Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2723
Peer-review started: December 27, 2017
First decision: February 10, 2017
Revised: February 21, 2017
Accepted: March 21, 2017
Article in press: March 21, 2017
Published online: April 21, 2017
Processing time: 114 Days and 20.2 Hours
To determine the optimal treatment strategy for Siewert type II and III adenocarcinoma of the esophagogastric junction.
We retrospectively reviewed the medical records of 83 patients with Siewert type II and III adenocarcinoma of the esophagogastric junction and calculated both an index of estimated benefit from lymph node dissection for each lymph node (LN) station and a lymph node ratio (LNR: ratio of number of positive lymph nodes to the total number of dissected lymph nodes). We used Cox proportional hazard models to clarify independent poor prognostic factors. The median duration of observation was 73 mo.
Indices of estimated benefit from LN dissection were as follows, in descending order: lymph nodes (LN) along the lesser curvature, 26.5; right paracardial LN, 22.8; left paracardial LN, 11.6; LN along the left gastric artery, 10.6. The 5-year overall survival (OS) rate was 58%. Cox regression analysis revealed that vigorous venous invasion (v2, v3) (HR = 5.99; 95%CI: 1.71-24.90) and LNR of > 0.16 (HR = 4.29, 95%CI: 1.79-10.89) were independent poor prognostic factors for OS.
LN along the lesser curvature, right and left paracardial LN, and LN along the left gastric artery should be dissected in patients with Siewert type II or III adenocarcinoma of the esophagogastric junction. Patients with vigorous venous invasion and LNR of > 0.16 should be treated with aggressive adjuvant chemotherapy to improve survival outcomes.
Core tip: We reviewed the medical records of 83 patients with Siewert type II and III adenocarcinoma of the esophagogastric junction. The median duration of observation was 73 mo. Lymph nodes along the lesser curvature, right and left paracardial lymph nodes, and lymph nodes along the left gastric artery should be dissected in patients with Siewert type II or III adenocarcinoma of the esophagogastric junction. Patients with vigorous venous invasion and lymph node ratio of > 0.16 should be treated with aggressive adjuvant chemotherapy to improve survival outcomes.
- Citation: Hosoda K, Yamashita K, Moriya H, Mieno H, Watanabe M. Optimal treatment for Siewert type II and III adenocarcinoma of the esophagogastric junction: A retrospective cohort study with long-term follow-up. World J Gastroenterol 2017; 23(15): 2723-2730
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2723.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2723
The incidence of adenocarcinoma of the esophagogastric junction (AEG) is increasing in both Western[1,2] and Eastern[3-5] countries. Rüdiger Siewert et al[6] have proposed a classification system for AEG and discussed its characteristics and treatment according to disease types.
Siewert types II and III are more common than Siewert type I AEG in Japan[7,8]. The JCOG-9502 trial demonstrated that a left thoracoabdominal approach, which enables thorough lower mediastinal LN dissection, does not improve survival and leads to increased morbidity in patients with Siewert type II or III AEG with esophageal invasion of ≤ 3 cm. The JCOG0110 trial showed that spleen preservation does not impair survival in patients with proximal gastric cancer without invasion of the greater curvature, which includes Siewert type II and III AEG with esophageal invasion of ≤ 3 cm[9]; the authors therefore concluded that spleen preservation is indicated for reasons of both operative safety and survival benefit. However, that trial was not solely for Siewert type II and III AEG and did not include AEG with invasion of the greater curvature. Several reports have addressed the optimal extent of LN dissection in patients with AEG[10-14]. The right and left paracardial, lesser curvature, and left gastric artery LNs should be dissected because of their high rate of metastatic involvement and the associated benefit from dissection. However, the above-cited reports focused on various types of cancers; none have evaluated the benefit of LN dissection for Siewert type II and III AEG independently.
The pathologic depth of invasion and number of LN metastases are recognized as independent prognostic factors for gastric and esophagogastric junction (EGJ) cancer. According to some studies, metastasis to mediastinal LNs is an independent risk factor for Siewert type II and III AEG[11,15]. Others have determined that the number of examined LNs and the ratio of LNs (LNR) with metastases are significant prognostic factors for gastrointestinal cancer[16,17]. However, because these studies were retrospective and had various biases, additional studies are needed to strengthen these findings.
We aimed to determine the optimal treatment strategy for Siewert type II and III AEG by clarifying the benefit of dissecting specific LN stations and identifying poor prognostic factors.
AEG is defined as adenocarcinoma that has invaded the EGJ. Siewert type II and III AEGs are defined, respectively, as having epicenters located 1 cm above to 2 cm below, and 2 cm below to 5 cm below, the EGJ. The location of the epicenter and proximal extent of the tumor are evaluated based on findings from resected specimens.
The medical records of 83 patients with Siewert type II or III AEG who had undergone R0 or R1 resection at Kitasato University between January 1998 and December 2010 were retrospectively reviewed. Patients who had received preoperative chemotherapy or chemoradiotherapy, or who had pT1a tumors, were excluded. The median duration of observation was 73 mo.
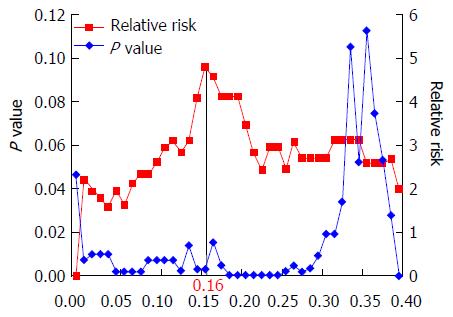
Tumor stage was classified according to the International Union Against Cancer TNM staging system, 7th edition[18]. LN stations were classified according to the Japanese Classification of Gastric Carcinoma[19]. The upper thoracic paraesophageal nodes and thoracic paratracheal nodes were classified as upper mediastinal LNs; the subcarinal nodes, middle thoracic paraesophageal nodes, and main bronchus nodes were classified as middle mediastinal LNs; and the lower thoracic paraesophageal nodes, supradiaphragmatic nodes, and posterior mediastinal nodes were classified as lower mediastinal LNs. Lymph node ratio (LNR) was defined as the ratio of the number of positive LNs to the total number of dissected LNs. Log-rank plot analysis was performed to determine the threshold value of LNR for prediction of overall survival (OS). Relative risks and P values were calculated by prognostic analysis using the log-rank method, classifying LNR at 0.01 intervals. The highest relative risk was considered the critical point in this analysis. The degrees of lymphatic and venous invasion were defined according to the Japanese Classification of Gastric Carcinoma. Venous invasion was evaluated by histopathological examination of hematoxylin-, eosin-, and Elastica-van-Gieson-stained operative specimens; lymphatic invasion was evaluated on hematoxylin- and eosin-stained specimens. In our hospital, the following definitions apply: v0, no venous invasion found on any slide examined; v1, one or two sites of venous invasion found throughout all slides examined; v2, intermediate between v1 and v3; v3, one or more sites of venous invasions found on every slide examined. Lymphatic invasion levels of ly0, ly1, ly2, and ly3 are defined in the same way.
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of Kitasato University School of Medicine. All study participants or their legal guardians provided informed written consent for personal and medical data collection prior to study enrollment.
Surgical procedures were determined on the basis of tumor location and length of esophageal invasion. A right thoracic approach was used to perform subtotal esophagectomy and mediastinal LN dissection through a right thoracotomy, and gastric conduit reconstruction was performed through a laparotomy. A left thoracic approach through a left thoracotomy and laparotomy, and a transhiatal approach after wide splitting of the esophageal hiatus were used to perform total gastrectomy, distal esophagectomy, D2 LN dissection including splenectomy, and lower-mediastinal LN dissection. For cT1 cancer, proximal gastrectomy with either jejunal interposition or esophagogastric anastomosis was performed if possible.
Postoperative adjuvant chemotherapy was given to 29 patients. The most commonly used regimen was S-1 monotherapy (17 out of 29 patients) followed by UFT monotherapy (6 out of 29 patients).
Student’s t-test was used to analyze continuous variables, and the χ2 or Fisher’s exact test to analyze categorical variables. Survival was calculated by the Kaplan-Meier method. Univariate analyses of prognostic factors for OS were performed using log-rank tests. Factors with P < 0.10 on univariate analysis were subjected to multivariate analysis using a Cox’s proportional-hazards model to identify independent prognostic factors. All calculations were performed using JMP® Pro 11.2.0 (SAS Institute, Cary, NC, United States). Values of P < 0.05 were considered to indicate statistical significance.
To evaluate the therapeutic value of LN dissection, we used a previously described method to circumvent the stage migration phenomenon[20]. In brief, the frequency of metastasis to each station was determined by dividing the number of patients with metastases to that station by the number of patients in whom the station had been dissected. The 5-year OS rates of patients with metastases at each nodal station were calculated irrespective of the presence or absence of metastases at other nodal stations. An index of estimated benefit from LN dissection (IEBLD) for each station was calculated by multiplying the frequency of metastasis to the station by the 5-year survival rate of patients with metastases to that station.
Tumor size was significantly larger and esophageal invasion significantly shorter in type III than in type II tumors (Table 1). Pathological classifications did not differ significantly between the groups (Table 2).
| Type II (n = 62) | Type III (n = 21) | P value | |
| Age (yr) | 0.72 | ||
| Median (range) | 68.5 (33-87) | 69 (54-80) | |
| Sex | 0.78 | ||
| Male | 49 (79) | 16 (76) | |
| Female | 13 (21) | 5 (24) | |
| Tumor size (mm) | 50 (10-168) | 70 (12-120) | 0.016 |
| Esophageal invasion (mm) | 9 (0-85) | 5 (0-15) | 0.024 |
| Total number of examined lymph nodes | 36.5 (5-96) | 51 (22-80) | 0.016 |
| Approach | |||
| Right thoracic | 8 (13) | 0 (0) | |
| Left thoracic | 8 (13) | 1 (5) | |
| Transhiatal | 46 (74) | 20 (95) | |
| Resection method | |||
| Subtotal esophagectomy | 8 (13) | 0 (0) | |
| Total gastrectomy with distal esophagectomy | 37 (60) | 21 (100) | |
| Proximal gastrectomy with distal esophagectomy | 17 (27) | 0 (0) | |
| Adjuvant chemotherapy | 0.24 | ||
| Yes | 19 (31) | 9 (43) | |
| No | 43 (69) | 12 (57) |
| Type II(n = 62) | Type III(n = 21) | P value | |
| T category | 0.06 | ||
| T1b | 11 (18) | 0 (0) | |
| T2 | 11 (18) | 2 (10) | |
| T3 | 40 (64) | 19 (90) | |
| N category | 0.41 | ||
| N0 | 21 (34) | 4 (19) | |
| N1 | 12 (19) | 6 (29) | |
| N2 | 13 (21) | 3 (14) | |
| N3 | 14 (23) | 6 (29) | |
| LNR | 0.063 (0-1) | 0.047 (0-0.41) | 0.74 |
| M category | 0.64 | ||
| M0 | 58 (94) | 19 (90) | |
| M1 | 4 (6) | 2 (10) | |
| TNM stage | 0.30 | ||
| IA | 10 (16) | 0 (0) | |
| IB | 5 (8) | 0 (0) | |
| IIA | 6 (10) | 4 (19) | |
| IIB | 5 (8) | 1 (5) | |
| IIIA | 8 (13) | 5 (24) | |
| IIIB | 11 (18) | 3 (14) | |
| IIIC | 12 (19) | 6 (29) | |
| IV | 5 (8) | 2 (10) | |
| ly category | 0.35 | ||
| ly0 | 8 (13) | 0 (0) | |
| ly1 | 16 (26) | 5 (24) | |
| ly2 | 22 (35) | 9 (43) | |
| ly3 | 16 (26) | 7 (33) | |
| v category | 0.55 | ||
| v0 | 7 (11) | 3 (14) | |
| v1 | 13 (21) | 4 (19) | |
| v2 | 24 (39) | 5 (24) | |
| v3 | 18 (29) | 9 (43) | |
| Histopathological grade | 0.56 | ||
| G1 + G2 | 37 (60) | 11 (52) | |
| G3 + G4 | 25 (40) | 10 (48) |
Seven patients with Siewert type II AEG had R1 resection: four of them had positive abdominal lavage cytology (CY1), one had positive pleural effusion cytology, one had positive proximal and one had positive circumferential margins. Two patients with Siewert type III AEG and R1 resection had positive abdominal lavage cytology.
Table 3 shows the number of patients with dissection and the number of patients with metastases for each LN station and the resultant IEBLDs, which in descending order were as follows (# denotes LN station): LNs along the lesser curvature (#3), 26.5; right paracardial (#1), 22.8; left paracardial (#2), 11.6; along the left gastric artery (#7), 10.6; along the distal splenic artery (#11d), 8.6; around the celiac artery (#9), 6.5; lower mediastinal LN, 6.3; and splenic hilum (#10), 5.0. No metastases were found in the lower mediastinal LNs in patients with Siewert type III AEG; such metastases were found only in patients with Siewert type II AEG.
| Lymph node | Type II | Type III | Total | |||||
| station | Number of patients with dissection | Number of patients with metastasis | Number of patients with dissection | Number of patients with metastasis | Number of patients with dissection | Number of patients with metastasis | 5-yr survival rate | IEBLD |
| 1 | 62 | 27 | 21 | 11 | 83 | 38 | 49.70% | 22.8 |
| 2 | 62 | 14 | 21 | 5 | 83 | 19 | 50.70% | 11.6 |
| 3 | 62 | 27 | 21 | 14 | 83 | 41 | 53.70% | 26.5 |
| 4sa | 42 | 1 | 18 | 0 | 60 | 1 | 0 | 0.0 |
| 4sb | 43 | 1 | 19 | 1 | 62 | 2 | 100% | 3.2 |
| 4d | 39 | 0 | 20 | 0 | 59 | 0 | NA | 0.0 |
| 5 | 25 | 0 | 14 | 1 | 39 | 1 | 0 | 0.0 |
| 6 | 31 | 0 | 21 | 0 | 52 | 0 | NA | 0.0 |
| 7 | 58 | 17 | 21 | 6 | 79 | 23 | 36.30% | 10.6 |
| 8 | 42 | 4 | 21 | 2 | 63 | 6 | 20.80% | 2.0 |
| 9 | 32 | 5 | 14 | 2 | 46 | 7 | 42.90% | 6.5 |
| 10 | 28 | 3 | 12 | 0 | 40 | 3 | 66.70% | 5.0 |
| 11p | 33 | 4 | 19 | 0 | 52 | 4 | 33.30% | 2.6 |
| 11d | 24 | 4 | 11 | 1 | 35 | 5 | 60% | 8.6 |
| 12 | 2 | 0 | 1 | 0 | 3 | 0 | NA | 0.0 |
| 16 | 2 | 0 | 0 | 0 | 2 | 0 | NA | 0.0 |
| LMLN | 13 | 5 | 3 | 0 | 16 | 5 | 20% | 6.3 |
| MMLN | 8 | 1 | 0 | 0 | 8 | 1 | 0 | 0.0 |
| UMLN | 7 | 1 | 0 | 0 | 7 | 1 | 0 | 0.0 |
Patients with lower mediastinal LN metastases who survived over 5 years all had Siewert type II tumors with esophageal invasion of more than 30 mm. Additionally, patients with splenic hilar LN metastases who survived over 5 years all had Siewert type II tumors with invasion of the greater curvature
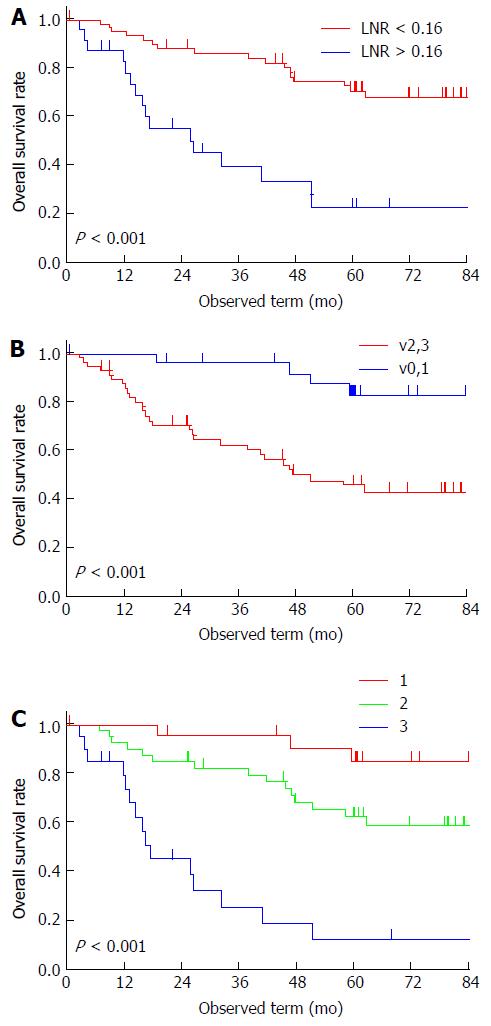
All patients were followed up for more than 5 years, except for 11 excluded patients who were lost to follow-up within the 5-year period. The 5-year OS rate was 58%. The threshold point for LNR was determined to be 0.16 (Figure 1). Univariate analysis revealed that pT, pN, mediastinal LN metastasis, LNR, lymphatic invasion, venous invasion, and residual tumor were potential prognostic factors for OS. According to Cox’s regression analysis, vigorous venous invasion (v2 or v3) (HR = 5.99, 95%CI: 1.71-24.90, P = 0.004) and LNR of > 0.16 (HR = 4.29, 95%CI: 1.79-10.89, P = 0.001) were independent prognostic factors for poor OS (Table 4). The 5-year survival rate of patients with v2 or v3 was 46% and of patients with LNR of > 0.16 was 23% (Figure 2A and B); whereas that of patients without either of the independent predictors of poor prognosis (v0 or v1 and LNR of < 0.16) was 85% and of patients with both of these predictors (v2 or v3, and LNR of > 0.16), 13% (Figure 2C).
| Category | Classification | Univariate analysis | Multivariate analysis | |||||
| n | Proportion | 5 yr-OS | P value | HR | 95%CI | P value | ||
| Age (yr) | < 65 | 29 | 35% | 67% | 0.084 | 0.47 | ||
| ≥ 65 | 54 | 65% | 52% | |||||
| Sex | Male | 65 | 78% | 56% | 0.57 | |||
| Female | 18 | 22% | 67% | |||||
| Siewert type | II | 62 | 75% | 58% | 0.88 | |||
| III | 21 | 25% | 57% | |||||
| Esophageal invasion | ≤ 3 cm | 73 | 88% | 60% | 0.21 | |||
| > 3 cm | 10 | 12% | 40% | |||||
| Tumor size | < 6 cm | 45 | 54% | 58% | 0.86 | |||
| ≥ 6 cm | 38 | 46% | 58% | |||||
| pT | pT1-2 | 24 | 29% | 77% | 0.01a | 0.88 | ||
| pT3 | 59 | 71% | 50% | |||||
| pN | pN0 | 25 | 30% | 78% | 0.028a | 0.30 | ||
| pN1-3 | 58 | 70% | 49% | |||||
| Mediastinal LN meta | No | 78 | 94% | 60% | 0.007a | 0.17 | ||
| Yes | 5 | 6% | 20% | |||||
| LNR | < 0.16 | 59 | 71% | 71% | < 0.001a | 0.001a | ||
| > 0.16 | 24 | 29% | 23% | 4.29 | 1.79-10.89 | |||
| ly | ly0, 1 | 29 | 35% | 69% | 0.059 | 0.13 | ||
| ly2, 3 | 54 | 65% | 52% | |||||
| v | v0, 1 | 27 | 33% | 83% | < 0.001a | 0.004a | ||
| v2, 3 | 56 | 67% | 46% | 5.99 | 1.71-24.90 | |||
| Histopathological Grade | G1 + G2 | 48 | 58% | 60% | 0.28 | |||
| G3 + G4 | 35 | 42% | 55% | |||||
| Adjuvant chemotherapy | No | 54 | 65% | 56% | 0.52 | |||
| Yes | 29 | 35% | 61% | |||||
| Residual tumor | R0 | 74 | 89% | 62% | < 0.001a | 0.056 | ||
| R1 | 9 | 11% | 22% | 2.61 | 0.97-6.59 | |||
Recurrence occurred in 30 of the study patients. In all six patients with CY1 the first recurrence site was peritoneal. The initial recurrence sites of the other patients were as follows: peritoneum, 8; liver, 6; lung, 3; bone, 1; adrenal, 1; abdominal LNs, 8; upper or middle mediastinal LNs, 3; and local, 3. Of the eight patients with first recurrence in abdominal LNs, three developed recurrence only in para-aortic LNs. All three patients with mediastinal LN recurrence had simultaneous liver, generalized LN, and para-aortic LN recurrence.
This study had two major findings. First, LNs in stations 1, 2, 3, and 7 had high IEBLDs, as previously reported[10-12] and should therefore be dissected in patients with Siewert type II or III AEG. Second, vigorous venous invasion (v2 or v3) and LNR of > 0.16 are independent predictors of poor OS.
Regarding splenic hilar LN dissection, the JCOG0110 study reported that splenectomy had little impact on survival of patients with proximal gastric cancer that did not invade the greater curvature[9]. Goto et al[21] concluded that splenectomy could be omitted from the treatment of AEG because of its high complication rate and minor impact on survival. However, the JCOG0110 study excluded patients with tumors that did invade the greater curvature. In addition, our current study revealed that splenic hilar LNs had a small, but not negligible, IEBLD. We therefore believe that splenectomy should not necessarily be omitted for patients with AEG who have extensive invasion of the greater curvature.
The JCOG9502 study reported no benefit for lower mediastinal LN dissection through a left thoracoabdominal approach in patients with ≤ 3 cm esophageal invasion[22]. Our current study confirmed that patients with tumors affecting ≤ 3 cm of the esophagus achieved no benefit from lower mediastinal LN dissection. In addition, a transthoracic approach was proven to increase pulmonary complications[22,23]. Therefore, thorough lower-mediastinal LN dissection through a right or left thoracic approach would be unnecessary for these patients. If we changed our approach to include only a laparotomy (laparoscopy) in patients with tumors affecting ≤ 3 cm of the esophagus, it appears that it would not impair survival outcomes and would reduce postoperative complications. However, Siewert type II AEG with ≥ 2 cm esophageal invasion is associated with metastasis to lower mediastinal LNs[24]. No standard surgical approach has been defined for AEG with esophageal invasion of > 3 cm. In the current study, patients who benefitted from lower mediastinal LN dissection had tumors with > 3 cm esophageal invasion. We therefore consider lower mediastinal LN dissection is likely important for patients with > 3 cm of esophageal invasion. We recommend a left or right thoracic approach for patients with esophageal invasion of > 3 cm because with a transhiatal approach, lower mediastinal LN dissection may be inadequate and the proximal margin may not be cancer-free. Relatively few patients underwent upper or middle mediastinal LN dissection; however, the IEBLDs for these LNs were zero. Additionally, patients whose first recurrence site was upper or lower mediastinal LNs had simultaneous distant metastases. Upper or middle mediastinal LN dissection needs to be performed cautiously in patients with Siewert type II or III AEG.
In the current study, v2 or v3 and LNR of > 0.16 were identified as independent predictors of poor OS. Because both v2 or v3 and LNR of > 0.16 cannot be diagnosed preoperatively, postoperative adjuvant chemotherapy would likely improve prognoses of such high-risk patients. Either v2 or v3 is reportedly an independent prognostic factor for Stage IB node-negative gastric cancer[25]. Venous invasion theoretically predicts hematological metastases, and lymphatic invasion, LN metastases. Therefore, it is unsurprising that v2 and v3 were identified as independent prognostic factors for Siewert type II and III AEG, which tend to metastasize hematologically.
LNR is reportedly an independent prognostic factor for esophagogastric cancer[16,26]. In Western countries, extended LN dissection is seldom performed for gastric or esophagogastric cancer; thus, an average of about 15 LNs are dissected, resulting in under-diagnosis of the number of LNs with metastases[17]. Therefore, LNR rather than the number of LNs with metastases is thought to be a more useful prognostic indicator. In the current study, though the total number of examined LNs was relatively large (median 36.5, range 5-96 for Siewert type II; median 51, range 22-80 for Siewert type III), the same was shown to be true. Where the extent of LN dissection is not standardized, LNR may be a more relevant prognostic indicator than the number of LNs with metastases in Siewert type II and III AEG.
In Japan, the standard treatment for resectable gastric adenocarcinoma, including AEG, is gastrectomy with D2 LN dissection followed by adjuvant chemotherapy using S1[27]. However, adjuvant chemotherapy with S1 reportedly has inadequate power to prevent hematological metastases and improve OS of patients with pStage IIIB gastric cancer[28]. In the CLASSIC trial, capecitabine plus oxaliplatin improved recurrence-free survival even in patients with pStage IIIB gastric cancer and well-controlled distant metastases[29]. Considering that hematological metastasis occurs relatively frequently in Siewert type II and III AEG, patients with AEG, and with mild or marked venous invasion and/or LNR of > 0.16, should receive aggressive adjuvant chemotherapy using a platinum-containing regimen such as capecitabine plus oxaliplatin.
This study has several limitations. First, it was a single-center, retrospective, observational study with various biases. Second, during the lengthy accrual period various regimens of adjuvant chemotherapy were used. Third, various surgical procedures, including subtotal esophagectomy, proximal gastrectomy with lower esophagectomy, and total gastrectomy with lower esophagectomy were performed, and the extent of LN dissection was not standardized, which may have resulted in bias in the IEBLD. Almost no patients underwent dissection of para-aortic LNs. The incidence of metastases in para-aortic LNs around the left renal vein is reportedly 17% and the 5-year survival rate of node-positive patients is 19%[30]. In the current study, para-aortic LNs were the first recurrence site in three patients. In Japan, a multicenter clinical study is evaluating the efficacy of mediastinal and para-aortic LN dissection in EGJ cancer (UMIN000013205). This study will provide useful information about the optimal extent of LN dissection for EGJ cancer.
In conclusion, based on our findings we recommend the following: LNs in stations 1, 2, 3, and 7 should be dissected in Siewert type II and III AEG because of their high IEBLDs. Patients with vigorous venous invasion or LNR of > 0.16 should receive aggressive adjuvant chemotherapy to improve survival outcomes. Patients with esophageal invasion of > 3 cm should undergo dissection of inferior mediastinal LNs, and those with invasion of the greater curvature should undergo dissection of splenic hilar LNs.
The incidence of adenocarcinoma of the esophagogastric junction (AEG) is increasing in both Western and Eastern countries. Siewert et al. have proposed a classification system for AEG and discussed its characteristics and treatment according to disease types.
In patients with Siewert type II or III AEG, a left thoracoabdominal approach, which enables thorough lower mediastinal lymph node (LN) dissection, was demonstrated not to improve survival and to lead to increased morbidity. The right and left paracardial, lesser curvature, and left gastric artery LNs should reportedly be dissected because of their high indices of estimated benefit of lymph node dissections (IEBLDs). The ratio of the number of positive LNs to the total number of dissected LNs (LNR) has been reported to be a significant prognostic factor for gastrointestinal cancer including AEG. However, these claims are based on clinical studies of various types of esophagogastric junction cancer and additional studies are needed to strengthen these findings specifically for Siewert type II or III AEG, which is a prevalent form in Eastern countries including Japan.
This study included only Siewert type II or III AEG. IEBLDs were again higher in the right and left paracardial, lesser curvature, and left gastric artery LNs. Vigorous venous invasion (v2 or v3) and LNR of > 0.16 were independent predictors of poor OS. IEBLDs of splenic hilar and lower mediastinal LN were 5.0 and 6.3, respectively. Patients who survived more than 5 years with splenic hilar LN and lower mediastinal LN involvement had tumors with esophageal invasion of more than 30 mm and with invasion of the greater curvature, respectively.
The right and left paracardial, lesser curvature, and left gastric artery LNs should be dissected in Siewert type II and III AEG because of their high IEBLDs. Patients with vigorous venous invasion or LNR of > 0.16 should receive aggressive adjuvant chemotherapy to improve survival outcomes. Patients with esophageal invasion of > 3 cm should undergo dissection of inferior mediastinal LNs, and those with invasion of the greater curvature should undergo dissection of splenic hilar LNs.
The LNR was the ratio of the number of positive LNs to the total number of dissected LNs. An IEBLD was calculated by multiplying the frequency of metastasis to a specified station by the 5-year OS rate of patients with metastases to that station.
Hosoda et al present a retrospective case series of esophagogastric junction tumors to show the prognostic of lymph node dissection. The manuscript is interesting with a significant number of patients and a long follow-up.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Herbella FAM S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Devesa SS, Blot WJ, Fraumeni JF. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049-2053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Pohl H, Welch HG. The role of overdiagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Natl Cancer Inst. 2005;97:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 956] [Cited by in RCA: 963] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 3. | Kusano C, Gotoda T, Khor CJ, Katai H, Kato H, Taniguchi H, Shimoda T. Changing trends in the proportion of adenocarcinoma of the esophagogastric junction in a large tertiary referral center in Japan. J Gastroenterol Hepatol. 2008;23:1662-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Blaser MJ, Saito D. Trends in reported adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur J Gastroenterol Hepatol. 2002;14:107-113. [PubMed] |
| 5. | Yamashita K, Sakuramoto S, Nemoto M, Shibata T, Mieno H, Katada N, Kikuchi S, Watanabe M. Trend in gastric cancer: 35 years of surgical experience in Japan. World J Gastroenterol. 2011;17:3390-3397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 6. | Rüdiger Siewert J, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353-361. [PubMed] |
| 7. | Hasegawa S, Yoshikawa T, Cho H, Tsuburaya A, Kobayashi O. Is adenocarcinoma of the esophagogastric junction different between Japan and western countries? The incidence and clinicopathological features at a Japanese high-volume cancer center. World J Surg. 2009;33:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T. Adenocarcinoma of the gastroesophageal junction in Japan: relevance of Siewert’s classification applied to 177 cases resected at a single institution. J Am Coll Surg. 1999;189:594-601. [PubMed] |
| 9. | Sano T, Sasako M, Mizusawa J, Yamamoto S, Katai H, Yoshikawa T, Nashimoto A, Ito S, Kaji M, Imamura H. Randomized Controlled Trial to Evaluate Splenectomy in Total Gastrectomy for Proximal Gastric Carcinoma. Ann Surg. 2017;265:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 10. | Yamashita H, Katai H, Morita S, Saka M, Taniguchi H, Fukagawa T. Optimal extent of lymph node dissection for Siewert type II esophagogastric junction carcinoma. Ann Surg. 2011;254:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Hosokawa Y, Kinoshita T, Konishi M, Takahashi S, Gotohda N, Kato Y, Daiko H, Nishimura M, Katsumata K, Sugiyama Y. Clinicopathological features and prognostic factors of adenocarcinoma of the esophagogastric junction according to Siewert classification: experiences at a single institution in Japan. Ann Surg Oncol. 2012;19:677-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Fujitani K, Miyashiro I, Mikata S, Tamura S, Imamura H, Hara J, Kurokawa Y, Fujita J, Nishikawa K, Kimura Y. Pattern of abdominal nodal spread and optimal abdominal lymphadenectomy for advanced Siewert type II adenocarcinoma of the cardia: results of a multicenter study. Gastric Cancer. 2013;16:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Goto H, Tokunaga M, Miki Y, Makuuchi R, Sugisawa N, Tanizawa Y, Bando E, Kawamura T, Niihara M, Tsubosa Y. The optimal extent of lymph node dissection for adenocarcinoma of the esophagogastric junction differs between Siewert type II and Siewert type III patients. Gastric Cancer. 2014; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Hosoda K, Yamashita K, Katada N, Moriya H, Mieno H, Sakuramoto S, Kikuchi S, Watanabe M. Impact of lower mediastinal lymphadenectomy for the treatment of esophagogastric junction carcinoma. Anticancer Res. 2015;35:445-456. [PubMed] |
| 15. | Nakamura M, Iwahashi M, Nakamori M, Naka T, Ojima T, Iida T, Katsuda M, Tsuji T, Hayata K, Mastumura S. Lower mediastinal lymph node metastasis is an independent survival factor of Siewert type II and III adenocarcinomas in the gastroesophageal junction. Am Surg. 2012;78:567-573. [PubMed] |
| 16. | Sisic L, Blank S, Weichert W, Jäger D, Springfeld C, Hochreiter M, Büchler M, Ott K. Prognostic impact of lymph node involvement and the extent of lymphadenectomy (LAD) in adenocarcinoma of the esophagogastric junction (AEG). Langenbecks Arch Surg. 2013;398:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Zhou Z, Zhang H, Xu Z, Li W, Dang C, Song Y. Nomogram predicted survival of patients with adenocarcinoma of esophagogastric junction. World J Surg Oncol. 2015;13:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Sobin L, Gospodarowicz M, Wittekind C; International Union Against Cancer. TNM Classification of Malignant Tumours. 7th ed. New York: Wiley-Blackwell, 2009. . |
| 19. | Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2869] [Article Influence: 204.9] [Reference Citation Analysis (0)] |
| 20. | Sasako M, McCulloch P, Kinoshita T, Maruyama K. New method to evaluate the therapeutic value of lymph node dissection for gastric cancer. Br J Surg. 1995;82:346-351. [PubMed] |
| 21. | Goto H, Tokunaga M, Sugisawa N, Tanizawa Y, Bando E, Kawamura T, Niihara M, Tsubosa Y, Terashima M. Value of splenectomy in patients with Siewert type II adenocarcinoma of the esophagogastric junction. Gastric Cancer. 2013;16:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 22. | Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, Nashimoto A, Hiratsuka M. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 303] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Hulscher JB, van Sandick JW, de Boer AG, Wijnhoven BP, Tijssen JG, Fockens P, Stalmeier PF, ten Kate FJ, van Dekken H, Obertop H. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med. 2002;347:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1232] [Cited by in RCA: 1144] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 24. | Kurokawa Y, Hiki N, Yoshikawa T, Kishi K, Ito Y, Ohi M, Wada N, Takiguchi S, Mine S, Hasegawa S. Mediastinal lymph node metastasis and recurrence in adenocarcinoma of the esophagogastric junction. Surgery. 2015;157:551-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Araki I, Hosoda K, Yamashita K, Katada N, Sakuramoto S, Moriya H, Mieno H, Ema A, Kikuchi S, Mikami T. Prognostic impact of venous invasion in stage IB node-negative gastric cancer. Gastric Cancer. 2015;18:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Zhang H, Wang W, Diao D, Cheng Y, Song Y, Zhu K, Dang C. Ratio of metastatic to examined lymph nodes, a helpful staging system and independent prognostic factor of esophagogastric junction cancer. PLoS One. 2013;8:e73238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1771] [Cited by in RCA: 1941] [Article Influence: 107.8] [Reference Citation Analysis (0)] |
| 28. | Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387-4393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1089] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 29. | Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1290] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 30. | Mine S, Sano T, Hiki N, Yamada K, Nunobe S, Yamaguchi T. Lymphadenectomy around the left renal vein in Siewert type II adenocarcinoma of the oesophagogastric junction. Br J Surg. 2013;100:261-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |