Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2276
Peer-review started: November 25, 2016
First decision: December 19, 2016
Revised: January 18, 2017
Accepted: March 15, 2017
Article in press: March 15, 2017
Published online: April 7, 2017
Processing time: 160 Days and 9.4 Hours
Pancreatic ductal adenocarcinoma is a devastating disease with a poor prognosis regardless of stage. To date the mainstay of therapy for advanced disease has been chemotherapy with little incremental improvements in outcome. Despite extensive research investigating new treatment options the current practices continue to utilise fluorouracil or gemcitabine containing combinations. The need for novel therapeutic approaches is mandated by the ongoing poor survival rates associated with this disease. One such approach may include manipulation of ribosome biogenesis and the nucleolar stress response, which has recently been applied to haematological malignancies such as lymphoma and prostate cancer with promising results. This review will focus on the current therapeutic options for pancreatic ductal adenocarcinoma and the complexities associated with developing novel treatments, with a particular emphasis on the role of the nucleolus as a treatment strategy.
Core tip: This manuscript is a review of the complexities involved in the treatment of advanced pancreatic ductal adenocarcinoma. It details the current approaches to therapy and the disease factors which have impacted on progress thus far. This review identifies the possible role of nucleolar stress as a treatment modality based on recent data from studies of haematological malignancies and some other solid organ cancers and explains the basic science involved in this process.
- Citation: Diwakarla C, Hannan K, Hein N, Yip D. Advanced pancreatic ductal adenocarcinoma - Complexities of treatment and emerging therapeutic options. World J Gastroenterol 2017; 23(13): 2276-2285
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2276.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2276
Advanced pancreatic ductal adenocarcinoma (PDAC) remains a significant cause of mortality accounting for up to 4% of all cancer related deaths world-wide[1]. Of concern, despite treatment, the mortality rates remain high and essentially unchanged over the last two decades[2]. Furthermore, even in the setting of adjuvant therapy, 5 year survival rates remain poor at only 25%[2], while for metastatic disease it is 1%[3]. Such poor statistics are no doubt compounded by the fact that current backbones to treatment, fluorouracil or gemcitabine, have also remained unchanged over the last 10-20 years and continue to form the basis for new combination approaches[4]. As a consequence, there is an ongoing need for the development of improved diagnostic techniques, treatment options and surveillance markers to improve the outcomes for this devastating disease.
In recent years, various studies have focused on trialling new chemotherapeutic drug combinations for the treatment of PDAC. The PRODIGY 4/ACCORD 11 trial[5,6] demonstrated that FOLFIRINOX, a combination of fluorouracil, oxaliplatin and irinotecan, provided superior progression free survival (PFS), overall survival (OS) and response compared with gemcitabine alone for patients with metastatic disease. Unfortunately, however, the drawback was that many patients displayed significant toxicity associated with this multidrug approach including an higher incidence of grade 3 or 4 neutropenia and thrombocytopenia[5].
Two years later, the MPACT trial compared the combination of gemcitabine and nab-paclitaxel against gemcitabine alone in a total of 842 patients and demonstrated a statistically significant improvement in median OS (8.7 mo vs 6.6 mo)[7] and was particularly interesting given that previous investigations comparing gemcitabine combinations against gemcitabine alone for PDAC had been negative. Importantly, this trial did not exclude more elderly patients unlike the ACCORD-11 study which only incorporated patients with a mean age of 61 years[5]. In keeping with PDAC demographics, MPACT included patients with a mean age for men of 71 and 75 for women[8]. Toxicities related to gemcitabine and nab-paclitaxel were overall very similar to those seen with FOLFIRINOX though there is a perception that FOLFIRINOX is best reserved for “fit” patients despite the two regimens never formally being compared.
More recently, the NAPOLI-1 study compared the effect of nanoliposomal irinotecan with or without fluorouracil and folinic acid as well as the combination of fluorouracil and folinic acid alone in patients with metastatic disease who progressed following a gemcitabine based approach[9]. In its nanoliposomal form, irinotecan has improved drug stability allowing its active metabolite, SN-38, to remain in circulation longer than free irinotecan, ultimately prolonging intra-tumoral levels of the active drug[10,11]. The median OS was significantly improved for those receiving nanoliposomal irinotecan, fluorouracil plus folinic acid (6.1 mo vs 4.2 mo) compared with patients who did not receive nanoliposomal irinotecan. Similarly, the PFS noted for those receiving combination therapy with nanoliposomal irinotecan was significantly improved compared to fluorouracil and folinic acid alone at 3.1 mo vs 1.5 mo. Differences between nanoliposomal irinotecan monotherapy and fluorouracil with folinic acid alone were not statistically significant[9]. Based on this study and others the FDA has approved the use of nanoliposomal irinotecan with fluorouracil and folinic acid for treatment of metastatic PDAC refractory to gemcitabine based therapy. Interestingly the toxicities seen with this newer regimen were relatively similar to those seen with FOLFIRINOX and gemcitabine with nab-paclitaxel including grade 3 or 4 toxicities in such domains as neutropenia, diarrhoea, vomiting and fatigue.
While new chemotherapy combinations have shown promise in the clinical setting, PDAC remains a difficult disease to manage due to multiple factors including patient age, medical co-morbidities and cancer related symptoms. As is often the case in advanced/metastatic malignancies, the art of medicine relies on achieving a balance between therapeutic gain, drug induced side effects and quality of life. To this end, novel approaches that are non, or at least minimally genotoxic are of particular interest. The potential advantages observed with targeted therapies in reducing systemic side effects and improving outcomes for other malignancies have gained interest in the realm of PDAC and will be briefly discussed in this review with a particular focus on the emerging role of nucleolar stress pathway and ribosome biogenesis as a potential treatment option given its promising results in the management of haematological malignancies and prostate cancer[12-14].
When evaluating the role of novel therapies, it is important to understand potential interactions with the disease in question, specifically focusing on disease factors that could impact on drug delivery, tolerability or dosing for example. PDAC is associated with multiple complexities which have precluded the development of novel approaches and therefore require due consideration.
PDAC is a disease of the elderly, presenting on average at 71 years of age[3]. Up to 9% of patients present with localised disease however, the majority are diagnosed with either locally advanced or metastatic disease at their first consultation[15]. Due its anatomical location the symptoms associated with PDAC tend to occur insidiously therefore contributing to the often delayed time to investigation and subsequent diagnosis[16]. Such patient factors have important implications on treatment decisions which may in part explain the limited use of FOLFIRINOX in many patients despite its improved median OS, PFS and objective response[5].
Given the advanced age and stage at diagnosis the traditional focus on clinical outcomes and survival for interventional studies of PDAC management have shifted with increasingly more importance being placed on patient reported outcomes such as pain management and appetite. This is of particular importance for advanced PDAC given its poor prognosis[17]. Without acknowledging the impact the treatment may have on patient reported outcomes, therapeutic advancements may be futile, thus developing drugs that are minimally toxic would be beneficial, yet to date, therapeutic advancements have continued to cause very similar side effect profiles to one another.
Inherited predispositions to PDAC account for 5%-10% of cases[18]. In ongoing studies through the Australasian Pancreatic Cancer Genome Initiative, Humphris et al[19] described the manifestations of inherited PDAC as occurring in 3 distinct settings, hereditary tumour predisposition syndromes, hereditary pancreatitis and familial pancreatic cancers which were further defined as occurring in a kindred in whom at least 2 first degree relatives have PDAC without diagnostic criteria for an inherited cancer syndrome[19,20]. Interestingly some of the genes identified in hereditary forms of PDAC affect pathways involved in DNA repair such as BRCA1/2 and PALB2 which have been noted in more recent detailed sequencing/mutational studies presented by Waddell et al[21] and Bailey et al[22] in two separate comprehensive analyses. While inherited forms of cancer are of interest the vast majority of genetic/metabolic pathway abnormalities are not inherited and form the basis of most PDAC cases.
Four major driver genes, KRAS, TP53, CDKN2A and SMAD4 have been identified in the development of PDAC[22], each sharing common oncogenic signalling pathways. KRAS mutations occur in > 90% of tumours and may represent the underlying insult to numerous subsequent events contributing to disease development. This mutation is widely accepted as a requirement for “reprogramming” pancreatic cell metabolism to facilitate the acidic environment needed for extracellular matrix breakdown and tumour invasion common to PDAC[23,24].
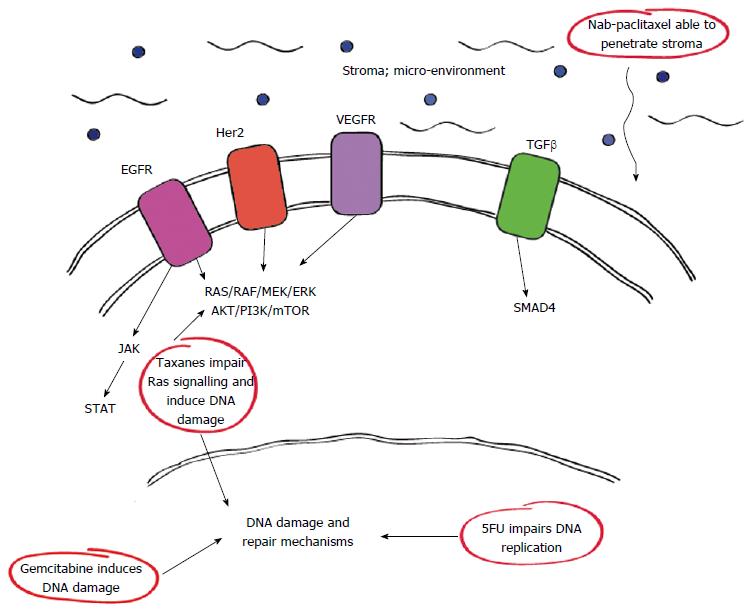
Of interest, the complex genetic and metabolic pathways associated with PDAC have identified various interactions and sites which can be utilised for therapeutic means. Among these include certain growth factor receptors such as epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor which have important roles in the RAS/RAF pathway as outlined in Figure 1. Furthermore it is now clear that drugs such as nab-paclitaxel have therapeutic actions against some of these pathways either directly or downstream accounting for their efficacy.
Recent data published in Nature by Waddell et al[21] reported the complex mutational landscape of PDAC, identifying multiple point mutations and structural variations in key genes. This data confirmed that KRAS abnormalities were almost ubiquitous while TP53 lesions were noted in 74% of samples, closely followed by CDKN2A lesions (35%) and SMAD4 abnormalities (31%). Through their analysis, PDAC was sub classified into 4 subtypes based on the distribution of events such that samples fell into either stable, scattered, unstable or locally arranged groupings. Of interest, the same study established a relationship between mutational load and abnormalities affecting DNA maintenance genes, specifically BRCA[25] and PALB2[26]. It is not surprising that PDAC associated with either PALB2 or BRCA2 mutations should behave in a similar fashion given their roles in DNA damage and repair. PALB2 binds and co-localizes with BRCA2 in order to facilitate double stranded DNA damage. In cases where PDAC harbours BRCA2 mutations there is good evidence for improved response to platinum containing therapies unlike other forms of PDAC[21]. For PALB2 mutant disease there have been a number of reports noting improved outcomes in response to platinum based therapies or mitomycin C when compared with gemcitabine[27,28] and there is potential for targeted treatment with poly ADP ribose polymerase (PARP) inhibitors especially in the setting of BRCA2 as seen in breast and ovarian cancers.
Further to the comprehensive studies of Waddell et al[21], Bailey et al[22] identified aggregates of point mutations in core molecular pathways affecting cellular functions including DNA damage and repair pathways, cell cycle regulation, transforming growth factor beta (TGFβ) signalling, chromatin regulation and axonal guidance. Based on the expression of 32 recurrently mutated genes found to aggregate into ten distinct pathways. From this analysis four subtypes were identified, comprising of, squamous, pancreatic progenitor, immunogenic and aberrantly differentiated endocrine and exocrine categories[22]. Similar to Waddell et al[21] the implications of these findings may potentially identify opportunities for therapeutic development. While these data have provided valuable insights into the complex molecular basis of PDAC many studies have previously attempted to manipulate some of these genes and/or pathways at various levels already and will be briefly discussed here.
Given the near ubiquitous nature of KRAS mutations in PDAC, targeting this gene and its associated pathways has been an area of interest however thus far this has not translated to clinically significant outcomes. In an effort to target as many known mutations/pathways as possible various studies have focused on novel therapies in combination with known chemotherapeutics in the hopes that treatment outcomes improve.
Selumetinib, an orally bioavailable selective MEK1/2 inhibitor showed promise in preclinical studies but failed to demonstrate a survival advantage in the second line setting when compared with the oral equivalent to fluorouracil, capecitabine[29]. Similarly, attempts at targeting other known PDAC genes/pathways including P13K/Akt/mTOR have been clinically disappointing[30,31].
Her2/neu amplification is well characterised in a number of malignancies which have shown response when used as druggable targets in breast and gastric malignancies for example[32-34]. In PDAC, Her2/neu is amplified in up to 45% of cases[35], particularly in the advanced setting. Unfortunately, while initial pre-clinical studies indicated a potential role for Her-2 directed therapy specifically trastuzumab as a monotherapy or in combination with gemcitabine[36] it did not prove clinically advantageous. Similarly, investigations combining trastuzumab with capecitabine were disappointing[35]. In a related fashion EGFR which is known to co-express with Her2 has also been investigated with statistical gains noted for the EGFR inhibitor erlotinib in conjunction with gemcitabine, however this was only in the order of two weeks survival benefit and little mention of the related quality of life impact secondary to treatment was reported[34].
The JAK/STAT pathway has also been implicated as a regulator in the development of PDAC via its role in activating signalling cascades and gene transcription. Stimulated by oxidative stress, this pathway ultimately induces the production of inflammatory cytokines as well as cell proliferation, malignant transformation and inhibition of apoptosis in the pancreas[37,38]. This association with inflammation has prompted trials of the JAK-1/2 inhibitor ruxolotinib in combination with capecitabine for patients with metastatic PDAC after failure of first line therapy if patients expressed elevated inflammatory markers as assessed by C-reactive protein[39]. In this phase II randomised trial, 127 patients were treated with either ruxolotinib and capecitabine or capecitabine and placebo. However, interim analyses failed to demonstrate sufficient efficacy with ruxolotinib combination therapy and further investigations of this drug has been suspended[40].
Together this information provides us with a raft of data for potential therapeutic targets, whether via drivers, alterations in signalling pathways or susceptibility genes. For example in the case of PALB2 mutation-associated disease there are multiple reports suggesting improved outcomes in response to platinum based therapy or mitomycin C when compared with gemcitabine[27,28] suggesting that a more comprehensive understanding of the genetic complexity of PDAC will assist in treatment decisions. Thus understanding the similarities and differences between the “poor” and “exceptional responders” may provide biomarkers to identify patients who might benefit from these treatments and improve outcomes[41].
PDAC is characterised by the surrounding cells, specifically activated fibroblasts, myofibroblasts and pancreatic stellate cells which contribute to the composition of the surrounding matrix, elements such as hyaluronan, growth factors (e.g., TGF-β) and secreted protein acidic rich in cysteine (SPARC)[42,43]. These result in a unique stromal microenvironment which may not only promote tumour initiation and progression but also create a barrier to drug delivery thus rendering PDAC relatively chemoresistant. Consequently, much effort has focused on ways to deplete or manipulate the stromal microenvironment and improve therapeutic outcomes.
SPARC is a glycoprotein believed to be involved in cancer development via its modulation of cell proliferation, progression, angiogenesis, migration, metastasis and apoptosis[44]. It’s normal role in cellular functions is thought to be multifactorial with effects on cell dispersion and chemosensitization as well as induction of apoptosis but also has antiangiogenic properties[45-48]. While the intricacies of SPARC and cancer are yet to be fully elucidated its potential to increase the invasive capacity of malignant cells and possible association with poor prognosis is recognised[7]. Of interest, SPARC methylation leading to pathogenesis correlates with both tobacco smoking and alcohol consumption, which are known associated modifiable risk factors for PDAC[49].
Interestingly gemcitabine has been reported to alter SPARC expression in a dose dependent manner in cell lines[50] however, there is also evidence to suggest that SPARC overexpression enhances PDAC cell chemosensitivity to gemcitabine[51]. In fact, SPARC may actually assist in the delivery of nab-paclitaxel to the tumour due to its affinity for albumin[52]. Nab-paclitaxel is formulated with human albumin at concentrations that closely resemble physiological albumin levels. This feature seems to enable nab-paclitaxel to penetrate the stromal environment and reach the tumour more efficiently[53]. Despite these studies however the role for SPARC within the stromal micro-environment and its implications on therapy remain controversial. For example data presented by Hidalgo et al[54] reported no clear association between SPARC levels and treatment efficacy with combination therapy using gemcitabine and nab-paclitaxel or gemcitabine alone in metastatic PDAC.
Growth factors such as TGF-β are produced by cells within the stromal microenvironment. Interestingly TGF-β levels correlate with tumour metastases and progression as well as poorer patient outcomes[55]. The specific role for TGF-β in this case is not clear but may involve regulation of cell cycle arrest, apoptosis, immune response and/or wound healing. Activated TGF-β signalling is mediated via SMADs, a known driver of PDAC, which is also reported to correlate with worse prognosis or disseminated disease[56,57].
Intriguingly TGF-β has been reported as a tumour suppressor in early stages of malignancy but a promoter in established disease[58] further emphasising the complex nature of PDAC.
Despite the steady accumulation of knowledge from studies into genetic and molecular pathways, complex stromal characteristics and better drug delivery remains an ongoing issue further prompting investigations of novel approaches such as nucleolar stress pathways and ribosome biogenesis.
Ribosome biogenesis is a highly coordinated process which takes place within a dynamic compartment of the nucleus termed the nucleolus. Long before the functional role of the nucleolus was established, Pianese[59] noted that malignant cells were often characterised by enlarged, abnormal nucleoli. Since then, numerous studies have correlated morphological changes of the nucleoli with malignant disease.
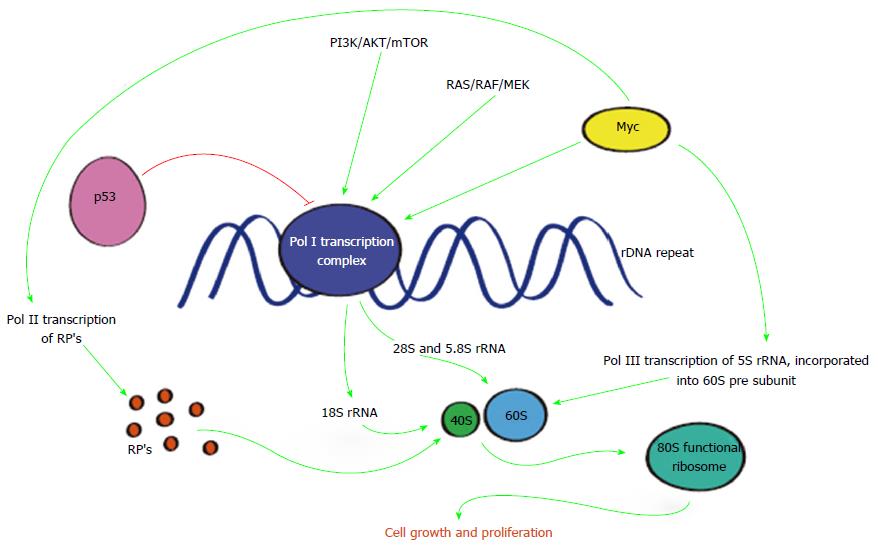
It is now clear that the morphological changes affecting nucleoli in malignant cells reflects hyperactivated transcription of ribosomal RNA (rRNA) genes by RNA polymerase I (Pol I) yielding the 47S rRNA precursor which is rapidly processed into mature 18, 5.8 and 28S rRNAs[60] while the 5S rRNA precursor is transcribed by RNA polymerase III (Pol III). These RNA’s together with ribosomal proteins (RPs) transcribed by RNA polymerase II (Pol II) are essential for ribosome assembly, central to the synthesis of cellular proteins[61,62]. Co-ordination of all three polymerases is necessary for the development of a functional mammalian ribosome (80S) composed of a small (40S) and large (60S) subunit[62,63].
During active cell division and proliferation ribosome biogenesis increases so as to maintain elevated cellular demands. It is not surprising that this process is a major consumer of cellular energy requiring vigilant regulation[64,65]. However, ribosome biogenesis is not only regulated by RNA polymerases, as depicted in Figure 2, but also involves a number of tumour suppressors or oncoproteins[66] including c-myc, considered a “master regulator” of protein synthesis[67]. Conversely p53 has an important place in inhibiting ribosome biogenesis[68] and promoting cell cycle arrest, senescence or apoptosis.
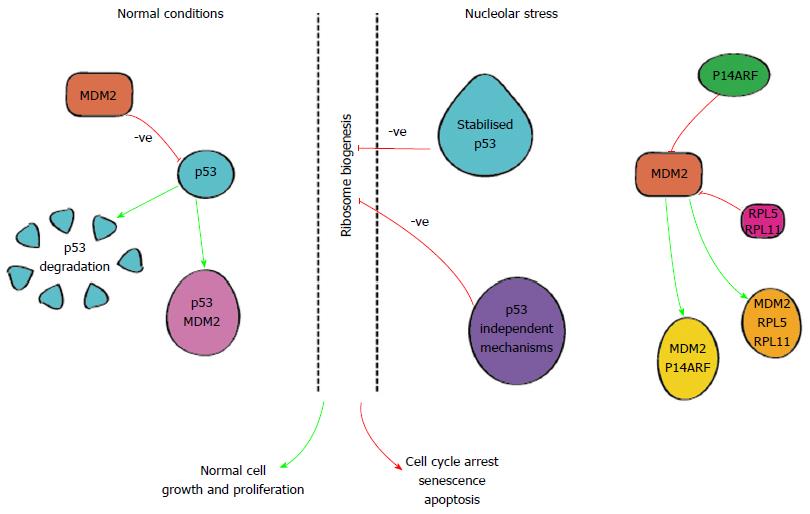
In normal cells, levels of p53 protein are typically low[69], kept in check by various mechanisms including MDM2, an E3 ubiquitin ligase[62], as shown in Figure 3, allowing cell division and proliferation.
Disruptions in ribosome synthesis lead to nucleolar stress which in turn results in nucleolar disruption and the release of RP’s i.e., RPL5 and RPL1. These RPs are able to bind to MDM2 and interrupt its interaction with p53[60,70]. Similarly, the product of the CDKN2A gene, p14ARF, is also able to exert effects on MDM2[60]. Ultimately, these pathways lead to stabilisation of p53 allowing it to act on various transcriptional targets and induce apoptosis, senescence and/or cell cycle arrest depicted in Figure 3. Importantly one of the transcriptional targets for p53 is the MDM2 gene setting up an autologous feedback loop to maintain genomic and cellular homeostasis[62,71,72] keeping the process of cell death and proliferation in check.
When the above mentioned homeostatic mechanisms are impaired however, ribosome biogenesis becomes dysregulated and the balance between cell growth and arrest is compromised leading to unchecked cellular proliferation and malignant transformation. Activation of the nucleolar stress pathway may therefore provide a therapeutic strategy against aggressive malignancies.
While p53 dependent nucleolar stress is well understood, more recent research by Quin et al[73] has identified p53 independent mechanisms involving activation of ATM/ATR signalling which occurs without the induction of global DNA damage[73]. Evidently, ribosome biogenesis is a highly complex and finely balanced process involving multiple key effectors. Disruption of any of these components can mediate the nucleolar stress pathway and have detrimental effects on cell growth and proliferation. It is this feature along with the potential for reduced global DNA damage that makes targeting dysregulated ribosome biogenesis an attractive therapeutic option.
Many regulators of ribosome biogenesis are also involved in malignant diseases including PDAC. For example, activation of RAS or PI3K pathways known to be involved in PDAC are critical for coordinating protein synthesising capacity (ribosome number) required for maintaining cellular growth and proliferation. Other genes and their products, such as c-myc and p53 also have significant roles in ribosome biogenesis and malignancy when overexpressed or mutated. Attempts at targeting these various genes and pathways have been disappointing in the realm of PDAC but tackling dysregulated ribosome biogenesis have shown more promise.
Pivotal studies conducted by Bywater et al[12], provided proof of principal for the benefits of targeting dysregulated ribosome biogenesis as demonstrated by CX5461, a novel small molecule Pol I inhibitor, investigated in the Eµ-Myc mouse model of Burkitt’s lymphoma. In this model, cells are highly proliferative and have elevated RNA levels, including rRNA, which correlates with accelerated Pol I transcription and cell growth. Pharmacological inhibition of Pol I transcription with CX5461 induced nucleolar stress leading to an increase in p53 levels resulting in apoptotic cell death of malignant cells. Most compelling however, CX5461 did not kill non-malignant B cells representing a valuable therapeutic option in the management of susceptible malignancies[12].
In addition to the Bywater study, Devlin et al[13] demonstrated that combination therapy with CX5461 and AKT-mTOR inhibitors worked synergistically leading to significantly extend survival in lymphoma bearing mice[61]. Importantly, the acute lymphoblastic leukaemia cells were more sensitive to rRNA synthesis inhibition compared with normal bone marrow cells[74] further emphasising the potential reduction in global toxicity underlying Pol I inhibition. Similarly, reduced global toxicity was noted by Hannan et al[75] review of lymphoma models in response to Pol I inhibition.
To date, the majority of studies of Pol I inhibitors have focussed on haematological malignancies, and as such CX-5461 is the subject of a phase I clinical trial at the Peter MacCallum Cancer Centre for treatment of patients with advanced haematological malignancies (Australian New Zealand Clinical Trials Registry 12613001061729). However, such studies are now being extended to various solid organ malignancies including metastatic/recurrent/locally advanced/unresectable triple negative breast cancer which is currently recruiting through the Canadian Cancer Trials group (NCT02719977). This is a logical extension as there is preliminary data for efficacy of CX-5461 in solid tumours, with pre-clinical data suggesting a role in prostate cancer[14]. Similarly, studies in PDAC cell lines have also shown promise[76], as documented by Drygin et al[76] using MIA PaCa-2 cell lines. This study demonstrated a process of both autophagy and cell senescence in response to Pol I inhibition. In xenograft mouse models of human MIA PaCa-2 cells, treatment with CX5461 demonstrated significant tumour growth inhibition deemed at least comparable to xenograft models treated with gemcitabine and again shows promise.
Given the promising result for Pol I inhibition in the treatment of haematological malignancies and emerging evidence of efficacy in various solid organ cancers the potential for targeting dysregulated ribosome biogenesis and manipulating the nucleolar stress pathway is tantalising, particularly for advanced PDAC given the slow progress in its treatment options so far. Whether this will be in the setting of combinations with already established chemotherapeutics or in more novel ways remains to be seen but the results from other malignancies are encouraging. Of particular note is the potential for less toxicity to healthy cells which thus far has been a major limitation in improving treatment outcomes for PDAC.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Alemar B S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. 2016. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. |
| 2. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8789] [Cited by in RCA: 9563] [Article Influence: 869.4] [Reference Citation Analysis (0)] |
| 3. | American Cancer Society. 2016. Available from: http://www.cancer.org/cancer/pancreaticcancer/detailedguide/pancreatic-cancer-key-statistics. |
| 4. | Lockhart AC, Rothenberg ML, Berlin JD. Treatment for pancreatic cancer: current therapy and continued progress. Gastroenterology. 2005;128:1642-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5609] [Article Influence: 400.6] [Reference Citation Analysis (1)] |
| 6. | Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Boige V. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 338] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 7. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 4857] [Article Influence: 404.8] [Reference Citation Analysis (0)] |
| 8. | Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, Seufferlein T, Haustermans K, Van Laethem JL, Conroy T. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v56-v68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 905] [Cited by in RCA: 925] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 9. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Kalra AV, Kim J, Klinz SG, Paz N, Cain J, Drummond DC, Nielsen UB, Fitzgerald JB. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014;74:7003-7013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 11. | Roy AC, Park SR, Cunningham D, Kang YK, Chao Y, Chen LT, Rees C, Lim HY, Tabernero J, Ramos FJ. A randomized phase II study of PEP02 (MM-398), irinotecan or docetaxel as a second-line therapy in patients with locally advanced or metastatic gastric or gastro-oesophageal junction adenocarcinoma. Ann Oncol. 2013;24:1567-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Bywater MJ, Poortinga G, Sanij E, Hein N, Peck A, Cullinane C, Wall M, Cluse L, Drygin D, Anderes K. Inhibition of RNA polymerase I as a therapeutic strategy to promote cancer-specific activation of p53. Cancer Cell. 2012;22:51-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 13. | Devlin JR, Hannan KM, Hein N, Cullinane C, Kusnadi E, Ng PY, George AJ, Shortt J, Bywater MJ, Poortinga G. Combination Therapy Targeting Ribosome Biogenesis and mRNA Translation Synergistically Extends Survival in MYC-Driven Lymphoma. Cancer Discov. 2016;6:59-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Rebello RJ, Kusnadi E, Cameron DP, Pearson HB, Lesmana A, Devlin JR, Drygin D, Clark AK, Porter L, Pedersen J. The Dual Inhibition of RNA Pol I Transcription and PIM Kinase as a New Therapeutic Approach to Treat Advanced Prostate Cancer. Clin Cancer Res. 2016;22:5539-5552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Institute NC. Surveillance, Epidemiology and End Results Program. SEER Stat Fact Sheets: Pnacreas Cancer. cited 2016-10-24. Available from: https://seer.cancer.gov/. |
| 16. | Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, Real FX, Van Laethem JL, Heinemann V. Addressing the challenges of pancreatic cancer: future directions for improving outcomes. Pancreatology. 2015;15:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 368] [Article Influence: 36.8] [Reference Citation Analysis (2)] |
| 17. | Gerritsen A, Jacobs M, Henselmans I, van Hattum J, Efficace F, Creemers GJ, de Hingh IH, Koopman M, Molenaar IQ, Wilmink HW. Developing a core set of patient-reported outcomes in pancreatic cancer: A Delphi survey. Eur J Cancer. 2016;57:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Zhen DB, Rabe KG, Gallinger S, Syngal S, Schwartz AG, Goggins MG, Hruban RH, Cote ML, McWilliams RR, Roberts NJ. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: a PACGENE study. Genet Med. 2015;17:569-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 221] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 19. | Humphris JL, Johns AL, Simpson SH, Cowley MJ, Pajic M, Chang DK, Nagrial AM, Chin VT, Chantrill LA, Pinese M. Clinical and pathologic features of familial pancreatic cancer. Cancer. 2014;120:3669-3675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 217] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 21. | Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, Johns AL, Miller D, Nones K, Quek K. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2130] [Cited by in RCA: 1981] [Article Influence: 198.1] [Reference Citation Analysis (1)] |
| 22. | Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, Miller DK, Christ AN, Bruxner TJ, Quinn MC. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2848] [Cited by in RCA: 2531] [Article Influence: 281.2] [Reference Citation Analysis (0)] |
| 23. | Cid-Arregui A, Juarez V. Perspectives in the treatment of pancreatic adenocarcinoma. World J Gastroenterol. 2015;21:9297-9316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 24. | Chiche J, Brahimi-Horn MC, Pouysségur J. Tumour hypoxia induces a metabolic shift causing acidosis: a common feature in cancer. J Cell Mol Med. 2010;14:771-794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 486] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 25. | Tutt A, Gabriel A, Bertwistle D, Connor F, Paterson H, Peacock J, Ross G, Ashworth A. Absence of Brca2 causes genome instability by chromosome breakage and loss associated with centrosome amplification. Curr Biol. 1999;9:1107-1110. [PubMed] |
| 26. | Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 645] [Cited by in RCA: 598] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 27. | Chan D, Clarke S, Gill AJ, Chantrill L, Samra J, Li BT, Barnes T, Nahar K, Pavlakis N. Pathogenic PALB2 mutation in metastatic pancreatic adenocarcinoma and neuroendocrine tumour: A case report. Mol Clin Oncol. 2015;3:817-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, De Jesus-Acosta A, Jones S, Maitra A, Hruban RH, Eshleman JR, Klein A, Laheru D. Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer. Mol Cancer Ther. 2011;10:3-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 29. | Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, Tebbutt NC. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 30. | Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 31. | Javle MM, Shroff RT, Xiong H, Varadhachary GA, Fogelman D, Reddy SA, Davis D, Zhang Y, Wolff RA, Abbruzzese JL. Inhibition of the mammalian target of rapamycin (mTOR) in advanced pancreatic cancer: results of two phase II studies. BMC Cancer. 2010;10:368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 32. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5290] [Article Influence: 352.7] [Reference Citation Analysis (3)] |
| 33. | Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177-182. [PubMed] |
| 34. | Kelley RK, Ko AH. Erlotinib in the treatment of advanced pancreatic cancer. Biologics. 2008;2:83-95. [PubMed] |
| 35. | Harder J, Ihorst G, Heinemann V, Hofheinz R, Moehler M, Buechler P, Kloeppel G, Röcken C, Bitzer M, Boeck S. Multicentre phase II trial of trastuzumab and capecitabine in patients with HER2 overexpressing metastatic pancreatic cancer. Br J Cancer. 2012;106:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 36. | Kimura K, Sawada T, Komatsu M, Inoue M, Muguruma K, Nishihara T, Yamashita Y, Yamada N, Ohira M, Hirakawa K. Antitumor effect of trastuzumab for pancreatic cancer with high HER-2 expression and enhancement of effect by combined therapy with gemcitabine. Clin Cancer Res. 2006;12:4925-4932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 37. | Yu JH, Kim H. Role of janus kinase/signal transducers and activators of transcription in the pathogenesis of pancreatitis and pancreatic cancer. Gut Liver. 2012;6:417-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Yu JH, Kim H. Oxidative stress and cytokines in the pathogenesis of pancreatic cancer. J Cancer Prev. 2014;19:97-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Hurwitz HI, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM, Nemunaitis JJ, Stella PJ, Pipas JM, Wainberg ZA. Randomized, Double-Blind, Phase II Study of Ruxolitinib or Placebo in Combination With Capecitabine in Patients With Metastatic Pancreatic Cancer for Whom Therapy With Gemcitabine Has Failed. J Clin Oncol. 2015;33:4039-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 40. | Wire B. Incyte Announces Decision to Discontinue JANUS Studies of Ruxolitinib plus Capecitabine in Patients with Advanced or Metastatic Pancreatic Cancer. Internet Press Release, 2016. Available from: http://www.businesswire.com/news/home/20160211005321/en/Incyte-Announces-Decision-Discontinue-JANUS-Studies-Ruxolitinib. |
| 41. | Chang DK, Grimmond SM, Evans TR, Biankin AV. Mining the genomes of exceptional responders. Nat Rev Cancer. 2014;14:291-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Neesse A, Algül H, Tuveson DA, Gress TM. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64:1476-1484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 417] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 43. | Matsuoka T, Yashiro M. Molecular targets for the treatment of pancreatic cancer: Clinical and experimental studies. World J Gastroenterol. 2016;22:776-789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (2)] |
| 44. | Nagaraju GP, Dontula R, El-Rayes BF, Lakka SS. Molecular mechanisms underlying the divergent roles of SPARC in human carcinogenesis. Carcinogenesis. 2014;35:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 45. | Lane TF, Sage EH. Functional mapping of SPARC: peptides from two distinct Ca+(+)-binding sites modulate cell shape. J Cell Biol. 1990;111:3065-3076. [PubMed] |
| 46. | Rahman M, Chan AP, Tang M, Tai IT. A peptide of SPARC interferes with the interaction between caspase8 and Bcl2 to resensitize chemoresistant tumors and enhance their regression in vivo. PLoS One. 2011;6:e26390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Funk SE, Sage EH. The Ca2(+)-binding glycoprotein SPARC modulates cell cycle progression in bovine aortic endothelial cells. Proc Natl Acad Sci USA. 1991;88:2648-2652. [PubMed] |
| 48. | Chlenski A, Liu S, Baker LJ, Yang Q, Tian Y, Salwen HR, Cohn SL. Neuroblastoma angiogenesis is inhibited with a folded synthetic molecule corresponding to the epidermal growth factor-like module of the follistatin domain of SPARC. Cancer Res. 2004;64:7420-7425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 49. | Gao J, Song J, Huang H, Li Z, Du Y, Cao J, Li M, Lv S, Lin H, Gong Y. Methylation of the SPARC gene promoter and its clinical implication in pancreatic cancer. J Exp Clin Cancer Res. 2010;29:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Toshimitsu H, Iizuka N, Yamamoto K, Kawauchi S, Oga A, Furuya T, Oka M, Sasaki K. Molecular features linked to the growth-inhibitory effects of gemcitabine on human pancreatic cancer cells. Oncol Rep. 2006;16:1285-1291. [PubMed] |
| 51. | Zhang J, Jiang H, Mao Z, Wang X, Fan X, Liu Y, Wang Y. [Mechanism of SPARC-enhanced chemosensitivity of pancreatic cancer cells to gemcitabine]. Zhonghua Zhongliu Zazhi. 2014;36:335-340. [PubMed] |
| 52. | Motamed K. SPARC (osteonectin/BM-40). Int J Biochem Cell Biol. 1999;31:1363-1366. [PubMed] |
| 53. | Vaz J, Ansari D, Sasor A, Andersson R. SPARC: A Potential Prognostic and Therapeutic Target in Pancreatic Cancer. Pancreas. 2015;44:1024-1035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 54. | Hidalgo M, Plaza C, Musteanu M, Illei P, Brachmann CB, Heise C, Pierce D, Lopez-Casas PP, Menendez C, Tabernero J. SPARC Expression Did Not Predict Efficacy of nab-Paclitaxel plus Gemcitabine or Gemcitabine Alone for Metastatic Pancreatic Cancer in an Exploratory Analysis of the Phase III MPACT Trial. Clin Cancer Res. 2015;21:4811-4818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 55. | Goel G, Sun W. Novel approaches in the management of pancreatic ductal adenocarcinoma: potential promises for the future. J Hematol Oncol. 2015;8:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130-3146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 517] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 57. | Iacobuzio-Donahue CA, Fu B, Yachida S, Luo M, Abe H, Henderson CM, Vilardell F, Wang Z, Keller JW, Banerjee P. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 852] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 58. | Tian M, Schiemann WP. The TGF-beta paradox in human cancer: an update. Future Oncol. 2009;5:259-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 59. | Pianese G. Beitrag zur Histologie und Aetiologie der Carcinoma. Histologische und experimentelle Untersuchungen. Beitr Pathol Anat Allgem Pathol. 1896;142:1-193. |
| 60. | Quin JE, Devlin JR, Cameron D, Hannan KM, Pearson RB, Hannan RD. Targeting the nucleolus for cancer intervention. Biochim Biophys Acta. 2014;1842:802-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 61. | Mayer C, Grummt I. Ribosome biogenesis and cell growth: mTOR coordinates transcription by all three classes of nuclear RNA polymerases. Oncogene. 2006;25:6384-6391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 419] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 62. | Brighenti E, Treré D, Derenzini M. Targeted cancer therapy with ribosome biogenesis inhibitors: a real possibility? Oncotarget. 2015;6:38617-38627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 63. | Pelava A, Schneider C, Watkins NJ. The importance of ribosome production, and the 5S RNP-MDM2 pathway, in health and disease. Biochem Soc Trans. 2016;44:1086-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 64. | Dang CV. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb Perspect Med. 2013;3:pii: a014217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 463] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 65. | Orsolic I, Jurada D, Pullen N, Oren M, Eliopoulos AG, Volarevic S. The relationship between the nucleolus and cancer: Current evidence and emerging paradigms. Semin Cancer Biol. 2016;37-38:36-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 66. | Dai MS, Zeng SX, Jin Y, Sun XX, David L, Lu H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol Cell Biol. 2004;24:7654-7668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 394] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 67. | Hsieh AL, Walton ZE, Altman BJ, Stine ZE, Dang CV. MYC and metabolism on the path to cancer. Semin Cell Dev Biol. 2015;43:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 68. | Pestov DG, Strezoska Z, Lau LF. Evidence of p53-dependent cross-talk between ribosome biogenesis and the cell cycle: effects of nucleolar protein Bop1 on G(1)/S transition. Mol Cell Biol. 2001;21:4246-4255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 284] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 69. | Golomb L, Volarevic S, Oren M. p53 and ribosome biogenesis stress: the essentials. FEBS Lett. 2014;588:2571-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 70. | James A, Wang Y, Raje H, Rosby R, DiMario P. Nucleolar stress with and without p53. Nucleus. 2014;5:402-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 207] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 71. | Toledo F, Wahl GM. Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer. 2006;6:909-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 973] [Cited by in RCA: 991] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 72. | Liu Y, Deisenroth C, Zhang Y. RP-MDM2-p53 Pathway: Linking ribosomal biogenesis and tumor surveillance. Trends Cancer. 2016;2:191-204. |
| 73. | Quin J, Chan KT, Devlin JR, Cameron DP, Diesch J, Cullinane C, Ahern J, Khot A, Hein N, George AJ. Inhibition of RNA polymerase I transcription initiation by CX-5461 activates non-canonical ATM/ATR signaling. Oncotarget. 2016;7:49800-49818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 84] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 74. | Negi SS, Brown P. rRNA synthesis inhibitor, CX-5461, activates ATM/ATR pathway in acute lymphoblastic leukemia, arrests cells in G2 phase and induces apoptosis. Oncotarget. 2015;6:18094-18104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 75. | Hannan KM, Sanij E, Rothblum LI, Hannan RD, Pearson RB. Dysregulation of RNA polymerase I transcription during disease. Biochim Biophys Acta. 2013;1829:342-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 76. | Drygin D, Lin A, Bliesath J, Ho CB, O’Brien SE, Proffitt C, Omori M, Haddach M, Schwaebe MK, Siddiqui-Jain A. Targeting RNA polymerase I with an oral small molecule CX-5461 inhibits ribosomal RNA synthesis and solid tumor growth. Cancer Res. 2011;71:1418-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 454] [Article Influence: 30.3] [Reference Citation Analysis (0)] |