Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.2086
Peer-review started: August 28, 2016
First decision: September 21, 2016
Revised: October 13, 2016
Accepted: October 31, 2016
Article in press: October 31, 2016
Published online: March 21, 2017
Processing time: 204 Days and 21.5 Hours
Massive global spread of multidrug-resistant (MDR) Salmonella spp. expressing extended-spectrum beta-lactamase (ESBL) and additional resistance to fluoroquinolones has often been attributed to high international mobility as well as excessive use of oral antibiotics in livestock farming. However, MDR Salmonella spp. have not been mentioned as a widespread pathogen in clinical settings so far. We demonstrate the case of a 25-year-old male with primary sclerosing cholangitis who tested positive for MDR Salmonella enterica serotype Choleraesuis expressing ESBL and fluoroquinolone resistance. The pathogen was supposedly acquired during a trip to Thailand, causing severe fever, cholangitis and pancreatitis. To our knowledge, this is the first report of Salmonella enterica serotype Choleraesuis in Europe expressing such a multidrug resistance pattern. ESBL resistance of Salmonella enterica spp. should be considered in patients with obstructive biliary tract pathology and travel history in endemic countries.
Core tip: We report a case of aggressive infection with a multidrug resistant strain of Salmonella choleraesuis in a patient with primary sclerosing cholangitis. Successful treatment involved repetitive ultrasound and endoscopic intervention, as well as multiple adjustments of the antibiotic regimen. This is the first case report addressing multidrug-resistant salmonellosis in patients with predisposing biliary disease in Europe. It illustrates how close interdisciplinary cooperation between clinicians and microbiologists is warranted in an era of emerging antibiotic resistance.
- Citation: Ferstl PG, Reinheimer C, Jozsa K, Zeuzem S, Kempf VA, Waidmann O, Grammatikos G. Severe infection with multidrug-resistant Salmonella choleraesuis in a young patient with primary sclerosing cholangitis. World J Gastroenterol 2017; 23(11): 2086-2089
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/2086.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.2086
Among a variety of pathogens that are known for colonizing the gallbladder, Salmonella spp. seem to benefit from its prevailing conditions in particular[1]. Bile has bactericidal properties, which Salmonella spp. manage to escape by several mechanisms[2]. Thus, the biliary system serves as a favorable reservoir for Salmonella spp. and they might bloom particularly well if the passage of bile into the intestine is impaired. In case of inflammation, the constitution of bile alters, thus supporting infection of the biliary tree with pathogenic Gram-negative organisms, e.g., Salmonella spp.[3]. Up to now, this phenomenon has mostly been attributed to gallstones[4], which have been known to be a common cause of infectious cholangitis. However, only few case reports of patients suffering from primary hepatobiliary diseases such as Caroli’s syndrome and shedding Salmonella are available up to now[5]. In case of severe cholangitis and acute pancreatitis in patients with a predisposition to cholestasis, Salmonella spp. should be considered as a causative pathogen. Since cases of infectious pancreatitis due to Salmonella spp, .e.g., Salmonella enterica serotype Typhimurium, have been reported earlier[6], we demonstrate here an infection with Salmonella enterica serotype Choleraesuis. Immunocompromising diseases, e.g., cirrhosis or inflammatory bowel disease, might promote invasive salmonellosis and bloodstream infection and lead to severe courses of infections[7].
A 25-year-old male student presented to the emergency department of the University Hospital Frankfurt with watery diarrhea at a frequency of ten stools per day, concomitant cramps in the lower abdomen, and fever up to 40 °C for six days. He had been diagnosed with primary sclerosing cholangitis and ulcerative colitis in 2005 and was on long-term medication with mesalazine and ursodesoxycholic acid. He had returned from a backpacking holiday to Thailand 16 d ago, which ended without any medical complaints or symptoms.
On admission, blood tests showed serum levels of bilirubin at 1.7 mg/dL, alkaline phosphatase (AP) at 276 U/L, alanine aminotransferase (ALT) at 56 U/L, lipase at 501 U/L, C-reactive protein (CRP) at 9.2 mg/dL, and 15000 leucocytes per milliliter of blood. Abdominal ultrasound showed an enlarged gallbladder, 11 cm in diameter, without any signs of cholecystitis or acute pancreatitis. Blood and stool cultures were taken and intravenous antibiotic therapy with ciprofloxacin 500 mg and metronidazole 400 mg three times daily was initiated. Stool cultures yielded detection of non-typhoidal Salmonella spp., hence antibiotic therapy was switched to ceftriaxone 2 g daily. Despite the adaptation of the antibiotic regimen, the patient’s general condition worsened and he reported increasing abdominal pain located in the epigastrium. Fever continued and diarrhea suspended.
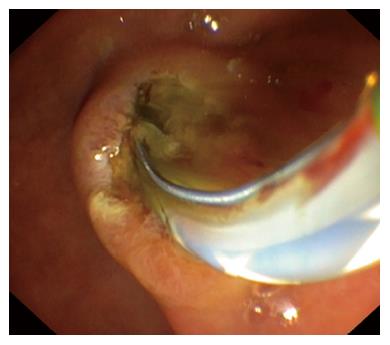
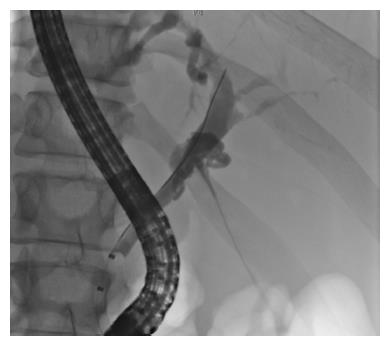
Repeated ultrasound of the abdomen displayed a mildly bloated pancreas with peripancreatic edema. Liver function tests showed rising bilirubin, ALT, AP, leucocytes, and CRP kept increasing. Antibiotic therapy was switched to imipenem 500 mg four times daily. Subsequently, an isolate of Salmonella group C with extended-spectrum beta-lactamase (ESBL) and additional resistance to fluoroquinolones was detected in stool cultures. Due to ongoing elevation of cholestatic enzymes and ultrasound evidence of a distended common bile duct (CBD), endoscopic retrograde cholangiopancreatography was performed, showing a high-grade stricture of the distal CBD (Figures 1 and 2). A 3 mm small stone and pus were extracted, and a stent was applied to the CBD. Fever ceased immediately and pain was easing during the following week. Gall cultures yielded detection of the same bacterial strain as was detected in the stool. Culture isolates were sent to the national reference center for salmonellosis in Wernigerode, Germany, in order to determine the exact species serotype. Imipenem was administered for 14 d and the patient was discharged with mild residual abdominal complaints. The following week, the reference center confirmed detection of Salmonella enterica serotype Choleraesuis [6,7,(c)1,5]. On readmission for planned stent extraction, the patient was free of symptoms and any Salmonella spp. in stool cultures. Thoracoabdominal magnetic resonance imaging showed no signs of mycotic aortic aneurism, which is frequently observed in Salmonella groups C and D infections[7].
Patient data and dates of treatment were anonymized prior to writing the case report. The patient provided informed written consent prior to report submission.
The rising global burden of multidrug-resistant (MDR) non-typhoidal Salmonella spp. has been attributed to increasing tourism to South-East Asia, where MDR non-typhoidal Salmonella spp. are endemic[8-10]. The prevalence of MDR non-typhoidal Salmonella spp. has been increasing worldwide for several years[7]. The global spread of these pathogens has been linked to use of antibiotics in livestock farming[11], consumption of raw or insufficiently cooked meat and vegetables, global food trade as well as travel to endemic areas[12,13]. The first case of serotype Choleraesuis resistant to both third-generation cephalosporins and fluoroquinolones has been reported in 2004[14]. Therefore, MDR Salmonella spp. are an issue of growing public health concern in Europe[11,13]. While antibiotic treatment is not recommended in asymptomatic shedders of Salmonella spp. or in uncomplicated gastroenteritis[15,16], MDR Salmonella is likely to have a critical impact in patients with obstructive biliary tract pathology and altered bile constitution. Since global burden of MDR Salmonella spp. keeps rising, this alarming development is reflected by our case report on travel-associated salmonellosis with serotype Choleraesuis expressing ESBL and additional resistance to fluoroquinolones.
We conclude that salmonellosis due to MDR Salmonella spp. should be considered in patients with immunosuppression or with hepatobiliary diseases, who can develop severe and complicated courses. Empiric treatment with carbapenems should be initiated in these patients upon clinical deterioration on common antibiotic regimens like fluoroquinolones and cephalosporins. Carbapenems cover MDR Salmonella spp., achieve higher concentrations within the pancreatic tissue, and thus reduce bacterial count[17]. Antibiotic treatment should be reserved for symptomatic patients. From the first day of treatment on, structured microbiological surveillance and close interdisciplinary cooperation between clinicians and microbiologists are warranted for best patient care.
A 25-year-old male with known primary sclerosing cholangitis and ulcerative colitis presented to our emergency ward with watery diarrhea at a frequency of ten stools per day, concomitant cramps in the lower abdomen, and fever up to 40 °C.
The authors diagnosed a case of severe salmonellosis due to an isolate of Salmonella choleraesuis expressing extended-spectrum beta-lactamase (ESBL) and fluoroquinolone resistance, which could be detected in both bile and stool cultures.
Initial differential diagnoses were infectious gastroenteritis, an atypical acute attack of ulcerative colitis, and obstructive cholangitis with febrile cholecystitis and pancreatitis.
Blood tests showed serum levels of bilirubin at 1.7 mg/dL, alkaline phosphatase at 276 U/L, alanine aminotransferase at 56 U/L, lipase at 501 U/L, c-reactive protein at 9.2 mg/dL, and 15000 leucocytes/mL.
Ultrasound of the abdomen displayed a distended gallbladder, a mildly bloated pancreas with peripancreatic edema, and a distended common bile duct (CBD), while endoscopic retrograde cholangiopancreatography showed a high-grade stricture of the distal CBD with discharge of a small stone and pus.
Upon unsuccessful antibiotic treatment with ciprofloxacin/metronidazole and later with ceftriaxone, the patient’s condition and laboratory values improved rapidly under therapy with imipenem, which was administered for 14 d in total.
ESBL resistance of Salmonella enterica spp. should be considered in patients with obstructive biliary tract pathology and travel history in endemic countries.
From an interdisciplinary perspective, this case report illustrates the features of multidrug-resistant non-typhoidal salmonellosis in a patient with primary sclerosing cholangitis, explains diagnostic pathways, and summarizes treatment recommendations for these patients.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Islek A, Sousa TCM, Upala S S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Zhang FF
| 1. | Verdier J, Luedde T, Sellge G. Biliary Mucosal Barrier and Microbiome. Viszeralmedizin. 2015;31:156-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Merritt ME, Donaldson JR. Effect of bile salts on the DNA and membrane integrity of enteric bacteria. J Med Microbiol. 2009;58:1533-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 3. | Vaishnavi C, Singh S, Kochhar R, Bhasin D, Singh G, Singh K. Prevalence of Salmonella enterica serovar typhi in bile and stool of patients with biliary diseases and those requiring biliary drainage for other purposes. Jpn J Infect Dis. 2005;58:363-365. [PubMed] |
| 4. | Crawford RW, Rosales-Reyes R, Ramírez-Aguilar Mde L, Chapa-Azuela O, Alpuche-Aranda C, Gunn JS. Gallstones play a significant role in Salmonella spp. gallbladder colonization and carriage. Proc Natl Acad Sci USA. 2010;107:4353-4358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 214] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 5. | Waldram R, Vahrman J, Williams R. Salmonella heidelberg infection in Caroli’s syndrome. Gastroenterology. 1975;68:151-153. [PubMed] |
| 6. | Rombolà F, Bertuccio SN. [Typhoid fever and acute pancreatitis: two cases]. Infez Med. 2007;15:63-65. [PubMed] |
| 7. | Chiu CH, Su LH, Chu C. Salmonella enterica serotype Choleraesuis: epidemiology, pathogenesis, clinical disease, and treatment. Clin Microbiol Rev. 2004;17:311-322. [PubMed] |
| 8. | Suwantarat N, Carroll KC. Epidemiology and molecular characterization of multidrug-resistant Gram-negative bacteria in Southeast Asia. Antimicrob Resist Infect Control. 2016;5:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 9. | Vlieghe ER, Phe T, De Smet B, Veng CH, Kham C, Bertrand S, Vanhoof R, Lynen L, Peetermans WE, Jacobs JA. Azithromycin and ciprofloxacin resistance in Salmonella bloodstream infections in Cambodian adults. PLoS Negl Trop Dis. 2012;6:e1933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Su LH, Teng WS, Chen CL, Lee HY, Li HC, Wu TL, Chiu CH. Increasing ceftriaxone resistance in Salmonellae, Taiwan. Emerg Infect Dis. 2011;17:1086-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Liebana E, Carattoli A, Coque TM, Hasman H, Magiorakos AP, Mevius D, Peixe L, Poirel L, Schuepbach-Regula G, Torneke K. Public health risks of enterobacterial isolates producing extended-spectrum β-lactamases or AmpC β-lactamases in food and food-producing animals: an EU perspective of epidemiology, analytical methods, risk factors, and control options. Clin Infect Dis. 2013;56:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 12. | Bae D, Cheng CM, Khan AA. Characterization of extended-spectrum β-lactamase (ESBL) producing non-typhoidal Salmonella (NTS) from imported food products. Int J Food Microbiol. 2015;214:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Burke L, Hopkins KL, Meunier D, de Pinna E, Fitzgerald-Hughes D, Humphreys H, Woodford N. Resistance to third-generation cephalosporins in human non-typhoidal Salmonella enterica isolates from England and Wales, 2010-12. J Antimicrob Chemother. 2014;69:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Chiu CH, Su LH, Chu C, Chia JH, Wu TL, Lin TY, Lee YS, Ou JT. Isolation of Salmonella enterica serotype choleraesuis resistant to ceftriaxone and ciprofloxacin. Lancet. 2004;363:1285-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Marzel A, Desai PT, Goren A, Schorr YI, Nissan I, Porwollik S, Valinsky L, McClelland M, Rahav G, Gal-Mor O. Persistent Infections by Nontyphoidal Salmonella in Humans: Epidemiology and Genetics. Clin Infect Dis. 2016;62:879-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 16. | Onwuezobe IA, Oshun PO, Odigwe CC. Antimicrobials for treating symptomatic non-typhoidal Salmonella infection. Cochrane Database Syst Rev. 2012;11:CD001167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 17. | Lankisch PG, Lerch MM. The role of antibiotic prophylaxis in the treatment of acute pancreatitis. J Clin Gastroenterol. 2006;40:149-155. [PubMed] |