Published online Apr 21, 2017. doi: 10.3748/wjg.v23.i15.2716
Peer-review started: December 6, 2016
First decision: January 19, 2017
Revised: February 14, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 21, 2017
Processing time: 138 Days and 19.2 Hours
To investigate the role of Δ133p53 isoform in nuclear factor-κB (NF-κB) inhibitor pyrrolidine dithiocarbamate (PDTC)-mediated growth inhibition of MKN45 gastric cancer cells.
The growth rate of MKN45 cells after treatment with different concentrations of only PDTC or PTDC in combination with cisplatin was detected by the CCK-8 assay. mRNA expression levels of Δ133p53, p53β, and the NF-κB p65 subunit and p65 protein levels were detected by reverse transcription-polymerase chain reaction (RT-PCR) and immunofluorescence, respectively. Growth of MKN45 cells was significantly inhibited by PDTC alone in a dose-dependent manner (P < 0.01). Moreover, the inhibitory effect of cisplatin was remarkably enhanced in a dose-dependent manner by co-treatment with PDTC (P < 0.01).
RT-PCR analysis revealed that mRNA expression of p65 was curbed significantly in a dose-dependent manner by treatment with only PDTC (P < 0.01), and this suppressive effect was further enhanced when co-treated with cisplatin (P < 0.01). With respect to the other p53 isoforms, mRNA level of Δ133p53 was significantly reduced in a dose-dependent manner by treatment with only PDTC or PTDC in combination with cisplatin (P < 0.01), whereas p53β mRNA expression was not altered by PDTC treatment (P > 0.05). A similar tendency of change in p65 protein expression, as observed for the corresponding mRNA, was detected by immunofluorescence analysis (P < 0.01). Pearson correlation analysis demonstrated that Δ133p53 and p65 mRNA expression levels were positively related, while no significant relationship was observed between those of p65 and p53β (r = 0.076, P > 0.01).
Δ133p53 isoform (not p53β) is required in PDTC-induced inhibition of MKN45 gastric cancer cells, indicating that disturbance in the cross-talk between p53 and NF-κB pathways is a promising target in pharmaceutical research for the development of treatment strategies for gastric cancer.
Core tip: Intestinal-type of gastric cancer develops from chronic gastritis. Δ133p53 isoform has been recently identified as an oncogenic actor that is pivotal in Helicobacter pylori-driven progression of chronic gastritis to gastric cancerogenesis. These results suggest that Δ133p53 isoform is required in pyrrolidine dithiocarbamate-induced inhibition of MKN45 gastric cancer cells, and disturbance in the cross-talk between p53 and nuclear factor-κB pathways is a promising target in pharmaceutical research for the development of treatment strategies against intestinal-type gastric cancer.
- Citation: Zhang HM, Sang XG, Wang YZ, Cui C, Zhang L, Ji WS. Role of Δ133p53 isoform in NF-κB inhibitor PDTC-mediated growth inhibition of MKN45 gastric cancer cells. World J Gastroenterol 2017; 23(15): 2716-2722
- URL: https://www.wjgnet.com/1007-9327/full/v23/i15/2716.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i15.2716
The association between chronic inflammation and cancer has been researched extensively. Few cases of gastric cancer, at early stages of cancerogenesis, have been ascribed to germline mutations in CDH1, encoding E-cadherins, or rarely other genes[1,2]. In fact, most cases of gastric carcinoma develop from chronic gastritis and its associated lesions and diseases. In addition, Helicobacter pylori (H. pylor) is considered the common pathogen of both chronic gastritis and gastric carcinoma. Thus, elucidation of the underlying molecular mechanisms of H. pylori-associated gastritis and cancerogenesis could contribute immensely to the treatment of associated diseases. Both the nuclear factor-kappa B (NF-κB) and p53 pathways display significant modifications, some of which are H. pylori-driven, in the progression of chronic gastritis to cancerogenesis[3-6]. A previous study reported that the interaction of mutant p53 and NF-κB promoted persistent tissue damage and extended inflammation and progressed to inflammation-associated human colorectal cancer[7]. Results from a recent study further indicated that Δ133p53 isoform is a crucial molecule in this process, and focusing on the changes in this isoform can provide insight into the association of H. pylori infection with chronic gastritis, precancerous lesions, and gastric cancerogenesis[8,9]. Therefore, understanding the crosstalk between NF-κB and p53 pathways could significantly contribute to cancer prevention and treatment strategies in inflammation-associated gastric cancerogenesis. Therefore, the present study was designed to evaluate the role of Δ133p53 isoform under an NF-κB-inhibited condition (using the NF-κB inhibitor pyrrolidine dithiocarbamate, PDTC) and the combined biological effect in MKN45 cells, a gastric cancer cell line with wild-type p53[10-12].
The MKN45 human gastric cancer cell line was supplied by the Cell Bank of Chinese Academy of Medical Science (Beijing, China) and used before the tenth passage. Cells were tested for mycoplasmic infection and cultured in RPMI1640 medium, supplemented with 10% fetal bovine serum, at 37 °C in an atmosphere containing 5% CO2. Cells in the exponential growth phase were collected for the following experiments.
MKN45 cells in the exponential growth phase were digested, seeded in a 96well plate (5 × 104 cells/well), and then cultured for 24 h. PDTC, at concentrations of 25, 50, and 75 μmol/L alone or in combination with 4 μg/mL cisplatin or an equal volume of phosphatebuffered saline (PBS), was added to the cells, which were then cultured for another 48 h. Medium without any cells or drugs was placed in the empty wells. Subsequently, the culture medium was discarded, 20 μL of a mixture of CCK-8 and the medium at a 1:10 ratio was added to each well, and the cells were cultured for another 1 h. Three replicate wells were established for each condition. Optical density (OD) of the cells was measured at 450 nm using a Multiskan FC microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA, United States), and the growth inhibition rate was calculated as follows:
IC =1 - [(AExp - AEmp)/(AC - AEmp)] × 100%
Where IC is the growth inhibition rate, AExp is the absorbance in the experimental group, AC is the absorbance in the control group, and AEmp is the absorbance in the empty group.
MKN45 cells in the exponential growth phase were digested, seeded in a 96well plate (5 × 104 cells/well), and then cultured for 24 h. PDTC, at concentrations of 25, 50, and 75 μmol/L alone or in combination with 4 μg/mL cisplatin or an equal volume of PBS (control wells), was added to the cells, which were then cultured for another 48 h. RNA extraction and cDNA synthesis were performed according to the manufacturer’s instructions provided along with the TRIzol extraction and reverse transcription PCR kits. PCR was performed to amplify Δ133p53, p53β, p65, and β-actin using the primers shown in Table 1. Lengths of the amplified products were 690 bp for p53, 1050 bp for p53β, and 539 bp for βactin. PCR conditions were set as 35 cycles at 94 °C for 1 min for denaturation, 58 °C for 50 s for annealing, and 72 °C for 1 min for extension. PCR was performed with a BIO-RAD IQ5 Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, United States). The cycle threshold (CT) value was detected, and the relative mRNA expression level was calculated as 2−ΔΔCT.
| Primer | Primer sequence (5’-3’) | Length (bp) | |
| p53β | Outer | Sense: GTCACTGCCATGGAGGAGCCGCA | 1050 |
| Antisense: GACGCACACCTATTGCAAGCAAGGGTTC | |||
| Inner | Sense: ATGGAGGAGCCGCAGTCAGAT | ||
| Antisense: TTGAAAGCTGGTCTGGTCCTGA | |||
| Δ133p53 | Outer | Sense: CTGAGGTGTAGACGCCAACTCTCTCTAG | 750 |
| Antisense: TGTCAGTCTGAGTCAGGCCCTTCTGTC | |||
| Inner | Sense: GCTAGTGGGTTGCAGGAGGTGCTTACGC | ||
| Antisense: CTCACGCCCACGGATCTGA | |||
| p65 | Sense: TATGACCACACATGACAG | 145 | |
| Antisense: CTGGATCTGTGAAACTTTGA | |||
| β-actin | Sense: GTGGGGCGCCCCAGGCACCA | 539 | |
| Antisense: CTCCTTAATGTCACGCACGATTTC |
MKN45 cells were digested as a single-cell suspension and plated in a 96-well plate. PDTC, at concentrations of 25, 50, and 75 μmol/L alone or in combination with 4 μg/mL cisplatin or an equal volume of PBS (control wells), was added to the cells, which were then cultured for another 48 h. Cells were fixed in 4% paraformaldehyde for 30 min, incubated with 0.1% TritonX-100 PBS solution for 10 min, and then with 3% H2O2 for another 10 min. Subsequently, 1% bovine serum albumin solution was added to block non-specific sites, and the cells were incubated with NF-κB p65 antibody (Abcam, E379 ab32536, Aibokang Corporation Ltd., Shanghai, China) at a 1:200 dilution overnight and then with a secondary antibody (goat-anti-rabbit-596nm, ZF0316, Zhongshan Jinqiao Biotechnology Corporation Ltd., Beijing, China) at a 1:200 dilution for another 2 h. Nuclei were stained with 4’,6-diamidino-2-phenylindole for 10 min, and the cells were examined under a fluorescence microscope (Olympus, Japan). p65 protein expression was measured as the integral optical value.
SPSS version 17.0 (International Business Machines, Armonk, NY, United States) software package was used for statistical analyses. Data are recorded as the mean ± SD. Differences between two groups were analyzed by the least significant difference t-test, and differences among more than two groups were evaluated by one-way analysis of variance. Correlations between variables were tested with Pearson linear relevancy analysis.
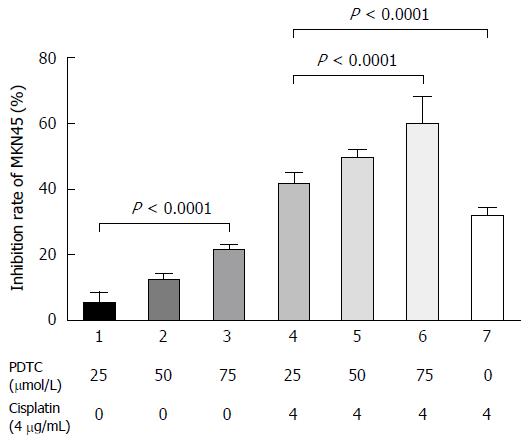
Growth inhibition rates of gastric cancer cells under different treatments are shown in Figure 1. PDTC significantly inhibited the growth of MKN45 cells in a dose-dependent manner (F = 167.10, P < 0.01). Moreover, the inhibitory effect of cisplatin was enhanced in a dose-dependent manner by co-treatment with PDTC (F = 52.23, P < 0.01).
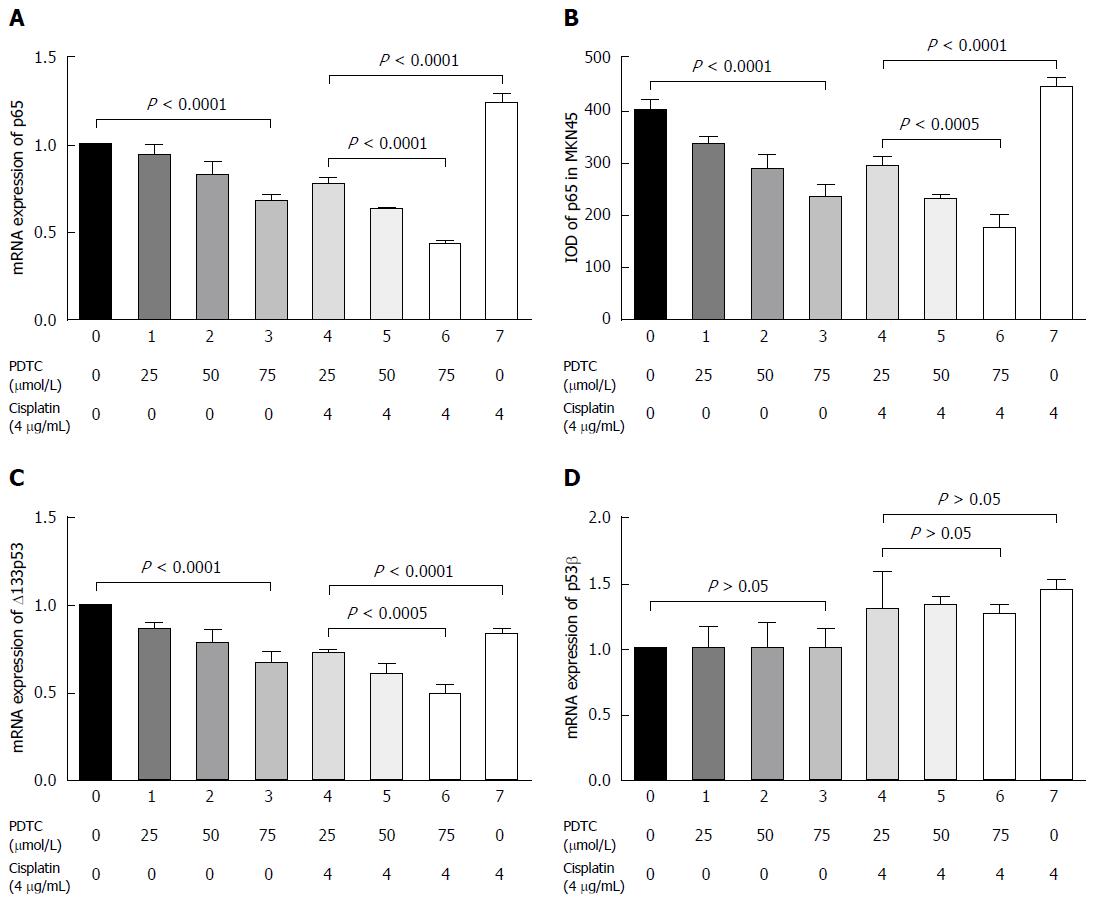
Results of reverse transcription-polymerase chain reaction (RT-PCR) analysis showed that PDTC significantly downregulated p65 mRNA expression in a dose-dependent manner (P < 0.01, Figure 2A). Although p65 mRNA expression was up-regulated by treatment with cisplatin alone, the combination of PDTC and cisplatin resulted in inhibitory enhancement (P < 0.01, Figure 2A). Similar trends in p65 protein level changes were detected (P < 0.01, Figure 2B).
RT-PCR analysis results showed that PDTC treatment significantly downregulated the expression of Δ133p53 mRNA in a dose-dependent manner (P < 0.01, Figure 2C), and inhibitory enhancement was observed after co-treatment with cisplatin (P < 0.01, Figure 2C). However, p53β mRNA remained stable in the presence of PDTC (P > 0.05, Figure 2D). It was down-regulated after cisplatin treatment, and no inhibitory enhancement was observed after co-treatment with cisplatin and PDTC (P > 0.05, Figure 2D).
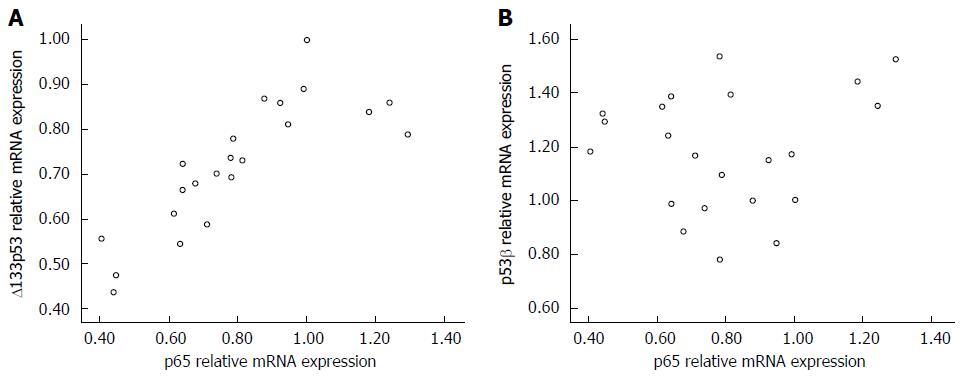
Changes in mRNA levels of p65 paralleled those of Δ133p53 mRNA in MKN45 cells treated with PDTC and/or cisplatin (Figure 3). Pearson linear relevancy analysis demonstrated that expression levels of p65 were positively correlated with those of Δ133p53 (r = 0.085, P < 0.01). However, there was no relationship between the expression levels of p65 and p53β (r = 0.076, P > 0.05).
Over the past two decades, p53 has proven to be a tumor-suppressor, but it loses this ability in various malignant diseases, such as gastric carcinoma. p53 mutations are commonly detected in gastric carcinoma, but are seldom clinically significant[13-19]. Recently, in the last decade, research focusing on the clinical application of p53 isoforms has progressed significantly. More than 12 p53 isoforms have been reported to date, namely, p53α (the canonical wild-type p53), p53β, p53γ, Δ40p53α, Δ40p53β, Δ40p53γ, Δ133p53α, Δ133p53β, Δ133p53γ, Δ160p53α, Δ160p53β, and Δ160p53γ. The p53 isoform expression pattern is remarkably altered in breast cancer[20], kidney cancer[21,22], colorectal cancer[23], ovarian malignancy[24-27], leukemia[28], medulloblastoma[29], and neuroblastoma[30]. Accumulating evidence has indicated that some of these isoforms function as p53 co-activators (e.g., p53β), while others function as its antagonists with oncogenic characteristics (e.g., Δ40p53α and Δ133p53α)[31-33].
A few years ago, our research group investigated the expression of p53 isoforms in different gastric tissues, and we observed a significant increasing trend for Δ133p53α expression but the opposite for p53β as the tissue progressed from superficial gastritis, atrophic gastritis, and para-cancerous lesion to gastric carcinoma[12]. As per the Lauren classification, most cases of gastric carcinoma belong to the intestinal type, gastritis-associated carcinogenesis. In 2015, a clear link between H. pylori infection, gastritis, and gastric cancerogenesis was demonstrated, indicating that Δ133p53 isoform plays an essential role in the process[8,9]. These results indicate that changes in the cross-talk between inflammatory and apoptotic pathways are likely to play an important role in gastric cancerogenesis, especially in cases of the intestinal type. Previously, the NF-κB pathway was reported to be essential in H. pylori-driven chronic gastritis and cancerogenesis. We now speculate that the oncogenic role of the NF-κB pathway cooperates with alterations in the expression patterns of p53 isoforms.
The present experiment was designed to evaluate changes in p53 isoforms (Δ133p53 and p53β) and their combined biological effect in MKN45 cells under an NF-κB-inhibited condition. We confirmed that NF-κB was effectively blocked by PDTC treatment in MKN45 cells, and cellular growth was significantly inhibited in a dose-dependent manner. In addition, the inhibitory effect of cisplatin was remarkably enhanced in a dose-dependent manner by co-treatment with PDTC. These inhibition results indicate that NF-κB signal blockade is a promising strategy in the treatment and prevention of intestinal-type gastric cancer.
However, it is important to explore the underlying molecular mechanisms further in future studies. In the cross-talk between NF-κB and p53 pathways, few molecules such as Δ133p53 have very distinct and significant functions. In the present study, down-regulation of the NF-κB p65 subunit (curbed by PDTC alone or in combination with cisplatin) correlated with growth inhibition of MKN45 cells and Δ133p53 expression. In addition, we found that the p53β isoform was not altered by PDTC intervention, reinforcing that Δ133p53 is a key molecule that is involved in the mediation of cross-talk between the NF-κB inflammatory and p53 apoptotic pathways, especially in gastritis-associated cancerogenesis. Thus, Δ133p53 is a useful marker and candidate target for diagnosis, treatment, and prognosis of intestinal-type gastric cancer.
In recent years, our research group completed a series of experiments to investigate the roles of p53 isoforms in gastric carcinoma for improved diagnosis and pharmacological development and found that the change of p53 isoforms is common in gastric cancerogenesis[12]. Furthermore, up-regulation of the p53β isoform is induced by cisplatin or fluorouracil[34], while down-regulation of Δ133p53 is induced by recombinant mutant human tumor necrosis factor[35], indicating that Δ133p53 and p53β isoforms are strong targets for further diagnosis and pharmacological research. Combined with inferences reported by other study groups[8,9], we now realize that Δ133p53 is an essential factor involved in gastritis-associated carcinoma. According to the Lauren classification[36,37], intestinal gastric carcinoma is roughly ascribed to chronic inflammation, and it develops from multi-genetic alterations and a multi-step process. Therefore, it might be feasible to refine the classification on the basis of molecules identified to be essential in the cross-talk between NF-κB and p53 pathways. Our previous and present results suggest that Δ133p53 is one such good candidate for refining gastric carcinoma classification. The latent value of these findings is that introduction of anti-inflammation agents that target these isoforms could enhance the effect of chemotherapy and improve the prognosis of intestinal-type gastric carcinoma. Further research should focus on two main aspects: development of a simple method to detect these markers and screening for effective agents based on this new perspective.
In summary, Δ133p53 (but not p53β) is a requisite factor in PDTC-induced inhibition of MKN45 gastric cancer cells, and the disturbed cross-talk between p53 and NF-κB pathways is a hopeful target for further diagnosis and pharmaceutical research of intestinal-type gastric cancer.
Accumulating evidence suggests that some p53 isoforms function as p53 co-activators (e.g., p53β); others, its antagonists that possess oncogenic characteristics (e.g., Δ133p53α). Results obtained by our research group and others demonstrate a clear link between Helicobacter pylori (H. pylori) infection, gastritis, and gastric cancerogenesis, with Δ133p53 isoform being essential in the process. Thus, changes in the cross-talk between inflammatory and apoptotic pathways likely play an important role in gastric cancerogenesis, especially in cases of the intestinal type.
Expression patterns of p53 isoforms instead of mutant p53 are significant for various malignant diseases. Δ133p53α has been proven to be a good indicator that could be used for the diagnosis and prognosis of breast and ovarian cancers. Moreover, Δ133p53α is also essential in H. pylori-driven gastritis and cancerogenesis.
Disturbed cross-talk between p53 and nuclear factor-κB (NF-κB) pathways is possibly involved in the progression of chronic gastritis to cancerogenesis. Δ133p53, among other p53 isoforms, is key to the association of H. pylori infection with gastritis and cancerogenesis. These results indicate that Δ133p53 is an important aspect of the NF-κB inhibitor PDTC-mediated biological effect.
Δ133p53 could serve as a promising target for diagnosis, treatment, and prognosis of gastric cancer.
p53 isoforms are produced by alternative splicing of TP53. At least twelve isoforms have been identified. Existence of these isoforms results in the regulation of p53 function. Disturbance in the pattern of these p53 isoforms has been observed to be involved in cancerogenesis in various tissues.
This is a very significant study that involved investigation of the role of delta133p53 isoform in NF-κB inhibitor pyrrolidine dithiocarbamate-mediated growth inhibition of MKN45 gastric cancer cells.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gazouli M, Lee DS S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | van der Post RS, Gullo I, Oliveira C, Tang LH, Grabsch HI, O’Donovan M, Fitzgerald RC, van Krieken H, Carneiro F. Histopathological, Molecular, and Genetic Profile of Hereditary Diffuse Gastric Cancer: Current Knowledge and Challenges for the Future. Adv Exp Med Biol. 2016;908:371-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | López M, Cervera-Acedo C, Santibáñez P, Salazar R, Sola JJ, Domínguez-Garrido E. A novel mutation in the CDH1 gene in a Spanish family with hereditary diffuse gastric cancer. Springerplus. 2016;5:1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Lin Q, Xu H, Chen X, Tang G, Gu L, Wang Y. Helicobacter pylori cytotoxin-associated gene A activates tumor necrosis factor-α and interleukin-6 in gastric epithelial cells through P300/CBP-associated factor-mediated nuclear factor-κB p65 acetylation. Mol Med Rep. 2015;12:6337-6345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Byun E, Park B, Lim JW, Kim H. Activation of NF-κB and AP-1 Mediates Hyperproliferation by Inducing β-Catenin and c-Myc in Helicobacter pylori-Infected Gastric Epithelial Cells. Yonsei Med J. 2016;57:647-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Reingewertz TH, Iosub-Amir A, Bonsor DA, Mayer G, Amartely H, Friedler A, Sundberg EJ. An Intrinsically Disordered Region in the Proapoptotic ASPP2 Protein Binds to the Helicobacter pylori Oncoprotein CagA. Biochemistry. 2015;54:3337-3347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Bhardwaj V, Noto JM, Wei J, Andl C, El-Rifai W, Peek RM, Zaika AI. Helicobacter pylori bacteria alter the p53 stress response via ERK-HDM2 pathway. Oncotarget. 2015;6:1531-1543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Cooks T, Pateras IS, Tarcic O, Solomon H, Schetter AJ, Wilder S, Lozano G, Pikarsky E, Forshew T, Rosenfeld N. Mutant p53 prolongs NF-κB activation and promotes chronic inflammation and inflammation-associated colorectal cancer. Cancer Cell. 2013;23:634-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 390] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 8. | Wei J, Noto J, Zaika E, Romero-Gallo J, Correa P, El-Rifai W, Peek RM, Zaika A. Pathogenic bacterium Helicobacter pylori alters the expression profile of p53 protein isoforms and p53 response to cellular stresses. Proc Natl Acad Sci USA. 2012;109:E2543-E2550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Wei J, O’Brien D, Vilgelm A, Piazuelo MB, Correa P, Washington MK, El-Rifai W, Peek RM, Zaika A. Interaction of Helicobacter pylori with gastric epithelial cells is mediated by the p53 protein family. Gastroenterology. 2008;134:1412-1423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Leroy B, Girard L, Hollestelle A, Minna JD, Gazdar AF, Soussi T. Analysis of TP53 mutation status in human cancer cell lines: a reassessment. Hum Mutat. 2014;35:756-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 11. | Leroy B, Fournier JL, Ishioka C, Monti P, Inga A, Fronza G, Soussi T. The TP53 website: an integrative resource centre for the TP53 mutation database and TP53 mutant analysis. Nucleic Acids Res. 2013;41:D962-D969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Ji W, Zhang N, Zhang H, Ma J, Zhong H, Jiao J, Gao Z. Expression of p53β and Δ133p53 isoforms in different gastric tissues. Int J Clin Exp Pathol. 2015;8:10468-10474. [PubMed] |
| 13. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 14. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 15. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21363] [Article Influence: 2136.3] [Reference Citation Analysis (3)] |
| 16. | Schirren R, Reim D, Novotny AR. Adjuvant and/or neoadjuvant therapy for gastric cancer? A perspective review. Ther Adv Med Oncol. 2015;7:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Ito S, Oki E, Nakashima Y, Ando K, Hiyoshi Y, Ohgaki K, Saeki H, Morita M, Sakaguchi Y, Maehara Y. Clinical significance of adjuvant surgery following chemotherapy for patients with initially unresectable stage IV gastric cancer. Anticancer Res. 2015;35:401-406. [PubMed] |
| 18. | Busuttil RA, Zapparoli GV, Haupt S, Fennell C, Wong SQ, Pang JM, Takeno EA, Mitchell C, Di Costanzo N, Fox S. Role of p53 in the progression of gastric cancer. Oncotarget. 2014;5:12016-12026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Yoshimura A, Sugihara H, Ling ZQ, Peng DF, Mukaisho K, Fujiyama Y, Hattori T. How wild-type TP53 is inactivated in undifferentiated-type gastric carcinomas: analyses of intratumoral heterogeneity in deletion and mutation of TP53. Pathobiology. 2006;73:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Avery-Kiejda KA, Morten B, Wong-Brown MW, Mathe A, Scott RJ. The relative mRNA expression of p53 isoforms in breast cancer is associated with clinical features and outcome. Carcinogenesis. 2014;35:586-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | van den Berg L, Segun AD, Mersch S, Blasberg N, Grinstein E, Wai D, Anlauf M, Gabbert HE, Mahotka C, Heikaus S. Regulation of p53 isoform expression in renal cell carcinoma. Front Biosci (Elite Ed). 2010;2:1042-1053. [PubMed] |
| 22. | Song W, Huo SW, Lü JJ, Liu Z, Fang XL, Jin XB, Yuan MZ. Expression of p53 isoforms in renal cell carcinoma. Chin Med J (Engl). 2009;122:921-926. [PubMed] |
| 23. | Fujita K, Mondal AM, Horikawa I, Nguyen GH, Kumamoto K, Sohn JJ, Bowman ED, Mathe EA, Schetter AJ, Pine SR. p53 isoforms Delta133p53 and p53beta are endogenous regulators of replicative cellular senescence. Nat Cell Biol. 2009;11:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 272] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 24. | Hofstetter G, Berger A, Fiegl H, Slade N, Zorić A, Holzer B, Schuster E, Mobus VJ, Reimer D, Daxenbichler G. Alternative splicing of p53 and p73: the novel p53 splice variant p53delta is an independent prognostic marker in ovarian cancer. Oncogene. 2010;29:1997-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Chambers SK, Martinez JD. The significance of p53 isoform expression in serous ovarian cancer. Future Oncol. 2012;8:683-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Hofstetter G, Berger A, Berger R, Zorić A, Braicu EI, Reimer D, Fiegl H, Marth C, Zeimet AG, Ulmer H. The N-terminally truncated p53 isoform Δ40p53 influences prognosis in mucinous ovarian cancer. Int J Gynecol Cancer. 2012;22:372-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Hofstetter G, Berger A, Schuster E, Wolf A, Hager G, Vergote I, Cadron I, Sehouli J, Braicu EI, Mahner S. Δ133p53 is an independent prognostic marker in p53 mutant advanced serous ovarian cancer. Br J Cancer. 2011;105:1593-1599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Ånensen N, Hjelle SM, Van Belle W, Haaland I, Silden E, Bourdon JC, Hovland R, Taskén K, Knappskog S, Lønning PE. Correlation analysis of p53 protein isoforms with NPM1/FLT3 mutations and therapy response in acute myeloid leukemia. Oncogene. 2012;31:1533-1545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Philipova T, Baryawno N, Hartmann W, Pietsch T, Druid H, Johnsen JI, Ekström TJ. Differential forms of p53 in medulloblastoma primary tumors, cell lines and xenografts. Int J Oncol. 2011;38:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Goldschneider D, Horvilleur E, Plassa LF, Guillaud-Bataille M, Million K, Wittmer-Dupret E, Danglot G, de Thé H, Bénard J, May E. Expression of C-terminal deleted p53 isoforms in neuroblastoma. Nucleic Acids Res. 2006;34:5603-5612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Chan WM, Poon RY. The p53 Isoform Deltap53 lacks intrinsic transcriptional activity and reveals the critical role of nuclear import in dominant-negative activity. Cancer Res. 2007;67:1959-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Courtois S, Verhaegh G, North S, Luciani MG, Lassus P, Hibner U, Oren M, Hainaut P. DeltaN-p53, a natural isoform of p53 lacking the first transactivation domain, counteracts growth suppression by wild-type p53. Oncogene. 2002;21:6722-6728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 206] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 33. | Marcel V, Perrier S, Aoubala M, Ageorges S, Groves MJ, Diot A, Fernandes K, Tauro S, Bourdon JC. Δ160p53 is a novel N-terminal p53 isoform encoded by Δ133p53 transcript. FEBS Lett. 2010;584:4463-4468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Ji W, Ma J, Zhang H, Zhong H, Li L, Ding N, Jiao J, Gao Z. Role of p53β in the inhibition of proliferation of gastric cancer cells expressing wild-type or mutated p53. Mol Med Rep. 2015;12:691-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Shang ZM, Tang JD, Jiang QQ, Guo A, Zhang N, Gao ZX, Ji WS. Role of Δ133p53 in Tumor Necrosis Factor-induced survival of p53 functions in MKN45 gastric cancer cell line. Eur Rev Med Pharmacol Sci. 2015;19:2416-2422. [PubMed] |
| 36. | Ma J, Shen H, Kapesa L, Zeng S. Lauren classification and individualized chemotherapy in gastric cancer. Oncol Lett. 2016;11:2959-2964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 37. | Riquelme I, Saavedra K, Espinoza JA, Weber H, García P, Nervi B, Garrido M, Corvalán AH, Roa JC, Bizama C. Molecular classification of gastric cancer: Towards a pathway-driven targeted therapy. Oncotarget. 2015;6:24750-24779. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |