Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2731
Peer-review started: December 30, 2016
First decision: February 10, 2017
Revised: February 23, 2017
Accepted: March 21, 2017
Article in press: March 21, 2017
Published online: April 21, 2017
Processing time: 112 Days and 18.9 Hours
To assess proportions, related conditions and survival of interval cancer (IC).
The programme has a linkage with different clinical databases and cancer registers to allow suitable evaluation. This evaluation involves the detection of ICs after a negative faecal inmunochemical test (FIT), interval cancer FIT (IC-FIT) prior to a subsequent invitation, and the detection of ICs after a positive FIT and confirmatory diagnosis without colorectal cancer (CRC) detected and before the following recommended colonoscopy, IC-colonoscopy. We conducted a retrospective observational study analyzing from January 2009 to December 2015 1193602 invited people onto the Programme (participation rate of 68.6%).
Two thousand five hundred and eighteen cancers were diagnosed through the programme, 18 cases of IC-colonoscopy were found before the recommended follow-up (43542 colonoscopies performed) and 186 IC-FIT were identified before the following invitation of the 769200 negative FITs. There was no statistically significant relation between the predictor variables of ICs with sex, age and deprivation index, but there was relation between location and stage. Additionally, it was observed that there was less risk when the location was distal rather than proximal (OR = 0.28, 95%CI: 0.20-0.40, P < 0.0001), with no statistical significance when the location was in the rectum as opposed to proximal. When comparing the screen-detected cancers (SCs) with ICs, significant differences in survival were found (P < 0.001); being the 5-years survival for SCs 91.6% and IC-FIT 77.8%.
These findings in a Population Based CRC Screening Programme indicate the need of population-based studies that continue analyzing related factors to improve their detection and reducing harm.
Core tip: Population based screening programmes are implemented when benefits are superior to harms and risks are acceptable to healthy population. However, programmes should continuously improve their quality and efficiency. This study shows by means of a well-accepted screening strategy that there is room for improvement and those programmes could be personalized or at least, stratified. Main results show that instead of a reduction in the cut-off points of faecal inmunochemical test, other strategies such as different follow up periods for sex, stage and previous location could be more effective and minimize risks at the same time that they increase benefits.
- Citation: Portillo I, Arana-Arri E, Idigoras I, Bilbao I, Martínez-Indart L, Bujanda L, Gutierrez-Ibarluzea I. Colorectal and interval cancers of the Colorectal Cancer Screening Program in the Basque Country (Spain). World J Gastroenterol 2017; 23(15): 2731-2742
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2731.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2731
The Basque Country is one of the 17 autonomous regions of Spain and has a population of approximately 2200000 inhabitants. Colorectal cancer (CRC) is the most common type of cancer when taking both sexes into account and is the most frequent in men[1]. The number of cases has increased since 1990 and there has been certain stability in mortality rate. In 2008, the year before the screening programme was implemented, there were 1869 new cases and 798 deaths were registered due to this type of cancer[2].
Following the 2003 European Guidelines[3] and the National Strategy against Cancer of 2006, validated in 2009[4,5], population based screening of CRC was approved by the Basque Autonomous Government and implemented in 2009. The screening is based on the detection of occult blood in faeces (FOB) using a biennial quantitative faecal immunochemical test (FIT), targeting women and men between 50 and 69 years of age (approximately 586700 inhabitants) and a colonoscopy under sedation for FIT positive cases. With the first invitation, almost 100% of the target population was reached at the beginning of 2014; by the end of 2015, 85% of the population had been invited at least twice and 56% a third time.
The characteristics of the programme and the main results of the first invitation were published in 2013, obtaining an average participation rate of (64.3%, 95%CI: 64.1%-64.5%) higher in women[6], finding significant differences in the rate of detection of Advanced Adenomas (AA) between women and men (OR = 0.45, 95%CI: 0.41-0.49) and CRC (OR = 0.80, 95%CI: 0.66-0.96), more frequent in men as well as the positive predictive value (PPV) for any type of adenoma which was significantly higher in men (72.4%, 95%CI: 71.2%-73.5%) than in women (48.8; 95%CI: 47.2%-50.5%), with differences depending on the age group and type of adenoma[7]. Likewise, there were differences in participation and detection of lesions according to the index of deprivation: men on the most deprived index, having a lower participation rate (60.2%) although a higher rate in the identification of lesions (55.7/1000) compared to the least deprived (41.0/1000)[8].
The characteristics of CRC detected by the programme in the first and second rounds after a negative result were observed by Bujanda et al[9]; significant differences being found in location, most frequently in the second round in the right-sided colon and in a less advanced stage.
Participation and the detection rate of advanced lesions and CRC were found to be within the parameters defined by the European Guidelines of Quality (2010)[10], which recommend 65% participation and an Adenoma detection rate between 13.3‰-22.3‰ and of CRC between 1.8‰-9.5‰. However, one aspect to bear in mind is the possible losses of the programme, which are interval cancers (ICs), and which are one of the biggest concerns of the screening programme as not only the capacity for detection is measured, but also the quality of the confirmatory diagnostic test, in this case a colonoscopy. As was pointed out by Robinson et al[11], in the Randomized Controlled Trial in Nottingham, both the positive effects (reduction of mortality because of screening) as well as the negative effects (false negatives among others) should be monitored and taken into account.
In fact, for a correct detection, it is necessary to have an organized Screening Programme and the possibility of individualized linkage with clinical databases (diagnostic procedures, pathological confirmation, hospital discharges and cancer registers), in such a way as to allow a suitable evaluation of the impact of the programmes[12].
A standardized methodology like the proposal for GISCoR 2013[13] is also required.
The aim of this study is to compare CRC detected by the Programme or screened cancers (SCs) and ICs detected from 2009-2015 both after a negative FIT and before the following invitation (IC-FIT), such as post-confirmatory colonoscopy cancers following a positive FIT and before a follow-up colonoscopy, depending on the lesion found (IC-colonoscopy).
The Basque Country’s Population Based Screening Programme has the support of a Coordinating Centre which plans, organizes, monitors and evaluates the individualized invitation process as well as the test results and follow-up of all positive cases. This is possible thanks to the interaction of software, specially designed for the programme, with clinical databases and cancer registries. The software contains a system of encryption and access in accordance with the current data protection laws as it is all systematically anonymized for analysis and subsequent publication. Participants in the programme are informed and consent to the use of their data. The FIT tests used were FOB Gold in 2009 and 2010 (Sentinel Diagnosis SpA, Milan Italy) and OC-Sensor from 2010 onwards (Eiken Chemical Co. Tokyo, Japan). Only one sample was collected, as the haemoglobin concentration cut-off (f-Hb) 100 ng Hb/mL of buffer, which is equivalent to 17 μg Hb/g faeces in Sentinel and to 20 μg Hb/g faeces in OC-Sensor, as the comparison of both tests showed[14].
The screening is based on the detection of occult blood in faeces (FOB) using a biennial quantitative FIT, targeting women and men between 50 and 69 years of age (approximately 586700 inhabitants) and a colonoscopy under sedation for FIT positive cases.
The tests were analyzed in the laboratories of the publicly-funded hospital system under strict internal and external quality control. The colonoscopies were also performed in publicly-funded hospitals by qualified and trained specialists in the digestive system; sedation was also provided by the same endoscopy team, although 20% included the presence of an anaesthetist. The recommendations of the European Guidelines (2010) and Spanish Guide for Quality Control (2011)[15] were followed in all cases. The resected polyps and CRC with biopsy and/or endoscopic or surgical resection were analyzed in laboratories of Pathological Anatomy by staff skilled in histopathology of gastrointestinal disease with specific emphasis on CRC. CRC was diagnosed when the neoplastic cells pass through the muscularis mucosae, invading the submucosae (≥ pT1). All CRCs were registered according to the criteria of the American Joint Committee on Cancer (AJCC)[16].
Detection of ICs after a negative FIT (IC-FIT): prior to a subsequent invitation, all negative cases from a previous round are linked to the register of hospital discharges with ICD-9 1530-1548, in primary and secondary diagnosis, ICDO-10 C18-C21 of hospital registers and population-based Cancer registries as well as codes of Pathology. In all coinciding cases, the qualified staff from the Programme’s Coordinating Centre checked the clinical history, including the cases as ICs which complied with the criteria of having a negative FIT result in the previous invitation (0-24 mo or more in case of a delay in the invitation to the screening programme). To ensure against any possible losses, this process was repeated on an annual basis with all negative FITs from the previous 24 mo.
Detection of ICs after a positive FIT and confirmatory diagnosis without CRC detected and before the following recommended colonoscopy (IC-colonoscopy): these data were annually cross-referenced with all the colonoscopies with a different result to detected CRC by the Programme or Screen-detected Colonoscopies (SCs), following the same methodology as the previous section. It was considered to be IC if a CRC was detected prior to the scheduled follow-up: 10 years with an invitation for a FIT in the case of a normal result/non-neoplastic polyps; 5 years with an invitation for a FIT in the case of a Low-Risk Adenoma (1-2 tubular adenomas and/or ≤ 10 mm and/or without high grade dysplasia); a colonoscopy after 3 years in the case of an Intermediate Risk Adenoma (3-4 adenomas and/or ≥ 10 mm and < 20 mm and/or a vellous component and/or a high grade dysplasia; colonoscopy in < 1 year in the case of a detected High-Risk Adenoma (≥ 5 adenomas and/or ≥ 20 mm). Given that the follow-up period is longer, cases detected up to May 2016 were taken into consideration, which means that the number could increase in the next five years.
In all the cases of SCs and ICs, the following variables were taken into account when analyzing: (1) type of participant (first invitation, regular - participated in the last two invitations, irregular - participated in at least one previous invitation), ICs were not considered in the case of not participating or refusing a colonoscopy after a positive FIT; (2) round of invitations; (3) sex and age; (4) deprivation index assigned to each patient - socioeconomic deprivation index (DI) of their area of residence, using the methodology of the MEDEA project[17]; (5) quantitative result of the test in μg Hemoglobin/g of faeces in invitations prior to a negative FIT; result of a confirmatory colonoscopy and date; diagnostic method in ICs and date; (6) details of CRC: location, size, morphology, TNM, degree of differentiation, state and type of first treatment; and (7) survival with a link to the Population Death Register until 1st December 2016.
For the description of qualitative variables frequency tables and percentages were used, for quantitative variables means and standard deviation or median and interquartile range (IQR). For comparison between two groups contrast of exploratory hypotheses have been made using exact test of Fisher or χ2 for categorical variables. To compare quantitative variables and categorical variables with 2 categories t-test or the non-parametric Mann Whitney U test was used. Logistic regression analysis was performed with interval vs screening detected CRC as the outcome variable. Overall survival after CRC diagnosis for the patients with interval CRC was compared, by Kaplan-Meier estimation, long-rank test and Cox proportional hazard ratios. The survival time was measured from the date of CRC diagnosis to date of death or censoring resulting from the end of the study period (December 1, 2016). Significance was set at the 5% level. The analysis was performed by a biomedical statistician using IBM SPSS Statistics 23.0.
From January 2009 to December 2015, 1193602 people were invited to the CRC Screening Programme, with a participation rate of 68.6%. Of the participants, 49687 obtained a positive result, with 2518 cancers diagnosed, with a 92.7% acceptance rate for screening colonoscopies. The global adenoma detection rate by the Programme for the period studied was 57.25‰.
Seven point five percent (204/2722) of the diagnosed cancers were IC. Of the colonoscopies performed, 18 cases of IC-colonoscopy were found before the recommended follow-up and of the 769200 negative FITs, 186 IC-FIT were identified before the following invitation. Table 1 shows the characteristics associated with the three types of cancers analyzed in this study. Significant statistical differences were observed by age, round of invitations and characteristics of the tumour such as: location, stage, morphology, degree of differentiation and size of the tumour.
| Interval cancers | Screen-detected (SCs) | P value | ||
| FIT | Post-colonoscopy | |||
| Total | 186 (6.8) | 18 (0.7) | 2518 (92.5) | - |
| Patient characteristics | ||||
| Sex | ||||
| Male | 125 (67.2) | 10 (55.6) | 1651 (65.6) | 0.601 |
| Female | 61 (32.8) | 8 (44.4) | 867 (34.4) | |
| Age (yr) | ||||
| Mean (SC) | 60.2 (4.9) | 62.0 (3.5) | 61.7 (3.4) | 0.042 |
| 50-54 | 28 (15.1) | 0 (0) | 375 (14.9) | |
| 55-59 | 53 (28.5) | 5 (27.8) | 539 (21.4) | 0.001 |
| 60-64 | 61 (32.8) | 10 (55.6) | 709 (28.2) | |
| 65-69 | 44 (23.7) | 3 (16.7) | 895 (35.5) | |
| Round of invitation | 0.001 | |||
| 1 | 143 (76.9) | 16 (88.9) | 1615 (64.1) | |
| 2 | 40 (21.5) | 2 (11.1) | 680 (27.0) | |
| 3 | 3 (1.6) | 0 (0) | 223 (8.9) | |
| Type participation | 0.086 | |||
| Initial | 149 (80.1) | 17 (94.4) | 1863 (74.0) | |
| Regular | 35 (18.8) | 1 (5.6) | 639 (25.4) | |
| Irregular | 2 (1.1) | 0 (0) | 16 (0.6) | |
| Deprivation Index | 0.192 | |||
| 1 (least deprived) | 42 (23.9) | 3 (16.7) | 521 (21.4) | |
| 2 | 43 (24.4) | 1 (5.5) | 503 (20.6) | |
| 3 | 29 (16.5) | 6 (33.4) | 539 (22.1) | |
| 4 | 38 (21.6) | 2 (11.1) | 461 (18.9) | |
| 5 (most deprived) | 24 (13.6) | 5 (27.8) | 414 (17.0) | |
| Unknown | 0 (0) | 1 (5.5) | 0 (0) | |
| Time to diagnosis (months) | - | < 0.0001 | ||
| Median (IQR) | 13.5 (8.5-18.9) | 28.1 (16.5-40.1) | ||
| Range | 1.5-39.4 | 5.6-61.2 | ||
| Cancer characteristics | ||||
| Location | < 0.0001 | |||
| Proximal1 | 68 (36.6) | 7 (38.9) | 478 (19.0) | |
| Distal2 | 58 (31.2) | 9 (50.0) | 1529 (60.7) | |
| Rectum | 58 (31.2) | 2 (11.1) | 404 (16.0) | |
| Unknown | 2 (1.1) | 0 (0) | 107 (4.2) | |
| Stage | < 0.0001 | |||
| I | 43 (23.1) | 4 (22.2) | 1376 (54.6) | |
| II | 36 (19.4) | 2 (11.1) | 408 (16.2) | |
| III | 57 (30.6) | 7 (38.9) | 566 (22.5) | |
| IV | 50 (26.9) | 5 (27.8) | 152 (6.0) | |
| Unknown | 0 (0) | 0 (0) | 16 (0.6) | |
| Morphology | 0.002 | |||
| ADC, NOS | 139 (74.7) | 15 (83.3) | 1823 (72.4) | |
| ADC in adenomatous polyp | 4 (2.2) | 0 (0) | 121 (4.8) | |
| Carcinoid tumor | 0 (0) | 0 (0) | 7 (0.3) | |
| ADC in villous adenoma | 6 (3.2) | 0 (0) | 154 (6.1) | |
| ADC in tubulovillous adenoma | 10 (5.4) | 0 (0) | 174 (6.9) | |
| Mucinous ADC | 5 (2.7) | 2 (11.1) | 42 (1.7) | |
| Mucin-producing ADC | 3 (1.6) | 0 (0) | 23 (0.9) | |
| Signet ring cell carcinoma | 0 (0) | 0 (0) | 4 (0.2) | |
| Other | 15 (8.1) | 0 (0) | 64 (2.5) | |
| Unknown | 4 (2.2) | 1 (5.6) | 106 (4.2) | |
| Degree of differentiation | < 0.0001 | |||
| Well differentiated | 76 (41.1) | 7 (38.9) | 988 (39.2) | |
| Moderately differentiated | 59 (31.9) | 7 (38.9) | 1061 (42.2) | |
| Poorly differentiated | 14 (7.6) | 1 (5.5) | 86 (3.4) | |
| Undifferentiated/anaplastic | 33 (17.8) | 2 (11.1) | 320 (12.7) | |
| Unknown | 3 (1.6) | 1 (5.5) | 63 (2.5) | |
| Size (mm) | 0.022 | |||
| Median (IQR) | 20 (8.0-40.0) | 38.0 (30.0-60.0) | 26.0 (19.7-40.0) | |
| Range | 2.0-90.0 | 9.0-80.0 | 2.0-95.0 | |
| Treatment | < 0.0001 | |||
| Endoscopic resection | 5 (2.7) | 0 (0) | 733 (29.1) | |
| Surgery | 56 (30.1) | 9 (50.0) | 182 (7.2) | |
| Surgery and neoadjuvant therapy | 106 (57.0) | 7 (38.9) | 1332 (52.9) | |
| Palliative procedure | 18 (9.7) | 2 (11.1) | 164 (6.6) | |
| Unknown | 1 (0.5) | 0 (0) | 107 (4.2) | |
Table 2 shows the medians of f-Hb and their corresponding IQR in the invitation, just before the diagnosis of IC. No differences between the variables analyzed were observed: sex, age, deprivation index, time to diagnosis; neither was there differences found in the characteristics of the tumour: location, state, morphology, degree of differentiation and size. What was observed in both the analysis of variables as well as in the global (Median: 2.8 μg Hb/faeces; IQR: 0.4-9.9) was that the values of the f-Hb of the FIT with a negative result prior to the diagnosis of IC-FIT were found to be very distant from the cut-off point established as positive (20 μg Hb/g faeces). Patients diagnosed after two rounds with a negative result, also presented low values in the first round (Median: 0.8 μg Hb/g faeces; IQR: 0.0-4.5).
| Median f-Hb (μg Hb/g faeces) | IQR | P value | |
| All | 2.8 | 0.4-9.9 | - |
| Sex | |||
| Male | 3.4 | 0.2-10.0 | 0.409 |
| Female | 1.9 | 0.4-9.8 | |
| Age (yr) | 0.380 | ||
| 50-54 | 1.9 | 0.2-6.9 | |
| 55-59 | 3.0 | 0.6-13.0 | |
| 60-64 | 2.8 | 0.2-8.6 | |
| 65-69 | 4.0 | 0.4-12.5 | |
| Deprivation Index | 0.887 | ||
| 1 (least deprived) | 3.0 | 0.6-11.9 | |
| 2 | 2.6 | 0.0-10.2 | |
| 3 | 4.2 | 1.1-12.4 | |
| 4 | 4.0 | 0.4-11.2 | |
| 5 (most deprived) | 2.6 | 0.0-8.7 | |
| Time to diagnosis (mo) | 0.795 | ||
| Within 1 yr | 3.4 | 0.2-10.6 | |
| 1-2 yr | 2.5 | 0.5-9.6 | |
| Location | 0.171 | ||
| Proximal1 | 1.9 | 0.0-9.3 | |
| Distal2 | 3.8 | 0.3-13.7 | |
| Rectum | 3.9 | 0.7-9.1 | |
| Unknown | 1.0 | 0.0-… | |
| Stage | 0.927 | ||
| I | 2.0 | 0.0-9.7 | |
| II | 2.6 | 0.2-10.6 | |
| III | 3.2 | 0.7-9.8 | |
| IV | 4.0 | 0.2-9.9 | |
| Unknown | |||
| Morphology | 0.550 | ||
| ADC, NOS | 3.0 | 0.6-10.0 | |
| ADC in adenomatous polyp | 10.0 | 2.0-17.2 | |
| ADC in villous adenoma | 0.5 | 0.0-9.8 | |
| ADC in tubulovillous adenoma | 2.4 | 0.0-9.3 | |
| Mucinous ADC | 0.4 | 0.0-12.5 | |
| Mucin-producing ADC | 14.0 | 0.0-… | |
| Other | 1.7 | 0.0-6.0 | |
| Unknown | 1.0 | 0.0-6.9 | |
| Degree of differentiation | 0.600 | ||
| Well differentiated | 2.8 | 1.0-9.0 | |
| Moderately differentiated | 4.4 | 0.6-13.0 | |
| Poorly differentiated | 0.6 | 0.0-5.6 | |
| Undifferentiated/anaplastic | 4.0 | 0.0-9.4 | |
| Unknown | 2.0 | 0.0-… | |
| Size (mm) | |||
| < 10 | 1.4 | 0.4-12.4 | 0.586 |
| 10-19.99 | 1.0 | 0.0-7.9 | |
| ≥ 20 | 2.9 |
In Table 3 the characteristics of negative colonoscopies are shown for CRC prior to the diagnosis of an interval cancer (IC-colonoscopy) and after a positive FIT result. More than a third of the diagnosed cancers were seen to be in the sigma location and 27.8% in the caecum. In 55.5% of colonoscopy cases prior to screening, polyps were detected and removed, although in two cases their removal was in the same location as the diagnosed cancer, in sigma. In 83.3% of the colonoscopies, the caecum was reached and the rate of colonic preparation was adequate in the majority of cases (66.7%). It should be clarified that out of the 18 IC-colonoscopies, 12 had an adequate preparation, 2 had a bad quality of colonic cleansing and 4 were not described in the report.
| n (%) | |
| Interval CRC tumor site | |
| Appendix and caecum | 5 (27.8) |
| Ascending | 0 (0) |
| Hepatic flexure | 1 (5.6) |
| Transverse | 1 (5.6) |
| Splenic flexure | 0 (0) |
| Descending | 2 (11.1) |
| Sigmoid | 7 (38.8) |
| Rectum | 2 (11.1) |
| Unknown | 0 (0) |
| Polyp found on the Screening colonoscopy | |
| Yes | 10 (61.1) |
| No | 8 (38.9) |
| Polyp frequency | |
| Median (IQR) | 2.0 (1.0-3.5) |
| Range | 1-6 |
| Previous resection in the same location | |
| Yes | 2 (11.0) |
| No | 16 (89.0) |
| Report of incomplete Screening colonoscopy | |
| Yes2 | 3 (16.7) |
| No | 15 (83.3) |
| Polyp size on the Screening colonoscopy | |
| ≥ 10 mm | |
| Yes | 2 (11.1) |
| No | 16 (88.9) |
| Polyp histology (n = 10) | |
| Hyperplastic polyp | 0 (0) |
| LRA | 6 (60.0) |
| AA | 4 (40.0) |
| Bowel preparation | |
| Inadequate | 2 (11.1) |
| Adequate1 | 12 (66.7) |
| Unknown | 4 (22.2) |
| Diverticulosis | |
| Yes | 5 (27.8) |
| No | 13 (72.2) |
On the other hand, consultations were made about 85% of these interval cancers for suspected symptomology - rectal bleeding (30.1%) and abdominal pain (29.6%) being the most frequent causes, respectively. On the other hand, in those cases where there were no symptoms, anaemia was the diagnostic sign in 5.4% of the cases.
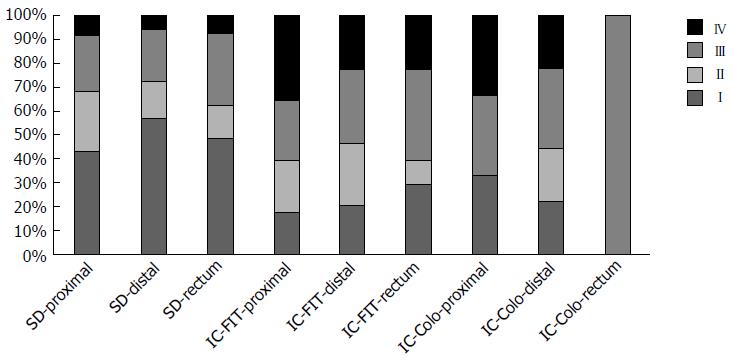
Figure 1 shows the distribution of the different types of colorectal cancer by stages at the time of diagnosis and its link to their location. A larger proportion of advanced stages were observed, firstly in the rectum and then in the proximal colon, in both the IC-FIT and IC-colonoscopy.
Table 4 shows that there was no statistically significant relation between the possible predictor variables of interval cancer by sex, age and deprivation index, but there was a relation between location and stage. However, it was observed that there was less risk when the location was distal rather than proximal (OR = 0.28, 95%CI: 0.20-0.40, P < 0.001), with no statistical significance when the location was in the rectum as opposed to proximal. The risk of having an advanced stage at the time of diagnosis was significantly higher in relation to the stage of the cancers detected out the programme said relation in Stage II: 2.73 (1.75-4.24); in Stage III 3.31 (2.24-4.88) and in Stage IV: 10.6 (6.93-16.18), respectively.
| % with CI | OR (95%CI) | P value | |
| Sex | |||
| Female | 69 (33.8) | 1 (ref.) | |
| Male | 135 (66.2) | 1.03 (0.76-1.39) | 0.860 |
| Age (yr) | |||
| 50-54 | 28 (13.7) | 1 (ref.) | |
| 55-59 | 58 (28.4) | 1.44 (0.90-2.31) | 0.127 |
| 60-64 | 71 (34.8) | 1.34 (0.84-2.11) | 0.206 |
| 65-69 | 47 (23.0) | 0.70 (0.43-1.14) | 0.153 |
| Deprivation | |||
| 1 (least deprived) | 45 (23.3) | 1 (ref.) | |
| 2 | 44 (22.8) | 1.01 (0.66-1.56) | 0.954 |
| 3 | 35 (18.1) | 0.75 (0.48-1.19) | 0.222 |
| 4 | 40 (20.7) | 1.00 (0.64-1.57) | 0.984 |
| 5 (most deprived) | 29 (15.0) | 0.81 (0.50-1.32) | 0.396 |
| Location | |||
| Proximal1 | 74 (36.8) | 1 (ref.) | |
| Distal2 | 67 (33.3) | 0.28 (0.20-0.40) | < 0.0001 |
| Rectum | 60 (29.9) | 0.96 (0.67-1.38) | 0.824 |
| Stage | |||
| I | 47 (23.0) | 1 (ref.) | |
| II | 38 (18.6) | 2.73 (1.75-4.24) | < 0.0001 |
| III | 64 (31.4) | 3.31 (2.24-4.88) | < 0.0001 |
| IV | 55 (27.0) | 10.59 (6.93-16.18) | < 0.0001 |
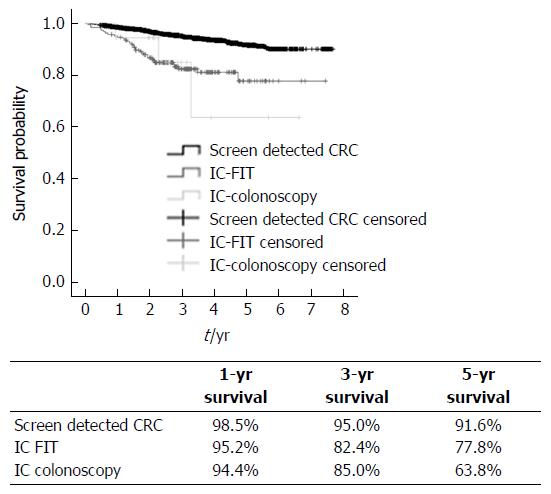
With regard to the analysis of survival rate, an average follow-up time was 3.6 ± 1.6 years (Range: 0.46-7.7 years). Significant differences in survival were found between groups (P < 0.0001), when comparing the screening-detected group (SCs) with the ICs. Figure 2 shows the survival graph and the percentages for groups at 1, 3 and 5 years, SCs having a better prognosis. It was also observed that for women, a distal rather than a rectal location, stages I-II has a significantly better survival prognosis as do SCs, as the risk of death is 3 times lower in relation to interval cancers (ICs).
Screening programmes are implemented into health systems to detect early and cure or improve the prognosis of the pathologies which they are aiming to address. There are two main aims in the case of early detection of cancer programmes: detecting the cancer at early stages to be able to cure it and, in the later stages, to begin the treatment to improve the chances of survival and the quality of life of the individuals concerned. There are a series of factors which determine the value and quality of the programmes. On the one hand, the cases which are not identified (false negatives) and the cases which are “wrongly identified” (false positives) and, on the other hand, the time periods in which there is no diagnosis and new premalignant or malignant lesions may occur. There are various screening methods and it is necessary to determine which is the most effective and which are the periods or typology of lesion (location, size...) to guarantee the best result for them to be effective and efficient. This study on the basis of the Population Based CCR screening Programme in the Basque Country, following a rigorous methodology in the diagnosis of both SCs and ICs, presents relevant data regarding both the methodology used as well as results of interest. The validity of these results is based on a programme with a high participation rate and acceptance of the diagnostic tests including colonoscopy, an effective cross-referencing between databases and adequate follow up in a public health system with universal coverage.
Various limitations can be found in the study’s findings. One of these is that when comparing FIT ICs to post-colonoscopy Interval Cancers, the latter are underestimated as they require a longer follow-up time and therefore the number of cases could increase in the next few years, hence the figures for post-colonoscopy ICs should be considered with caution. The same is not true regarding the data related to each individual case and their characteristics which justify the importance of this study.
Another limitation of this study is not having taken other risk factors of patient-related ICs into account, such as habits or other biochemical or genetic markers in order to provide a more detailed analysis. These factors are to be explored in prospective future studies. Advanced Adenomas were not analyzed either, as they were in the study by Stegeman et al[18]. In the FIT arm (OC Sensor) of the clinical trial, with a cut off of 50 ng/100 mL, defined a person with an advanced neoplasia (AA + CRC) found in a colonoscopy as a false negative. In Stegeman et al[18] study, of 1112 participants, 65 (64%) had a false negative result, age (OR = 1.04 per additional year) and smoking (OR = 2.02) were found to be risk factors and sensitivity in women was lower. However, these results coincide with our programme's regarding age and sex. The classification and definition used by Sanduleanu et al[19] was also employed our this study, using a modified Delphi methodology defining Interval Cancer as a “colorectal cancer diagnosed after a screening or surveillance exam in which no cancer is detected, and before the date of the next recommended exam” (Table 5). Classification was established based on the systematic revision from January 2004 to January 2014, checking the time of diagnosis, location, stage and histology as well as the etiology (missed, incompletely resected polyps or biological factors associated with a more rapid progression). In the afore-mentioned revision, people with hereditary CRC syndromes or inflammatory bowel diseases were excluded. This classification used in our study will allow a more standardized comparison of the results, which has not happened in previous studies.
| Hazard ratio | 95% CI | P value | |
| Sex | |||
| Female (ref) | |||
| Male | 1.39 | 1.01-1.93 | 0.049 |
| Age (yr) | |||
| 50-54 (ref) | 0.451 | ||
| 55-59 | 1.37 | 0.79-2.36 | 0.258 |
| 60-64 | 1.22 | 0.72-2.07 | 0.463 |
| 65-69 | 1.47 | 0.89-2.44 | 0.137 |
| Deprivation Index | |||
| 1 (least deprived) (ref) | 0.839 | ||
| 2 | 0.89 | 0.54-1.46 | 0.639 |
| 3 | 1.01 | 0.63-1.62 | 0.956 |
| 4 | 0.90 | 0.54-1.51 | 0.696 |
| 5 (most deprived) | 1.16 | 0.72-1.88 | 0.537 |
| Location | |||
| Rectum (ref) | < 0.001 | ||
| Proximal1 | 1.17 | 0.78-1.77 | 0.456 |
| Distal2 | 0.56 | 0.39-0.82 | 0.003 |
| Stage | |||
| I (ref) | < 0.001 | ||
| II | 1.99 | 1.11-3.54 | 0.020 |
| III | 3.67 | 2.31-5.83 | < 0.001 |
| IV | 26.54 | 17.37-40.56 | < 0.001 |
| CRC type | |||
| Screen detected CRC (ref) | < 0.0001 | ||
| IC FIT | 3.31 | 2.25-4.85 | < 0.0001 |
| IC colonoscopy | 3.49 | 1.11-10.97 | 0.032 |
Our results show similarities to those found by Zorzi et al[20] in Italian CRC screening programmes. In these studies, ICs which had had a negative result with only a FIT (OC-Sensor in 4 programmes and FOB Gold in one of them with a cut-off of 100 ng/100 mL) were analyzed. The follow-up period was from 2002-2007 in 267789 invitations via linkage with hospital discharges and an active search in clinical histories and pathological anatomy reports. Of the 126 ICs identified, compared with the expected 572 cases, 15.3% and 31.0% were found in the first and second period (interval-years), respectively. Of the total number of cases identified, in 86 cases with known stages, 21.2% were stage I, 22.3% stage II and 56.5% stages III/IV. The most advanced stage was found in the proximal colon (76.6% as opposed to 46.8% in the distal colon above the splenic flexure). The sensitivity for the proximal colon was 68.3% (95%IC: 57.7%-76.8%). Even though cases of those not invited and not participants were not analyzed, the most frequent cases in men with ICs and in the left-sided colon (distal colon and rectum) coincided exactly with those shown by Gill et al[21] in their comparative study of SCs (322 cases), ICs (192 cases), controls never invited (511) and non-participants (311) in the National Health Service. Although these were done with gFOBT, in which ICs were found to be more frequent in men (60.4%, P = 0.003), in the left-sided colon (66.7%, P = 0.003). However, the study undertaken by Steel et al[22], points out that where location is concerned, screening cancers are diagnosed in earlier stages than interval cancers in the colon (I-II: 62.2% vs 21.5%) but not in the rectum (I/II: 54.3% vs 49.9%); unlike in our study in which all screening cancers were diagnosed in earlier stages than those of interval cancers.
There was a significant reduction in the detection rate of SCs in subsequent rounds, which was also seen in ICs. When comparing the three rounds of screening with gFOBT of 48500 invitations with an average participation rate of 61.8%, 57.0% and 58.7% respectively, Moss et al[23] found a sensitivity of 71% and 50% in men and women, respectively, in the first round and 65% and 51%, respectively, in the second round, observing the same pattern of reduction. In our study, the detection rates of ICs were found to have the same trend, which points to the fact that screening is a protective factor, corroborated in our study by the high participation rate. In this sense, no significant differences were found regarding participation in SCs and ICs, unlike those found by Steele et al[24], who categorized the cancers detected in Scotland with the gFOBT Programme according to the pattern of participation, finding that of the 1927 CRC detected, 405 were SCs, 529 were ICs and 993 CRC in people who had not participated in over 2 years, and of which 658 had never participated. The stage was similar in those who had participated one, two or three times, indicating that it was not likely that the prognosis of SCs would be worse if it had not been detected in the first invitation. Similarly, differences were found between SCs and ICs in the pattern of participation.
Regarding the study by Garcia et al[25], differences were found in the detection rate of ICs in rounds. An increase was observed, even though different tests were used in four invitations with gFOBT and FIT, and a shorter follow-up period than in our case, 30 mo of monitoring (30480 tests carried out), finding 97 SCs, 74 ICs after a negative test result, 17 after an inconclusive result and 2 in post-colonoscopy follow-up. The rate of ICs increased in the rounds (32.4%-46.0%). In their study, they also found that the ICs were found predominantly in the rectum (OR = 3.66, 95%CI: 1.51-8.88), as opposed to our study in which they were more commonly found in a proximal location in the case of IC-FIT and distal in IC-colonoscopy. However, very similar data were found regarding the most advanced stages of ICs (P = 0.025) and there were no significant differences regarding sex or location.
Many studies published over the last few years have tried to study the impact of ICs in screening programmes as well as the factors associated with its appearance. Robertson et al[26] study, which monitored 9167 patients who had had a colonoscopy with adenomas diagnosed after a follow-up of an average of 47.2 mo, identified 58 ICs, 0.6%, similar to our findings of IC-colonoscopy (0.7%). Fifty-two percent of the CRC were classified as possible missed lesions, 24% as probable new lesions and 19% possibly related to a previous and incomplete resection of polyp. One of the risk factors associated with an IC-colonoscopy could therefore be an incomplete resection, which was shown in 11% of the cases in our study, which is an important fact in the quality of the programme and its possible consequences. le Clercq et al[27], in their follow-up of people diagnosed with CRC (5107 patients) five years after an index colonoscopy, where 147 ICs were identified or postcolonoscopy CRCs (PCCRCs) found that 8.8% were seen to have had an incomplete resection in the previous colonoscopy. Location could also be another risk factor to be taken into account. In this study as well as others which have been published in recent years [Brenner et al[28], Samadder et al[29] and Richter et al[30], a proximal location or right-sided colon seem to be a risk factor when developing an IC; similar to our study in which a proximal location was significantly more frequent than distal]. These locations would benefit from further targeted research.
Moreover, the study by Samadder et al[29], carried out on 26851 patients who had had a colonoscopy, found 159 ICs which developed between 6-60 mo after the colonoscopy. In 57.2% of the cases, previous adenomas had been identified, which is a similar percentage to our study (61.1%). As in other studies, another factor associated with IC is the stage. In the study by Samadder et al[29], as well as that by Brenner et al[28], ICs are diagnosed in more advanced stages than screening cancers, which also coincides with our study.
Neither sex nor age seem to play an important part in the diagnosis of an IC. Samadder et al[29], like our study, did not find any relation between these two factors. On the other hand, Richter et al[30] identify being over the age of 60 as a risk factor. These differences could be due to the context of implementation of the programmes, but new studies should corroborate or reject this hypothesis.
Another key point in screening programmes is the f-Hb cut-off. In a study carried out by Digby et al[31] with FIT analyzing interval cancers, they concluded that the average value of f-Hb just before the round prior to the diagnosis of interval cancer (2.8 μgr f-Hb/g faeces) is much lower than the cut-off used most frequently in screening programmes (20 μgr f-Hb/g Faeces). In this study, by reducing the cut-off to 10 μgr f-Hb/g Faeces, the rate of positives would increase from 2.4% to 9.4%, with an important increase in the need for colonoscopies (increasing the number of false positives), which would increase the proportion of interval cancers by 38.3%. In this study, a similar average of f-Hb to this study was observed, so interval cancers do not seem to have f-Hb levels close to positive in previous rounds. This fact could reduce both cut-off and sensitivity without an important increase in the number of colonoscopies needed. Moreover, it is considered that an increase in false negatives would affect the balance risks/benefits of the programmes, increasing the risk for healthy people unnecessarily and reducing the number needed to harm (NNH).
In our study, a survival pattern was seen to be greater in SCs and also in women, even though significant differences were not found in the deprivation index. These data are in keeping with those analyzed by Gill et al[32], although a different classification of the stage was used in our study. In their study, 322 SCs were compared with 192 ICs with gFOBT, according to their stage, and differences were found in survival in stages Dukes C and D, higher in SCs than in ICs (P = 0.014 and P = 0.04, respectively). In fact, Cox’s proportional hazards regression showed that Dukes’ stage, location of tumour and diagnostic group (HR = 0.45, 95%CI: 0.29-0.69, P < 0.0001) as SCs were all found to have a significant impact on patients' survival.
These data also coincide with the study carried out by Morris et al[33], in which a better prognosis is estimated, with earlier stages in screening cancers (95.9% one-year survival rate) and interval cancers (78.4% one-year survival rate), which is the greatest difference when compared to our study. Patients with screening cancers were offered a higher percentage of treatments with curative intent than those with interval cancers, which is the same as in our study.
On the basis of our results, there are a wealth of options, among which a comparison of CRCs in people who were not invited (in fact, total coverage was not achieved until the beginning of 2014), people who have not participated in any of the rounds of the programme, people who refused a colonoscopy after a positive test and CRC detected during the scheduled follow-up stand out. These comparisons would help us to know the programme’s outcomes and quality? more precisely and the impact of early detection by screening as opposed to other strategies, as developed by Morris et al[33].
Thank to Cancer Registries and Research and Innovation Directorate of the Basque Ministry for Health for their help and support. Thanks to all staff of Primary Care Centers, Biochemistry and Pathology Laboratories, Endoscopy Units, Information and Communication Technology Centralized Unit, Documentation Departments and to the Colorectal Cancer Screening staff of the Basque Health Service, Osakidetza for their involvement.
Colorectal cancer (CRC) is one of the main leading causes of death in the world. There is a consensus that population based screening programmes help improving life expectancy and quality of life of those suffering from CRC by early detecting CRCs and early management of patients. Interval Cancers in CRC screening programmes could be seen as failures of detection and they are due to the inexistence of diagnostic tools that ensure 100% sensitivity without harming healthy people (false positives).
Except for those well-known genetic disorders that are directly linked to CRC (5% of the CRC) to whom personalised strategies are proposed, the rest of the population are managed equally in CRC population based CRC screening programmes. In this sense, there is a need to know the characteristics of interval cancer (ICs), in order to achieve a better understanding of CRC development and thus, propose context and patients’ tailored strategies that could improve the efficiency of CRC screening programmes while innovators are trying to find more accurate diagnostic tools.
This research has studied the differences among ICs and Screening-detected cancers (SCs) on the basis of a high rate participation and population based screening programme (100% coverage and more than 70% participation rate). When studying ICs and SCs we have found that the survival rate of SCs is higher than ICs. Furthermore, we observed relation between ICs, lesion location and stage.
These findings help designing more efficient and tailored strategies that reduce unnecessary harm and improve current achievements regarding quality of life and overall life expectancy. Under current diagnostic paradigm, these findings can define a less harmful and more efficient alternative to those that propose an increase of diagnostic cut-off points to improve detection while increasing harms (false positives) and costs (increasing number of unnecessary colonoscopies).
Interval cancer refers to lesions that are detected within the periods in which no diagnostic strategies are performed. Interval Cancers FIT (IC-FIT) refers to interval cancers that are detected after a negative FIT and before the following invitation inside a CRC screening programme. In the case, the period among invitations is two years. Interval cancers colonoscopy (IC-colonoscopy) refers to interval cancers that are post-confirmatory colonoscopy cancers following a positive FIT and before a follow-up colonoscopy. SCs refer to lesions that have been detected within the programme in each round.
It’s an interesting and informative manuscript, although the manuscript has a little complicated design to understand, especially in terms of data presentation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kanat O, Kupeli S S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Lopez de Munain A. Incidencia del cáncer en la comunidad autónoma de Euskadi, 2013. Vitoria-Gasteiz: Departamento de Salud, Servicio de Registros e Información Sanitaria. Available from: http://www.osakidetza.euskadi.eus/contenidos/informacion/estado_salud/es_5463/adjuntos/INFORME_Bilingue_2013nuevo.pdf. |
| 2. | Department of Health and Consumer Affairs. Cancer in the Basque Country. Incidence, mortality, survival and their trends. 1st edn. Gasteiz: Eusko Jaurlaritzaren Argitalpen Zerbitzu Nagusia, 2010. Available from: http://www.osakidetza.euskadi.eus/contenidos/informacion/estado_salud/es_5463/adjuntos/cancer_en.pdf. |
| 3. | von Karsa L, Anttila A, Ronco G, Ponti A, Malila N, Arbyn M, Segnan N, Castillo-Beltran M, Boniol M, Ferlay J. Report on the implementation of the Council Recommendation on cancer screening. Available from: http://ec.europa.eu/health/ph_determinants/genetics/documents/cancer_screening.pdf. |
| 4. | Ministry of Health and Consumer Affairs. The National Health System Cancer Strategy. Ministerio de Sanidad y Consumo: Madrid, 2006. Available from: http://www.msc.es/organizacion/sns/planCalidadSNS/docs/NHS_cancerStrategy.pdf. |
| 5. | Ministry of Health, Social Services and Equality. The National Health System Cancer Strategy. Madrid: Ministerio de Sanidad y Consumo, 2009. Available from: http://www.msssi.gob.es/organizacion/sns/planCalidadSNS/pdf/Cancer_Strategy_of_the_Spanish_2009.pdf. |
| 6. | Portillo I, Idígoras I, Ojembarrena E, Arana-Arri E, Zubero MB, Pijoán JI, López Urrutia A, Marqués ML. [Main results of the colorectal cancer screening program in the Basque Country (Spain)]. Gac Sanit. 2013;27:358-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Portillo I, Idígoras I, Ojembarrena E, Arana E, Luis Hurtado J, Basurko R, Tapia M, Luz Peña M. [Lesions detected in a colorectal cancer screening program in the Basque Country: first round (2009-2011)]. Gastroenterol Hepatol. 2013;36:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Hurtado JL, Bacigalupe A, Calvo M, Esnaola S, Mendizabal N, Portillo I, Idigoras I, Millán E, Arana-Arri E. Social inequalities in a population based colorectal cancer screening programme in the Basque Country. BMC Public Health. 2015;15:1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Bujanda L, Sarasqueta C, Castells A, Pellisé M, Cubiella J, Gil I, Cosme A, Arana-Arri E, Mar I, Idigoras I. Colorectal cancer in a second round after a negative faecal immunochemical test. Eur J Gastroenterol Hepatol. 2015;27:813-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Segnan N, Patnick J, von Karsa L. European guidelines for quality assurance in colorectal cancer screening and diagnosis. Brussels: European Commission; 2011: 386. Available from: http://www.kolorektum.cz/res/file/guidelines/CRC-screening-guidelines-EC-2011-02-03.pdf. |
| 11. | Robinson MHE, Hardcastle JD, Moss SM, Amar SS, Chamberlain JO, Armitage NCM, Scholefield JH, Mangham CM. The risk of screening: data from the Nottingham randomized controlled trial of faecal occult blood screening for colorectal cancer. Gut. 1999;45:588-592. |
| 12. | Anttila A, Lönnberg S, Ponti A, Suonio E, Villain P, Coebergh JW, von Karsa L. Towards better implementation of cancer screening in Europe through improved monitoring and evaluation and greater engagement of cancer registries. Eur J Cancer. 2015;51:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | GISCoR Working Group, Zorzi M (Coordinator). Detection of the interval cancers and estimate of the sensitivity of colorectal cancer screening programmes. Working report. Epidemiol Prev 2013; 37 (2-3) suppl 1. Available from: http://www.epiprev.it/materiali/2013/EP2-3/S1_GISCOR/GISCOR_2013_Eng_def.pdf. |
| 14. | Zubero MB, Arana-Arri E, Pijoan JI, Portillo I, Idigoras I, López-Urrutia A, Samper A, Uranga B, Rodríguez C, Bujanda L. Population-based colorectal cancer screening: comparison of two fecal occult blood test. Front Pharmacol. 2014;4:175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Jover R and Grupo de trabajo de la AEG-SEED. Programa de calidad en la colonoscopia de cribado. Ed. EdimSa. Madrid 164p. Available from: http://www.aegastro.es/sites/default/files/archivos/guia-clinica/guia_clinica_-_calidad_en_la_colonoscopia.pdf. |
| 16. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC. AJCC Cancer Staging Manual. Chicago: Springer 7th ed. 2010: 615-649. . |
| 17. | Domínguez-Berjón MF, Borrell C, Cano-Serral G, Esnaola S, Nolasco A, Pasarín MI, Ramis R, Saurina C, Escolar-Pujolar A. [Constructing a deprivation index based on census data in large Spanish cities(the MEDEA project)]. Gac Sanit. 2008;22:179-187. [PubMed] |
| 18. | Stegeman I, de Wijkerslooth TR, Stoop EM, van Leerdam M, van Ballegooijen M, Kraaijenhagen RA, Fockens P, Kuipers EJ, Dekker E, Bossuyt PM. Risk factors for false positive and for false negative test results in screening with fecal occult blood testing. Int J Cancer. 2013;133:2408-2414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Sanduleanu S, le Clercq CM, Dekker E, Meijer GA, Rabeneck L, Rutter MD, Valori R, Young GP, Schoen RE. Definition and taxonomy of interval colorectal cancers: a proposal for standardising nomenclature. Gut. 2015;64:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Zorzi M, Fedato C, Grazzini G, Stocco FC, Banovich F, Bortoli A, Cazzola L, Montaguti A, Moretto T, Zappa M. High sensitivity of five colorectal screening programmes with faecal immunochemical test in the Veneto Region, Italy. Gut. 2011;60:944-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Gill MD, Bramble MG, Rees CJ, Lee TJ, Bradburn DM, Mills SJ. Comparison of screen-detected and interval colorectal cancers in the Bowel Cancer Screening Programme. Br J Cancer. 2012;107:417-421. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 22. | Steele RJ, Stanners G, Lang J, Brewster DH, Carey FA, Fraser CG. Interval cancers in a national colorectal cancer screening programme. United European Gastroenterol J. 2016;4:587-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Moss SM, Campbell C, Melia J, Coleman D, Smith S, Parker R, Ramsell P, Patnick J, Weller DP. Performance measures in three rounds of the English bowel cancer screening pilot. Gut. 2012;61:101-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 24. | Steele RJ, McClements PL, Libby G, Carey FA, Fraser CG. Patterns of uptake in a biennial faecal occult blood test screening programme for colorectal cancer. Colorectal Dis. 2014;16:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 25. | Garcia M, Domènech X, Vidal C, Torné E, Milà N, Binefa G, Benito L, Moreno V. Interval cancers in a population-based screening program for colorectal cancer in catalonia, Spain. Gastroenterol Res Pract. 2015;2015:672410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Robertson DJ, Lieberman DA, Winawer SJ, Ahnen DJ, Baron JA, Schatzkin A, Cross AJ, Zauber AG, Church TR, Lance P. Colorectal cancers soon after colonoscopy: a pooled multicohort analysis. Gut. 2014;63:949-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 338] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 27. | le Clercq CM, Bouwens MW, Rondagh EJ, Bakker CM, Keulen ET, de Ridder RJ, Winkens B, Masclee AA, Sanduleanu S. Postcolonoscopy colorectal cancers are preventable: a population-based study. Gut. 2014;63:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 268] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 28. | Brenner H, Chang-Claude J, Seiler CM, Hoffmeister M. Interval cancers after negative colonoscopy: population-based case-control study. Gut. 2012;61:1576-1582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Samadder NJ, Curtin K, Tuohy TM, Pappas L, Boucher K, Provenzale D, Rowe KG, Mineau GP, Smith K, Pimentel R. Characteristics of missed or interval colorectal cancer and patient survival: a population-based study. Gastroenterology. 2014;146:950-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 233] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 30. | Richter JM, Campbell EJ, Chung DC. Interval colorectal cancer after colonoscopy. Clin Colorectal Cancer. 2015;14:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Digby J, Fraser CG, Carey FA, Lang J, Stanners G, Steele RJ. Interval cancers using a quantitative faecal immunochemical test (FIT) for haemoglobin when colonoscopy capacity is limited. J Med Screen. 2016;23:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Gill MD, Bramble MG, Hull MA, Mills SJ, Morris E, Bradburn DM, Bury Y, Parker CE, Lee TJ, Rees CJ. Screen-detected colorectal cancers are associated with an improved outcome compared with stage-matched interval cancers. Br J Cancer. 2014;111:2076-2081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Morris EJ, Whitehouse LE, Farrell T, Nickerson C, Thomas JD, Quirke P, Rutter MD, Rees C, Finan PJ, Wilkinson JR. A retrospective observational study examining the characteristics and outcomes of tumours diagnosed within and without of the English NHS Bowel Cancer Screening Programme. Br J Cancer. 2012;107:757-764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |