062 |
| Non-cirrhotic | Child Pugh C | 0.267 | 0.165-0.433 | < 0.001 |
| Child Pugh A | Child Pugh C | 0.403 | 0.287-0.566 | < 0.001 |
| Child Pugh B | Child Pugh C | 0.705 | 0.501-0.992 | 0.045 |
| Gender | Male | 0.978 | 0.748-1.280 | 0.874 |
| MELD | MELD score | 1.016 | 0.999-1.033 | 0.072 |
| Resection | Yes | 2.212 | 1.434-3.413 | < 0.001 |
| Dominant lesion < 1 cm | Dominant lesion > 5 cm | 0.375 | 0.156-0.898 | 0.028 |
| Dominant lesion 1-3 cm | Dominant lesion > 5 cm | 0.527 | 0.394-0.704 | < 0.001 |
| Dominant lesion 3-5 cm | Dominant lesion > 5 cm | 0.740 | 0.550-0.995 | 0.046 |
| Sorafenib | Yes | 1.118 | 0.688-1.817 | 0.652 |
| TACE | Yes | 1.371 | 0.938-2.004 | 0.103 |
| Transplant | Yes | 8.486 | 5.185-13.889 | < 0.001 |
| Tumor extent < 50% | Tumor extent > 50% | 0.690 | 0.476-1.000 | 0.050 |
| Vascular invasion | Yes | 0.555 | 0.393-0.784 | 0.001 |
HCC is a disease of rising incidence and mortality among many developed countries. Screening of high-risk candidates for HCC is recommended by international guidelines[20] to allow for early detection and effective treatment, thus reducing the burden of this disease. Previous studies show that screening in patients with NVLD occurs less frequently when compared to patients with HBV and HCV[16,19].
Many series reporting on outcomes of HCC are predominantly limited to populations of predominantly hepatitis B (from Asian countries) or hepatitis C (from Japan, North America or Europe). Liver disease due to alcohol often accounts for a smaller proportion of patients in these series[1,5], and often the proportion attributable to NAFLD is not reported, or termed cryptogenic liver disease[5,11,12]. The strength of our study population is that includes almost equal proportions of patients with hepatitis B, hepatitis C and NVLD, and includes large numbers of both Asian and Caucasian patients reflecting the multi-ethnic mix of a large Australian city. This unique balance of etiologies allows for an examination of the relationship between presenting features and outcomes according to the cause of underlying liver disease.
It was somewhat reassuring that patients with HCV cirrhosis were likely to be diagnosed with HCC within a screening program, and perhaps this is why these patients had a lower tumor burden at diagnosis. In Australia, it is estimated that 85% of patients with HCV have been diagnosed as part of a screening program, a much higher percentage than in most other countries[21,22]. It is likely that many of these patients are under specialist care, and those with advanced disease have been identified and entered into screening programs. Despite severe underlying liver disease, many of the patients with HCV-associated HCC were diagnosed within transplant criteria, and over 20% underwent transplantation.
In contrast the diagnosis rates of hepatitis B among migrants is likely to be somewhat lower, and many patients, even if diagnosed, may be considered as “healthy carriers” and not referred for specialist care or entered into routine HCC surveillance programs. These factors may account for the relatively higher rates of advanced tumor presentations, despite less severe liver disease in patients with HBV-associated HCC. Of note, 22% of patients with HBV-associated HCC were non-cirrhotic and probably did not have clinically apparent liver disease. Screening recommendations for patients with HBV include patients without cirrhosis, patients older than 50, those with a family history of HCC, and those with active hepatitis[20]. It seems likely that these screening recommendations are not followed by non-specialists. Furthermore, if patients are identified with HBV cirrhosis or active hepatitis, the introduction of antiviral therapy may significantly reduce the chance of developing HCC in the future[23,24].
Non-alcoholic liver disease and alcoholic liver disease, the main causes of NVLD, are relatively common in the community, and it is likely that many of these patients are not under regular specialist care. Particularly in patients with NAFLD, advanced fibrosis and cirrhosis are under-recognized in primary care. Over reliance on ultrasound and a tendency to dismiss mildly abnormal liver tests as benign fatty liver disease, without the use of non-invasive investigations, such as the Fibroscan result in a missed opportunity to identify cirrhosis and appropriately refer or screen patients at risk. Furthermore 13% of patients with NVLD (predominantly NAFLD patients) did not appear to have cirrhosis at presentation with HCC, although a role for HCC surveillance in this group of patients has not been established.
Another important determinant of outcome may have been the age at diagnosis of HCC. Patients with either HBV, most of whom would have been infected in the perinatal period, or HCV which was mostly acquired in early adulthood from injecting drug use, presented at a younger age than those with NVLD. It is unclear whether age had a modifying effect on disease presentation, however it may have been important in determining appropriate treatment. Interestingly, age was just outside the threshold for statistical significance when analyzed as a predictor of survival, using the multivariate analysis.
In our cohort, univariate analysis showed that survival was poorer in patients with NVLD compared to those with chronic viral hepatitis. Poorer survival in patients with NVLD is likely to be multifactorial, related to more advanced liver disease, a greater tumor burden, and presentation at an older age, compared to patients with viral hepatitis, all of which were demonstrated in this study.
Indeed, in multivariate analysis, etiology was not an independent predictor of survival, but was related to tumor and liver disease severity. Related to poor characteristics at presentation, patients with NVLD and HCC were less likely to be amenable to curative, or even effective palliative management approaches and were more likely to receive best supportive care. Almost a third of patients with NVLD were not suitable for any active management, including Sorafenib. This is compared to 21% of HCV patients who were suitable for liver transplantation.
Our study has several limitations. We did not differentiate between death due to HCC from death due to other causes. Patients with NVLD were older and were more likely to have comorbidities, in particular cardiovascular disease. It is likely that the majority of deaths were liver-related, when one considers that these patients had more advanced liver disease, more advanced HCC, and were more likely to be managed with supportive therapy from the time of diagnosis.
Another limitation in our study relates to how our subjects came to be diagnosed with HCC. We documented if patients were diagnosed as result of screening by scrutinizing their medical records. If patients were found to have a small asymptomatic lesion, during an imaging procedure performed to evaluate their liver disease, even if not enrolled in a formal screening program, we categorized them in the screened group. This was to distinguish them from patients who presented with larger symptomatic HCC lesions. In our country, as in many others, screening is often not well documented, and may be done on an ad hoc basis rather than in a routine surveillance program.
In conclusion, in a large cohort of patients with HCC, those with NVLD had a greater tumor burden, worse liver function and older age at diagnosis, all resulting in a lower likelihood of receiving curative therapies such as surgical resection or liver transplantation. Importantly, patients with NVLD were more likely to present with symptomatic disease, rather than be diagnosed as a consequence of routine surveillance while asymptomatic. Our results highlight the need for early identification of liver disease in patients at risk of HCC, so that risk stratification and appropriate specialist referral and enrolment within an HCC surveillance program can be undertaken.
Hepatocellular carcinoma (HCC) is one of the leading causes of cancer deaths worldwide. Subjects with chronic Hepatitis B and subjects with cirrhosis from hepatitis C or non viral hepatitis (predominantly alcoholic liver disease and non alcoholic steatohepatitis) are at risk of developing HCC. These subjects should be screened regularly.
Effective screening of patients at risk of developing HCC, leads to the detection of HCC at an early stage, when curative therapy is possible.
Subjects with non viral liver disease are less likely to be screened for HCC. These subjects are more likely to present with a greater burden of HCC, and have less curative treatment options.
This article emphasises the importance of: (1) Identifying cirrhosis among subjects with non viral liver disease, by using objective diagnostic tools like fibroscan; and (2) Surveillance of subjects at risk of developing HCC, using six monthly abdomen ultrasounds.
The study is of interest for researchers in the field. The analysis is well performed, the results are relevant and the paper is well written. Mohsen et al may consider excluding patients without alcoholic cirrhosis and non alcoholic steatohepatitis from the non viral liver disease cohort.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Makisalo H, Nakajima H, Ocker M, Silva ACS S- Editor: Ma YJ L- Editor: A E- Editor: Zhang FF
| 1. | Kemp W, Pianko S, Nguyen S, Bailey MJ, Roberts SK. Survival in hepatocellular carcinoma: impact of screening and etiology of liver disease. J Gastroenterol Hepatol. 2005;20:873-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012;4:19-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2508] [Article Influence: 192.9] [Reference Citation Analysis (2)] |
| 4. | El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med. 2003;139:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 714] [Cited by in RCA: 684] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 5. | Yang JD, Harmsen WS, Slettedahl SW, Chaiteerakij R, Enders FT, Therneau TM, Orsini L, Kim WR, Roberts LR. Factors that affect risk for hepatocellular carcinoma and effects of surveillance. Clin Gastroenterol Hepatol. 2011;9:617-623.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Tokushige K, Hashimoto E, Horie Y, Taniai M, Higuchi S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: report of the nationwide survey. J Gastroenterol. 2011;46:1230-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Reddy SK, Steel JL, Chen HW, DeMateo DJ, Cardinal J, Behari J, Humar A, Marsh JW, Geller DA, Tsung A. Outcomes of curative treatment for hepatocellular cancer in nonalcoholic steatohepatitis versus hepatitis C and alcoholic liver disease. Hepatology. 2012;55:1809-1819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 8. | Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 9. | Stickel F, Hellerbrand C. Non-alcoholic fatty liver disease as a risk factor for hepatocellular carcinoma: mechanisms and implications. Gut. 2010;59:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1478] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 11. | White DL, Kanwal F, El-Serag HB. Association between nonalcoholic fatty liver disease and risk for hepatocellular cancer, based on systematic review. Clin Gastroenterol Hepatol. 2012;10:1342-1359.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 576] [Article Influence: 44.3] [Reference Citation Analysis (2)] |
| 12. | Hashimoto E, Yatsuji S, Tobari M, Taniai M, Torii N, Tokushige K, Shiratori K. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44 Suppl 19:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 13. | Altekruse SF, McGlynn KA, Dickie LA, Kleiner DE. Hepatocellular carcinoma confirmation, treatment, and survival in surveillance, epidemiology, and end results registries, 1992-2008. Hepatology. 2012;55:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 213] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 14. | Witjes CD, Karim-Kos HE, Visser O, van den Akker SA, de Vries E, Ijzermans JN, de Man RA, Coebergh JW, Verhoef C. Hepatocellular carcinoma in a low-endemic area: rising incidence and improved survival. Eur J Gastroenterol Hepatol. 2012;24:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54:1998-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 16. | Patwardhan V, Paul S, Corey KE, Mazhar SM, Richter JM, Thiim M, Chung RT. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialists. Dig Dis Sci. 2011;56:3316-3322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 308] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Amarapurkar D, Han KH, Chan HL, Ueno Y. Application of surveillance programs for hepatocellular carcinoma in the Asia-Pacific Region. J Gastroenterol Hepatol. 2009;24:955-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Akahoshi H, Taura N, Ichikawa T, Miyaaki H, Akiyama M, Miuma S, Ozawa E, Takeshita S, Muraoka T, Matsuzaki T. Differences in prognostic factors according to viral status in patients with hepatocellular carcinoma. Oncol Rep. 2010;23:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 21. | Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, Amarapurkar D, Chen CH, Dou X, El Khayat H. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31 Suppl 2:61-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 400] [Cited by in RCA: 395] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 22. | Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, Dalgard O, Dillion JF, Flisiak R, Forns X. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31 Suppl 2:30-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 287] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 23. | Robotin MC, Kansil M, Howard K, George J, Tipper S, Dore GJ, Levy M, Penman AG. Antiviral therapy for hepatitis B-related liver cancer prevention is more cost-effective than cancer screening. J Hepatol. 2009;50:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Strasser SI. Managing hepatitis B to prevent liver cancer: recent advances. Expert Rev Gastroenterol Hepatol. 2014;8:409-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |