Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2396
Peer-review started: January 7, 2017
First decision: February 9, 2017
Revised: February 20, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 7, 2017
Processing time: 92 Days and 4.5 Hours
To evaluate the safety and efficacy of tenofovir disoproxil fumarate (TDF) as a first-line therapy in decompensated liver disease.
We enrolled 174 chronic hepatitis B-related liver cirrhosis patients treated with 300 mg/d TDF at six Korean centers. Of the 174 cirrhosis patients, 57 were assigned to the decompensated cirrhosis group and 117 were assigned to the compensated cirrhosis group. We followed the patients for 12 mo and evaluated clinical outcomes, including biochemical, virological, and serological responses. We also evaluated changes in hepatic and renal function and compared the decompensated and compensated cirrhosis groups.
The 1-year complete virological response (CVR) and Hepatitis B e antigen (HBeAg) seroconversion were seen in 70.2% and 14.2% in the decompensated cirrhosis group, respectively. The rates of HBeAg seroconversion/loss and ALT normalization at month 12 were similar in both groups. TDF treatment was also effective for decreasing the level of hepatitis B virus (HBV) DNA in both groups, but CVR was higher in the compensated group (88.9% vs 70.2%, P = 0.005). Tenofovir treatment for 12 mo resulted in improved Child-Turcotte-Pugh (CTP) and model for end-stage liver disease (MELD) scores in decompensated group (P < 0.001). Of the 57 decompensated patients, 39 (68.4%) achieved CTP class A and 27 (49.1%) showed improvement in the CTP score of 2 points after 12 mo of TDF. The observed rate of confirmed 0.5 mg/dL increases in serum levels of creatinine in the decompensated and compensated cirrhosis group were 7.0% and 2.5%, respectively (P < 1.000).
TDF therapy in decompensated cirrhosis patients was effective for decreasing HBV DNA levels and improving hepatic function with relatively lower CVR than in compensated cirrhosis. Thus, physicians should carefully monitor not only renal function but also treatment responses when using TDF in decompensated cirrhosis patients.
Core tip: We evaluated the safety and efficacy of disoproxil fumarate (TDF) treatment in patients with treatment-naïve chronic hepatitis B related decompensated cirrhosis. TDF therapy for 12 mo in decompensated cirrhosis patients was effective for decreasing hepatitis B virus DNA levels and improving hepatic function with relatively lower complete virological response (CVR) than in compensated cirrhosis (70.2% vs 88.9%). The elevation of serum creatinine (> 0.5 mg/dL) in the decompensated cirrhosis relatively higher compared to compensated cirrhosis patients (7.0% vs 2.5%, respectively, P < 1.000). Therefore, TDF therapy is useful in decompensated cirrhosis and needed for close monitoring of CVR and renal function.
- Citation: Lee SK, Song MJ, Kim SH, Lee BS, Lee TH, Kang YW, Kim SB, Song IH, Chae HB, Ko SY, Lee JD. Safety and efficacy of tenofovir in chronic hepatitis B-related decompensated cirrhosis. World J Gastroenterol 2017; 23(13): 2396-2403
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2396
Chronic hepatitis B virus (CHB) infection is a the major public health problem because of its worldwide distribution and its substantial morbidity, and mortality due to complications of cirrhosis and hepatocellular carcinoma (HCC)[1]. In cirrhosis, the 5year probability of decompensation is 15%-20%, with higher risks associated with high viral replication[2]. The 5year survival rate is 14%-35% for decompensated cirrhosis[2].
Decompensation usually presents with at least one episode of ascites, jaundice, hepatic encephalopathy, or variceal bleeding. Patients with decompensated cirrhosis should be treated with potent nucleos(t)ide analogues (NUCs) with good resistance profiles (e.g., entecavir or tenofovir)[3,4]. Treatment is indicated even if hepatitis B virus (HBV) DNA level is low to prevent recurrent reactivation[3,4]. However, there is little information on the safety of tenofovir in decompensated cirrhosis. In addition, tenofovir is cleared primarily by the kidneys, and there have been reports of renal impairment, including Fanconi syndrome. Appropriate monitoring and dosing adjustments are recommended for patients with baseline high renal risk, including one or more of the following: decompensated cirrhosis, creatinine clearance < 60 mL/min, poorly controlled hypertension, proteinuria, or uncontrolled diabetes[3].
Currently, there is little information regarding 1-year treatment efficacy and safety with tenofovir in CHB-related decompensated cirrhosis. In Korea, for 48 wk, we evaluated the safety and efficacy of tenofovir disoproxil fumarate (TDF) in patients with decompensated cirrhosis and those with compensated cirrhosis.
These retrospective cohort analyses were conducted among 180 treatment-naïve patients with CHB-related cirrhosis who were treated with 300 mg/d TDF from January 2013 to January 2014 at six medical centers in Korea. The study was approved by our institutional ethics review board and was conducted in compliance with the Declaration of Helsinki.
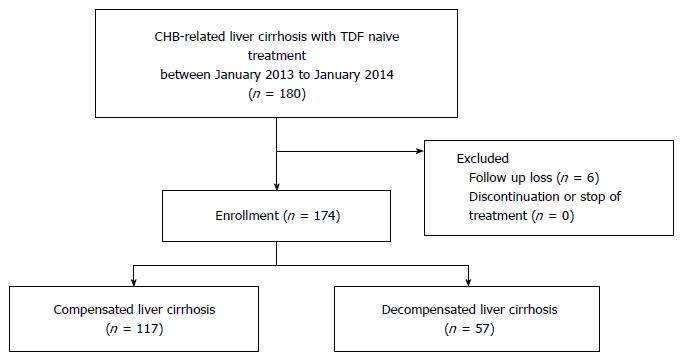
Of these patients, 6 (3.3%) were lost to follow-up. There were no hepatic failure-related deaths during the follow-up period. We analyzed data from the remaining 57 patients with decompensated cirrhosis and 117 with compensated cirrhosis who were treated with 300 mg/d TDF for at least 48 wk in the same period (Figure 1). All patients did not take any other antiviral agent except tenofovir during follow-up period.
Eligibility criteria were as follows: confirmed liver cirrhosis based on clinical tests or radiological imaging [ultrasonography (USG) or liver dynamic computed tomography (CT)][5], serum levels of HBV DNA ≥ 104 in HBeAg-negative or 105 copies/mL in HBeAg-positive CHB, alanine aminotransferase (ALT) < 10 times the upper limit of normal (ULN, 40 IU/L), calculated creatinine clearance (eGFR) ≥ 50 mL/min, and no evidence of HCC. Exclusion criteria included HCV-positive serologies; prior oral NUC use, including lamivudine, telbivudine, adefovir, or entecavir; current grade 2 or higher hepatic encephalopathy; history of variceal bleeding within 2 mo; and hepatorenal syndrome or use of hepatotoxic or nephrotoxic drugs, including those affecting renal tubular secretion.
Decompensated cirrhosis was defined as a Child-Turcotte-Pugh (CTP) score ≥ 7 (Child B and C) or at least one episode of ascites, jaundice, hepatic encephalopathy, or variceal bleeding.
All patients were monitored at least every 3 mo during the antiviral treatment period. Biochemical (serum AST, ALT) and virological parameters (HBeAg, HBeAb status, and quantitative HBV DNA) were assessed at every visit. Imaging studies with USG or liver dynamic CT were performed at every 6 mo. Renal safety in TDF-treated patients was also evaluated in terms of serum levels of creatinine and eGFR every 3 mo.
The primary efficacy endpoints were biochemical, virological, and serological responses during 48 wk of antiviral treatment. Biochemical response was defined as a normalized ALT (≤ 1 times ULN). Complete virological response (CVR) was defined as a decline in HBV-DNA levels to < 116 copies/mL. Serum HBV DNA was measured using the Roche COBAS TaqMan assay (lower limit of quantification of 116 copies/mL). Selected secondary efficacy end points included the difference between compensated and decompensated cirrhosis in biochemical, virological, and serological responses during 48 wk of antiviral treatment. The mean values of HBV DNA were calculated at baseline, week 12, and at 12-wk intervals through week 48. The incidence of virological breakthrough (confirmed ≥ 1 log10 increase in HBV DNA level from nadir) and cumulative probability of HBV antiviral drug resistance were determined through week 48.
Serological response was defined as the disappearance of HBeAg positivity (HBeAg loss) and then HBeAb positivity (HBeAg seroconversion). Serum HBeAg and HBeAb were measured with a radioimmunoassay (RIA) according to the manufacturer’s protocol (Abbott Laboratories, Chicago, IL, United States).
Cumulative safety was assessed through week 48. HBV-related outcomes, including ALT flares, hepatic decompensation, and HCC, were also assessed. The occurrences of serious adverse events and deaths were reported for all enrolled patients. Serum levels of creatinine and creatinine clearance (eGFR using CKD-EPI) were evaluated as categorical end points (confirmed serum levels of creatinine increase from baseline ≥ 0.5 mg/dL and creatinine clearance < 50 mL/min) and creatinine was evaluated as a continuous variable.
Continuous variables are expressed as mean ± SD. Serum HBV DNA levels were expressed on a logarithmic scale. Between group comparisons were performed using Student t test or the Mann-Whitney U test for continuous variables, and the χ2 test or Fisher’s exact test for categorized variables, as appropriate. The multivariate analysis using a logistic regression model was used to determine predictive factors for CVR among various variables including age, pretreatment ALT levels, and viral status. A P value of < 0.05 was considered to be significant (SPSS 17, Chicago, IL, United States).
In total, 174 patients were examined in this study from January, 2013 to January 2014. Of these patients, 57 were assigned to the decompensated cirrhosis group and 117 to the compensated cirrhosis group. The baseline characteristics of the two groups are shown in Table 1. The mean age was 52 years old and 65.5% of the patients were males. Mean serum levels of ALT were higher in the decompensated cirrhosis than compensated cirrhosis group (124.4 IU/L vs 77.2 IU/L, P = 0.014). The proportion of HBeAg positivity was similar in both groups (42.7% vs 49.1%, P = 0.516). The decompensated group had higher CTP and MELD scores, hepatic function (total bilirubin, albumin, PT INR), and platelet counts (P < 0.001 for all). In the decompensated cirrhosis group, 20 (35.0%) patients had ascites, 1 (1.7%) had episodes of hepatic encephalopathy, and 4 (7.0%) experienced variceal bleeding.
| All (n = 174) | Compensated LC (n = 117) | Decompensated LC (n = 57) | P value | |
| Male/female | 114/60 | 73/44 | 41/16 | 0.238 |
| Age (yr) | 52.2 ± 11.0 | 52.3 ± 11.0 | 51.9 ± 11.0 | 0.889 |
| HBeAg positive | 78/96 (44.8%) | 50/67 (42.7%) | 28/29 (49.1%) | 0.516 |
| HBV DNA (log10 copies/mL) | 6.49 ± 1.40 | 6.34 ± 1.40 | 6.79 ± 1.35 | 0.065 |
| ALT (IU/L) | 92.8 ± 165.6 | 77.2 ± 115.2 | 124.4 ± 235.5 | 0.014 |
| TB (mg/dL) | 1.71 ± 2.40 | 1.06 ± 0.97 | 3.04 ± 3.61 | 0.000 |
| PT INR | 1.27 ± 0.29 | 1.14 ± 0.12 | 1.49 ± 0.36 | 0.000 |
| Albumin (g/dL) | 3.9 ± 2.95 | 4.35 ± 3.51 | 3.03 ± 0.66 | 0.000 |
| Platelet count(103/μL) | 115.0 ± 48.5 | 127.7 ± 47.4 | 90.9 ± 41.1 | 0.000 |
| Child score | 6.1 ± 1.6 | 5.2 ± 0.5 | 8.0 ± 1.5 | 0.000 |
| MELD score | 10.1 ± 4.0 | 8.3 ± 1.87 | 13.4 ± 4.76 | 0.000 |
| Ascites | 20 | |||
| Episode of HE | 1 | |||
| Episode of varix bleeding | 4 |
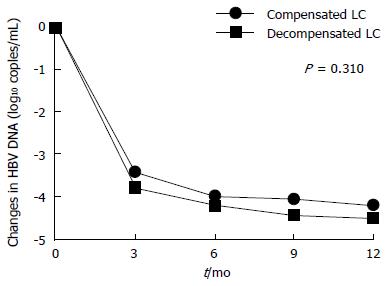
Virological, serological, and biochemical responses are presented in Table 2. Overall, TDF treatment over 12 mo resulted in a progressive reduction in serum levels of HBV DNA (-4.07 log10 copies/mL at 6 mo, and -4.30 log10 copies/mL at 12 mo).
| All (n = 174) | Compensated LC (n = 117) | Decompensated LC (n = 57) | P value | |
| Virological response | ||||
| Change in serum HBV DNA level | ||||
| Month 6 | -4.07 ± 1.39 | -4.00 ± 1.42 | -4.20 ± 1.32 | 0.581 |
| Month 12 | -4.30 ± 1.38 | -4.20 ± 1.39 | -4.49 ± 1.35 | 0.310 |
| Serum HBV DNA undetectable1 | ||||
| Month 6 | 96 (55.2) | 67 (57.3) | 29 (50.9) | 0.516 |
| Month 12 | 144 (82.8) | 104 (88.9) | 40 (70.2) | 0.005 |
| Serological | ||||
| HBeAg seroconversion | ||||
| Month 6 | 6/78 (7.7) | 3/50 (6.0) | 3/28 (10.7) | 0.664 |
| Month 12 | 7/78 (8.9) | 3/50 (6.0) | 4/28 (14.2) | 0.373 |
| HBeAg loss | ||||
| Month 6 | 2/78 (2.5) | 2 (4.0) | 0 (0) | 0.427 |
| Month 12 | 2/78 (2.5) | 2 (4.0) | 0 (0) | 0.517 |
| Biochemical | ||||
| ALT normalization | ||||
| Month 6 | 108 (62.0) | 72 (61.5) | 36 (63.2) | 0.869 |
| Month 12 | 121 (69.5) | 77 (65.8) | 44 (77.2) | 0.161 |
Moreover, undetectable HBV DNA levels were observed in 144 of 174 (82.8%) patients during the 12 mo of TDF treatment. The mean reductions in HBV DNA levels at 6 and 12 mo did not significantly differ between the groups (P = 0.31; Figure 2). However, there was a significant difference in CVR; it was higher in the compensated group (88.9% vs 70.2%, P = 0.005). There was no virological breakthrough in any patient during the follow-up period. Regarding the biochemical response, ALT normalization was similar between the groups after 12 mo of TDF therapy (77.2% vs 65.8%, P = 0.161).
Of the 78 HBeAg-positive patients, 6 (7.7%) and 7 (8.9%) exhibited HBeAg seroconversion at months 6 and 12, respectively, with similar proportions being observed in each group (10.7% vs 6% at 6 mo, P = 0.664, and 14.2% vs 6% at 12 mo, P = 0.373). This distribution was consistent with the HBeAg loss (0% vs 4% at 6 mo, P = 0.427; and 0% vs 4% at 12 mo, P = 0.517). Subgroup analyses according to HBeAg status showed that CVR in HBeAg-positive patients were significantly lower in the decompensated group (P = 0.01, Table 3). During 12 mo of TDF therapy, the cumulative rate of CVR was significantly higher in HBeAg-negative than in HBeAg-positive patients (93.7% vs 74.3%, P < 0.001).
| All | Compensated LC (n = 50) | Decompensated LC (n = 28) | P value | |
| Virological response of HBeAg positive patients (n = 78) | ||||
| Change in serum HBV DNA level | ||||
| Month 6 | -4.32 ± 1.33 | -2.89 | -3.18 | 0.926 |
| Month 12 | -4.65 ± 1.33 | -4.56 ± 1.45 | -4.82 ± 1.08 | 0.701 |
| Serum HBV DNA undetectable | ||||
| Month 6 | 31 (39.7) | 20/50 (40) | 11/17(39.2) | 1.000 |
| Month 12 | 54 (74.3) | 40/10(80) | 14/14(50) | 0.010 |
| Compensated LC (n = 67) | Decompensated LC (n = 29) | |||
| Virological response of HBeAg negative patients (n = 96) | ||||
| Change in serum HBV DNA level | ||||
| Month 6 | -3.86 ± 1.40 | -3.78 ± 1.39 | -4.04 ± 1.43 | 0.576 |
| Month 12 | -4.01 ± 1.37 | -3.94 ± 1.30 | -4.19 ± 1.51 | 0.534 |
| Serum HBV DNA undetectable | ||||
| Month 6 | 65 (67.7) | 47/20 (70.1) | 18/11 (62.0) | 0.481 |
| Month 12 | 90 (93.7) | 64/3 (95.5) | 26/3 (89.6) | 0.362 |
Logistic regression analyses with adjustments for potential baseline confounders (age, sex, HBeAg status, initial HBV DNA levels, and categories of liver disease) showed that baseline HBeAg seropositivity and decompensated liver disease status were the only independent predictive factors that adversely affected CVR during TDF therapy (OR = 5.617, 95%CI: 2.011-15.689, P = 0.001, OR = 0.340; 95%CI: 0.139-0.829, P = 0.018, respectively; Table 4).
| Regression coefficient | Standard error | OR (95%CI) | P value | |
| Age (per year) | -0.018 | 0.022 | 0.982 (0.941-1.025) | 0.412 |
| Sex | 0.427 | 0.543 | 1.533 (0.529-4.439) | 0.431 |
| ALT level (per 1 IU/L) | 0.001 | 0.001 | 1.001 (0.999-1.003) | 0.342 |
| HBeAg | 1.726 | 0.524 | 5.617 (2.011-15.689) | 0.001 |
| Positivity | ||||
| Negativity | ||||
| Baseline HBV DNA (per 1 log10 copies/mL) | 0.117 | 0.184 | 1.124 (0.784-1.611) | 0.525 |
| Diagnosis | -1.080 | 0.456 | 0.340 (0.139-0.829) | 0.018 |
| Compensated | ||||
| Decompensated |
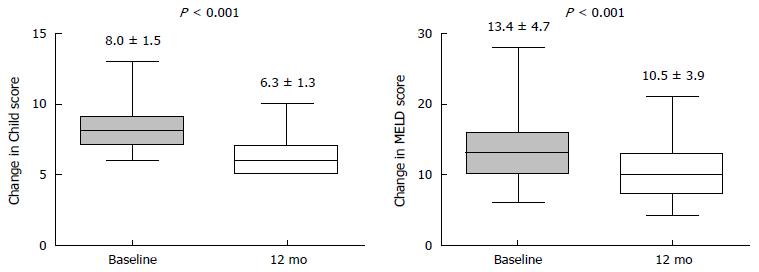
To evaluate the influence of TDF therapy on hepatic function reserve in the decompensated group, we measured the CTP and MELD score and compared these values of pre- and post-treatment (Figure 3). For all 57 patients, the mean CTP score (8.0 vs 6.3), and MELD score (13.4 vs 10.5) improved after 12 mo of TDF treatment vs baseline (all P < 0.001). Of them, 27 (49.1%) patients showed an improvement of ≥ 2 points in CTP score. Of the remaining 30 (50.9%) patients, 12 achieved CTP class A with a 1-point of improvement; 4 did not achieve CTP class A despite 1 point in improvement; 11 showed no change, and 3 experienced aggravation. As a result, 39 of the 57 (68.4%) patients achieved CTP class A (score 5 or 6) after 12 mo of TDF.
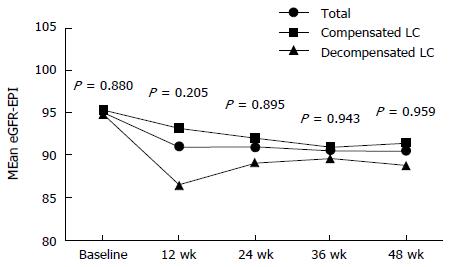
Changes of creatinine clearance (eGFR) during TDF therapy are shown in Figure 4 and Table 5. There were no statistically significant differences in eGFR between the groups during TDF therapy. Seven patients (three in the compensated vs four in the decompensated group, 2.5% vs 7.0%, P < 1.000, respectively) had confirmed ≥ 0.5 mg/dL increases from baseline in serum levels of creatinine (all also had confirmed CLcr< 50 mL/min). Of these seven patients, two patients had increased creatinine at 12 wk, one had it at 24 wk, and four had it at 48 wk.
| All (n = 174) | Mean eGFR (CKD-EPI equation) | P value | ||
| Compensated LC (n = 117) | Decompensated LC (n = 57) | |||
| Baseline | 95.2 ± 17.8 | 95.4 ± 14.8 | 94.8 ± 23.0 | 0.880 |
| Week 12 | 91.1 ± 16.2 | 93.2 ± 13.0 | 86.6 ± 21.1 | 0.205 |
| Week 24 | 91.1 ± 17.5 | 92.2 ± 14.2 | 89.2 ± 22.0 | 0.895 |
| Week 36 | 90.6 ± 16.6 | 91.1 ± 14.4 | 89.7 ± 20.3 | 0.943 |
| Week 48 | 90.7 ± 18.4 | 91.6 ± 14.9 | 88.9 ± 23.8 | 0.959 |
We examined the safety and efficacy of TDF for 48 wk in treatment-naïve decompensated cirrhosis patients compared to compensated patients. We found that TDF was effective both groups. Moreover, TDF monotherapy significantly improved underlying liver function. Previous studies have shown that entecavir is also effective for decompensated cirrhosis and improves liver function[6]. However, the long-term treatment efficacy and safety of TDF in decompensated cirrhosis has not been well established.
In our study, the HBeAg seroconversion rate and the HBeAg loss rate were similar between the groups but were considerably lower than in a previous Liaw’s study[7]. That study was a phase 2, multi-center, randomized trial conducted at several hospitals in Europe, Canada, Singapore, Taiwan, and the United States. However, our study represented real-world data from Korea. The differences may also be due to the high prevalence of the HBV genotype C, acquired through vertical transmission.
HBV DNA levels after TDF treatment over 12 mo markedly decreased in both the compensated and decompensated groups. CVR after 12 mo was 70.2% in the decompensated cirrhosis patients, significantly lower than in the compensated group (88.9%, P = 0.005). The rate of DNA negativity in the former group was similar to a previous report[7]. Moreover, logistic regression analyses showed that baseline HBeAg seropositivity and decompensated cirrhosis were independent predictive factors adversely affecting CVR. No studies to date have compared decompensated and compensated groups treated with TDF. The entecavir study reported by Shim et al[6] showed no significant difference in HBV DNA negativity between compensated and decompensated cirrhosis patients. The lower CVR in the decompensated group of our study than in the entecavir study may be due to the higher baseline MELD score. Moreover, the stricter cut-off level (HBV-DNA level < 116 copies/mL) could also have contributed to this result. However, even considering these factors, the lower CVR in the decompensated group suggests that there would be a higher risk of hepatic events and mortality in such patients, because entecavir-treated cirrhosis patients without CVR have a higher risk of hepatic events and mortality than CVR patients[8]. Moreover, patients without CVR show comparable risks of hepatic events and mortality to untreated patients[8]. Thus, we should follow HBV DNA levels more carefully in TDF-treated decompensated patients and check for possible hepatic events more frequently in patients without CVR.
We also measured changes of CTP and MELD scores in the decompensated group to evaluate the effects of TDF on hepatic function. All patients in that group showed improvements in the mean CTP and MELD scores. Moreover, 49.1% of patients had an improvement of ≥ 2 points in CTP score and 68.4% of patients achieved CTP class A. This result is similar several previous studies[9,10]. Here we showed that TDF therapy not only decreased the HBV DNA level effectively but also improved the hepatic function in the decompensated group.
In terms of renal function, TDF therapy was safe in both groups, consistent with some previous studies[10,11]. However, Tsai et al[12] reported that TDF is an independent predictor of renal dysfunction and the AASLD guidelines recommend follow-up of renal function in patients after TDF treatment[4]. In our study, the decompensated group showed a higher rate of decreasing renal function (7.0% vs 2.5%) but the difference was not statistically significant. Thus, we should be cautious about TDF therapy, particularly in high-risk groups for renal dysfunction, such as patients with uncontrolled diabetes, proteinuria, and poorly controlled hypertension[3]. Recently, tenofovir alafenamide (TAF) was introduced as an effective and safe drug[13-15]. TAF therapy can be safe and effective for groups at high risk for renal dysfunction. In the future, a TAF study in decompensated cirrhosis patients is needed.
This study had several limitations. First, the study was retrospective design and the patient number was small, thus a prospective study is needed. Second, the follow up period was 1 year, which was sufficient, but data from a longer follow-up period are needed.
In conclusion, we showed that TDF therapy in decompensated cirrhosis patients was effective for decreasing HBV DNA levels and improving hepatic function with relatively lower CVR than in compensated cirrhosis. Thus, physicians should carefully monitor not only renal function but also treatment responses when using TDF in decompensated cirrhosis patients.
Currently, there is little information regarding 1-year treatment efficacy and safety with tenofovir in chronic hepatitis B virus-related decompensated cirrhosis.
Authors aim to evaluate the safety and efficacy of tenofovir disoproxil fumarate (TDF) as a first-line therapy in decompensated liver disease.
TDF therapy in decompensated cirrhosis patients was effective for decreasing hepatitis B virus DNA levels and improving hepatic function with relatively lower complete virological response than in compensated cirrhosis. Thus, physicians should carefully monitor not only renal function but also treatment responses when using TDF in decompensated cirrhosis patients.
Overall, this is a clear and well-written manuscript. The introduction is relevant and theory based. The methods are appropriate and the results are clear. The authors make contribution to the research literature in this area of investigation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Fimmel CJ, Gao ZL, Li J, Utku AC, Wang X S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Fattovich G. Natural history and prognosis of hepatitis B. Semin Liver Dis. 2003;23:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 285] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 2. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 967] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 3. | EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 4. | Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1532] [Cited by in RCA: 1588] [Article Influence: 176.4] [Reference Citation Analysis (2)] |
| 5. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 6. | Shim JH, Lee HC, Kim KM, Lim YS, Chung YH, Lee YS, Suh DJ. Efficacy of entecavir in treatment-naïve patients with hepatitis B virus-related decompensated cirrhosis. J Hepatol. 2010;52:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 211] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 7. | Liaw YF, Sheen IS, Lee CM, Akarca US, Papatheodoridis GV, Suet-Hing Wong F, Chang TT, Horban A, Wang C, Kwan P. Tenofovir disoproxil fumarate (TDF), emtricitabine/TDF, and entecavir in patients with decompensated chronic hepatitis B liver disease. Hepatology. 2011;53:62-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 8. | Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 396] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 9. | Köklü S, Tuna Y, Gülşen MT, Demir M, Köksal AŞ, Koçkar MC, Aygün C, Coban S, Ozdil K, Ataseven H, Akin E, Pürnak T, Yüksel I, Ataseven H, Ibiş M, Yildirim B, Nadir I, Küçükazman M, Akbal E, Yüksel O, Başar O, Alkan E, Baykal O. Long-term efficacy and safety of lamivudine, entecavir, and tenofovir for treatment of hepatitis B virus-related cirrhosis. Clin Gastroenterol Hepatol. 2013;11:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Miquel M, Núñez Ó, Trapero-Marugán M, Díaz-Sánchez A, Jiménez M, Arenas J, Canós AP. Efficacy and safety of entecavir and/or tenofovir in hepatitis B compensated and decompensated cirrhotic patients in clinical practice. Ann Hepatol. 2013;12:205-212. [PubMed] |
| 11. | Cholongitas E, Papatheodoridis GV, Goulis J, Vlachogiannakos J, Karatapanis S, Ketikoglou J, Vasiliadis T, Kontos G, Karlaftis A, Akriviadis E. The impact of newer nucleos(t)ide analogues on patients with hepatitis B decompensated cirrhosis. Ann Gastroenterol. 2015;28:109-117. [PubMed] |
| 12. | Tsai MC, Chen CH, Tseng PL, Hung CH, Chiu KW, Wang JH, Lu SN, Lee CM, Chang KC, Yen YH. Comparison of renal safety and efficacy of telbivudine, entecavir and tenofovir treatment in chronic hepatitis B patients: real world experience. Clin Microbiol Infect. 2016;22:95.e1-95.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Lin CL, Yang HC, Kao JH. Hepatitis B virus: new therapeutic perspectives. Liver Int. 2016;36 Suppl 1:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Bam RA, Yant SR, Cihlar T. Tenofovir alafenamide is not a substrate for renal organic anion transporters (OATs) and does not exhibit OAT-dependent cytotoxicity. Antivir Ther. 2014;19:687-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Agarwal K, Fung SK, Nguyen TT, Cheng W, Sicard E, Ryder SD, Flaherty JF, Lawson E, Zhao S, Subramanian GM. Twenty-eight day safety, antiviral activity, and pharmacokinetics of tenofovir alafenamide for treatment of chronic hepatitis B infection. J Hepatol. 2015;62:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |