Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.2077
Peer-review started: October 18, 2016
First decision: December 2, 2016
Revised: December 26, 2016
Accepted: January 17, 2017
Article in press: January 17, 2017
Published online: March 21, 2017
Processing time: 157 Days and 21.6 Hours
To analyze cytokine levels and to identify their association with outcome in patients with hepatocellular carcinoma (HCC) treated with radiotherapy (RT).
Patients with HCC who were treated with RT were eligible for this prospective study. Blood samples were collected before and after RT, and serum cytokine levels including interleukin (IL)-1, IL-6, IL-8, IL-10, IL-12, and tumor necrosis factor-α were analyzed.
Between 2008 and 2009, 51 patients were enrolled in this study. Baseline IL-6 level was high in patients with a history of pre-RT treatment. Median survival was 13.9 mo with alpha-fetoprotein (AFP) as a significant factor (P = 0.020). Median failure-free survival (FFS) for infield, outfield-intrahepatic and extrahepatic failures were 23.3, 11.5 and 12.0 mo, respectively. Sex and baseline IL-6 level were associated with infield FFS, and baseline IL-10 level was correlated with outfield-intrahepatic FFS. For extrahepatic FFS, AFP was significant (P = 0.034). Patients with a baseline IL-6 level of ≥ 9.7 pg/mL showed worse infield FFS (P = 0.005), and this significance was observed only in treatment-non-naïve patients (P = 0.022).
In addition to AFP, cytokines seem useful in predicting infield and outfield-intrahepatic failure. Serum cytokines could be useful biomarkers for predicting RT outcome in HCC.
Core tip: A prospective study to identify associations between serum cytokine levels and radiotherapy (RT) outcomes was performed in 51 patients with hepatocellular carcinoma. Baseline serum interleukin (IL)-6 levels were higher in patients with treatment failure than in those without treatment failure. This significant difference was observed only in treatment-non-naïve patients. To predict RT outcomes, analysis of baseline serum IL-6 levels may be helpful.
- Citation: Cha H, Lee EJ, Seong J. Multi-analyte analysis of cytokines that predict outcomes in patients with hepatocellular carcinoma treated with radiotherapy. World J Gastroenterol 2017; 23(11): 2077-2085
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/2077.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.2077
The liver is an organ that is enriched with immune cells. Chronic inflammation caused by infection with hepatitis B or C virus or by steatohepatitis is a risk factor for the development of hepatocellular carcinoma (HCC). Continued cytokine-induced hepatocyte damage followed by hepatocyte regeneration leads to HCC development. The involvement of interleukin (IL)-1, IL-2, IL-6, IL-10, IL-12, tumor necrosis factor (TNF)-α, and transforming growth factor (TGF)-β in hepatocarcinogenesis has been reported. As levels of these cytokines are more increased in patients with HCC than in healthy individuals or patients with cirrhosis, they have been studied as biomarkers for the detection of HCC[1-5].
It has been reported that cytokine levels are associated with the radiation response of the tumor. In general, elevated pro-inflammatory cytokine levels before radiotherapy (RT) correlated with treatment resistance and poor treatment outcome in several cancers. Radiation-induced pro-inflammatory cytokine pathways within tumor cells may help the cells survive RT[6]. Recently, the application of RT in the treatment of HCC has increased[7]. However, there have been few studies on the role of cytokines in the RT response in patients with HCC.
Several biomarkers in cancer detection or treatment have been studied. As mentioned, cytokines play a role in cancer development and treatment response. Cytokine levels in serum or plasma are easily accessible and may be useful as biomarkers in screening for cancer or predicting outcomes[8]. The aim of this study was to analyze serum cytokine levels and to identify the significance of serum cytokines in treatment outcomes for patients with HCC treated with RT.
This study was conducted prospectively and conformed to the ethical guidelines of the Declaration of Helsinki. It was approved by the Institutional Review Board at Yonsei Severance Hospital (IRB number: 4-2008-2012). All patients were enrolled after providing written informed consent. The number of patients was calculated using a one-sample t-test. Based on an expected dropout rate of 10%, we planned to enroll 109 patients. However, the study was closed early due to poor accrual. Ultimately, 51 patients were included in this study between September 2008 and October 2009.
RT was performed using 3D conformal RT or intensity-modulated RT. Blood samples were collected from patients before the start and at completion of the RT schedule. We selected serum cytokines, including IL-1, IL-6, IL-8, IL-10, IL-12 and TNF-α, for analysis that were known to be associated with HCC. Serum cytokine levels were measured using Cytokine Bead Array kits according to the manufacturer’s instructions (BD Biosciences, San Jose, CA, United States) using a fluorescence-activated cell sorting (FACS) Verse flow cytometer (Becton Dickinson, Franklin Lakes, NJ, United States). The baseline level was defined as the value before RT.
We classified patterns of failure into three categories: infield failure, outfield-intrahepatic failure, and extrahepatic failure. Infield failure was defined as progression of the tumor within the RT field that covered the planning target volume. Intrahepatic failure was defined as progression of the tumor within the liver but outside of the RT field. Extrahepatic failure was a distant metastasis in lung, bone, etc. Overall survival (OS) and failure-free survival (FFS) were calculated from the first date of RT to the date of death and disease progression, respectively.
The statistical significance of differences in mean values according to patient and tumor characteristics was determined using Student’s t-test or a one-way analysis of variance. Survival rates were evaluated using a Kaplan-Meier analysis, and the correlation between FFS and clinical factors or serum cytokine level as a continuous variable was analyzed using the Cox hazard proportional model. Multivariate analysis was performed using the Cox stepwise regression model. The cut-off value of IL-6 was obtained from a receiver operating characteristic (ROC) curve based on the Youden index. Statistical analysis was performed using SPSS 20.0 (IBM, Armonk, NY, United States).
The median follow-up duration for the entire patient population was 13.3 mo (range, 1.6-63.5 mo).
The patient, tumor and treatment characteristics are described in Table 1. The median age was 54 years (range, 32-75 years). Most patients (92.2%) were diagnosed with International Union Against Cancer (UICC) stage III or IV HCC, and half of the patients had Barcelona Clinic Liver Cancer (BCLC) stage C disease at the time of RT. Twenty-two patients (43.1%) had a history of pre-RT treatment and were defined as treatment-non-naïve patients. All treatment-non-naïve patients received transarterial chemoembolization (TACE) or transarterial chemoinfusion before RT. Additionally, surgery, radiofrequency ablation, holmium, or intra-arterial chemotherapy was performed in 7 patients. The median serum levels of alpha-fetoprotein (AFP) and PIVKA-II were 141.9 IU/mL (range, 1.53-83000 IU/mL) and 600 mAU/mL (range, 12-2000 mAU/mL), respectively. The median total prescribed dose of RT was 50.4 Gy (range, 45-64.8 Gy) and median duration of RT was 34 d (range, 25-56 d).
| Characteristic | n | Percent | |
| Age (yr), median | 54 (range, 32-75) | ||
| Sex | Male | 44 | 86.3 |
| Female | 7 | 13.7 | |
| Viral type | B | 44 | 86.3 |
| C | 4 | 7.8 | |
| NBNC | 3 | 5.9 | |
| Child-Pugh class | A | 49 | 96.1 |
| B | 2 | 3.9 | |
| Modified UICC stage | II | 4 | 7.8 |
| III | 32 | 62.7 | |
| IV | 15 | 29.5 | |
| BCLC stage | A | 14 | 27.5 |
| B | 12 | 23.5 | |
| C | 25 | 49.0 | |
| PVT | No | 31 | 60.8 |
| Yes | 20 | 39.2 | |
| Multiplicity | No | 25 | 49.0 |
| Yes | 26 | 51.0 | |
| Tumor size in cm, median | 8.5 (range, 1.0-19.6) | ||
| AFP (IU/mL), median | 141.9 (range, 1.53-83000) | ||
| PIVKA-II (mAU/mL), median | 600 (range, 12-2000) | ||
| Treatment-naïve | Yes | 29 | 56.9 |
| No | 22 | 43.1 | |
| Total dose of RT (Gy), median | 50.4 (range, 45-64.8) | ||
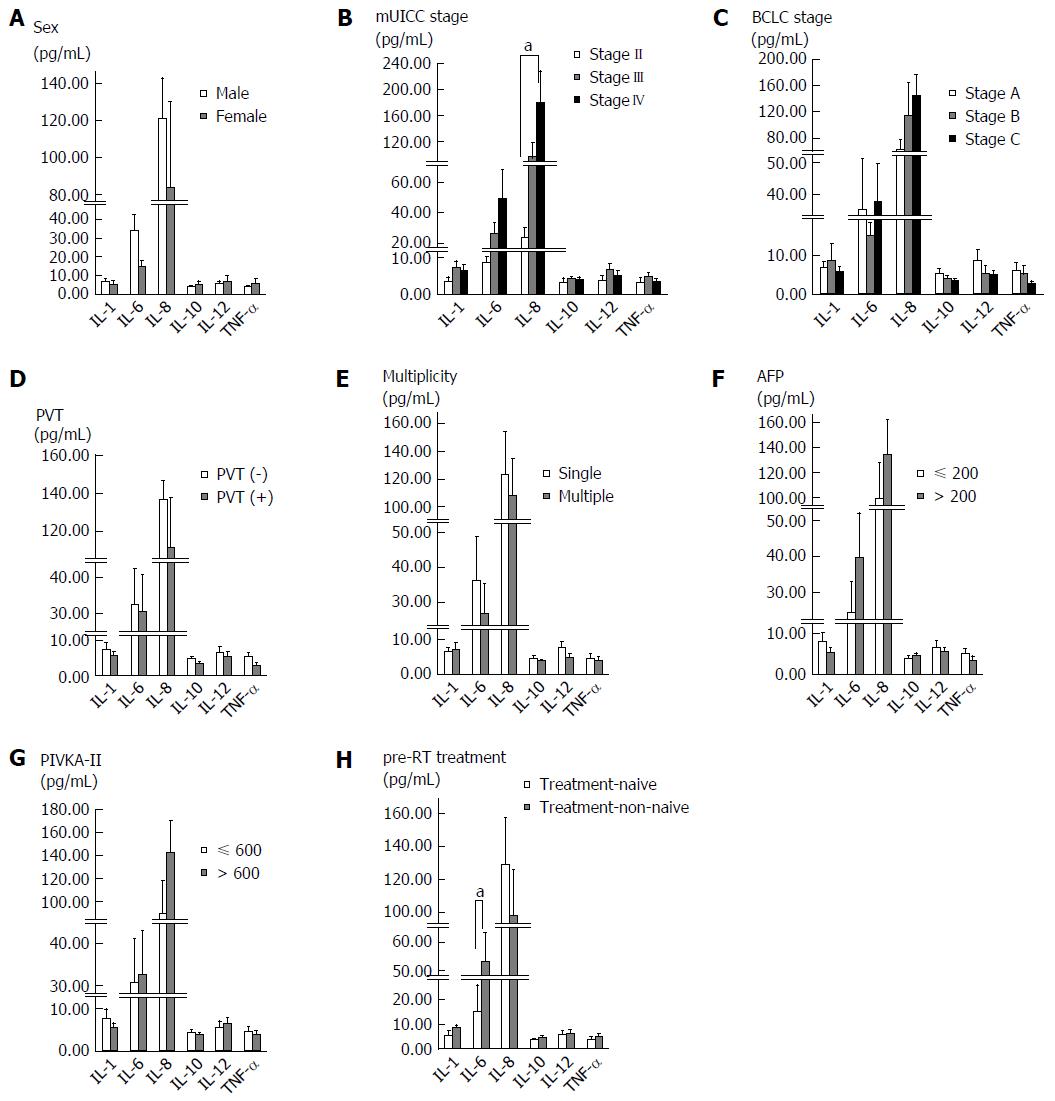
We evaluated the correlation between baseline serum cytokine levels and the characteristics of patients and tumors (Figure 1). Baseline IL-6 and IL-8 levels were found to increase with the UICC stage. When comparing stage II with stage IV, there was a significant difference in the IL-8 level (P = 0.006), and IL-6 showed borderline significance (P = 0.051). Treatment-non-naïve patients showed higher baseline serum IL-6 levels than treatment-naïve patients (P = 0.028). There were no significant differences in serum cytokine levels according to sex, BCLC stage, portal vein thrombosis, tumor multiplicity, and pre-RT tumor marker level.
Forty-five patients (88.2%) died during the follow-up period. Median OS was 13.9 mo (range, 2.2-63.5 mo) and 1-year and 2-year survival rates were 56.9% and 33.3%, respectively. The AFP level was only a significant factor for OS (P = 0.020, RR, 1.001, 95%CI: 1.000-1.002). There was no correlation between OS and baseline serum cytokine level (Table 2).
| Univariate | Multivariate | |||
| P value | RR (95%CI) | P value | RR (95%CI) | |
| Sex | 0.580 | 1.276 (0.538-3.027) | ||
| Age | 0.954 | 1.001 (0.970-1.033) | ||
| Stage | 0.861 | 1.052 (0.600-1.844) | ||
| BCLC | 0.224 | 1.252 (0.872-1.799) | ||
| Size | 0.308 | 1.037 (0.967-1.113) | ||
| PVT | 0.132 | 1.581 (0.871-2.871) | ||
| LN metastasis | 0.867 | 1.092 (0.389-3.061) | ||
| Multiplicity | 0.957 | 1.016 (0.561-1.841) | ||
| AFP | 0.020 | 1.001 (1.000-1.002) | 0.020 | 1.001 (1.000-1.002) |
| PIVKA-II | 0.211 | 1.022 (0.988-1.056) | ||
| Pre-RT treatment | 0.693 | 0.887 (0.487-1.608) | ||
| Baseline IL-1 | 0.262 | 0.979 (0.942-1.016) | ||
| Baseline IL-6 | 0.692 | 1.001 (0.996-1.007) | ||
| Baseline IL-8 | 0.770 | 1.000 (0.998-1.002) | ||
| Baseline IL-10 | 0.909 | 0.994 (0.890-1.109) | ||
| Baseline IL-12 | 0.449 | 1.014 (0.978-1.052) | ||
| Baseline TNF-α | 0.573 | 0.984 (0.930-1.041) | ||
During the follow-up period, treatment failures occurred in 40 patients (79.4%), and infield, outfield-intrahepatic and extrahepatic failures were observed in 19, 31 and 31 patients, respectively. The median FFS for infield, outfield-intrahepatic, and extrahepatic failures were 23.3, 11.5 and 12.0 mo, respectively. The correlation of FFS with tumor characteristics and baseline cytokine level was described in Tables 3-5. For infield FFS, sex (P < 0.001, RR, 47.505, 95%CI: 7.384-305.601) and baseline serum IL-6 level (P < 0.001, RR, 1.019, 95%CI: 1.011-1.028) were statistically significant. Baseline serum IL-10 level was a significant factor for outfield-intrahepatic FFS (P = 0.026, RR, 0.830, 95%CI: 0.705-0.978), and AFP was associated with extrahepatic failure (P = 0.034, RR, 1.001, 95%CI: 1.000-1.003).
| Univariate | Multivariate | |||
| P value | RR (95%CI) | P value | RR (95%CI) | |
| Sex | 0.001 | 10.262 (2.479-42.481) | < 0.001 | 47.505 (7.384-305.601) |
| Age | 0.198 | 0.968 (0.921-1.017) | ||
| Stage | 0.082 | 2.106 (0.911-4.871) | ||
| BCLC | 0.441 | 1.257 (0.702-2.250) | ||
| Size | 0.903 | 1.007 (0.904-1.121) | ||
| PVT | 0.247 | 1.741 (0.681-4.452) | ||
| LN metastasis | 0.408 | 1.867 (0.425-8.191) | ||
| Multiplicity | 0.944 | 1.033 (0.412-2.591) | ||
| AFP | 0.653 | 0.999 (0.996-1.002) | ||
| PIVKA-II | 0.889 | 0.996 (0.947-1.048) | ||
| Pre-RT treatment | 0.290 | 1.627 (0.660-4.013) | ||
| Baseline IL-1 | 0.895 | 0.997 (0.955-1.041) | ||
| Baseline IL-6 | 0.000 | 1.011 (1.005-1.018) | < 0.001 | 1.019 (1.011-1.028) |
| Baseline IL-8 | 0.985 | 1.000 (0.997-1.003) | ||
| Baseline IL-10 | 0.437 | 1.060 (0.915-1.227) | ||
| Baseline IL-12 | 0.903 | 0.996 (0.938-1.059) | ||
| Baseline TNF-α | 0.864 | 0.993 (0.918-1.074) | ||
| Univariate | Multivariate | |||
| P value | RR (95%CI) | P value | RR (95%CI) | |
| Sex | 0.387 | 1.539 (0.579-4.086) | ||
| Age | 0.834 | 0.996 (0.959-1.035) | ||
| Stage | 0.601 | 0.834 (0.423-1.646) | ||
| BCLC | 0.383 | 1.210 (0.788-1.859) | ||
| Size | 0.534 | 0.974 (0.898-1.058) | ||
| PVT | 0.326 | 1.433 (0.699-2.940) | ||
| LN metastasis | 0.387 | 0.530 (0.126-2.230) | ||
| Multiplicity | 0.303 | 1.464 (0.709-3.021) | ||
| AFP | 0.649 | 1.000 (0.999-1.002) | ||
| PIVKA-II | 0.920 | 1.002 (0.963-1.043) | ||
| Pre-RT treatment | 0.745 | 1.126 (0.550-2.307) | ||
| Baseline IL-1 | 0.573 | 1.015 (0.964-1.069) | ||
| Baseline IL-6 | 0.271 | 0.950 (0.985-1.004) | ||
| Baseline IL-8 | 0.942 | 1.000 (0.998-1.002) | ||
| Baseline IL-10 | 0.026 | 0.830 (0.705-0.978) | 0.026 | 0.830 (0.705-0.978) |
| Baseline IL-12 | 0.197 | 0.959 (0.901-1.022) | ||
| Baseline TNF-α | 0.240 | 0.951 (0.876-1.034) | ||
| Univariate | Multivariate | |||
| P value | RR (95%CI) | P value | RR (95%CI) | |
| Sex | 0.153 | 2.043 (0.768-5.438) | ||
| Age | 0.502 | 0.987 (0.951-1.025) | ||
| Stage | 0.196 | 1.562 (0.795-3.070) | ||
| BCLC | 0.782 | 0.942 (0.615-1.441) | ||
| Size | 0.830 | 1.010 (0.926-1.101) | ||
| PVT | 0.988 | 0.994 (0.465-2.125) | ||
| LN metastasis | 0.936 | 0.952 (0.289-3.141) | ||
| Multiplicity | 0.622 | 1.197 (0.586-2.448) | ||
| AFP | 0.034 | 1.001 (1.000-1.003) | 0.034 | 1.001 (1.000-1.003) |
| PIVKA-II | 0.449 | 1.016 (0.976-1.057) | ||
| Pre-RT treatment | 0.878 | 1.057 (0.520-2.147) | ||
| Baseline IL-1 | 0.725 | 1.005 (0.977-1.034) | ||
| Baseline IL-6 | 0.529 | 1.002 (0.996-1.007) | ||
| Baseline IL-8 | 0.546 | 1.001 (0.999-1.003) | ||
| Baseline IL-10 | 0.309 | 1.062 (0.946-1.193) | ||
| Baseline IL-12 | 0.726 | 1.009 (0.962-1.058) | ||
| Baseline TNF-α | 0.956 | 1.002 (0.942-1.066) | ||
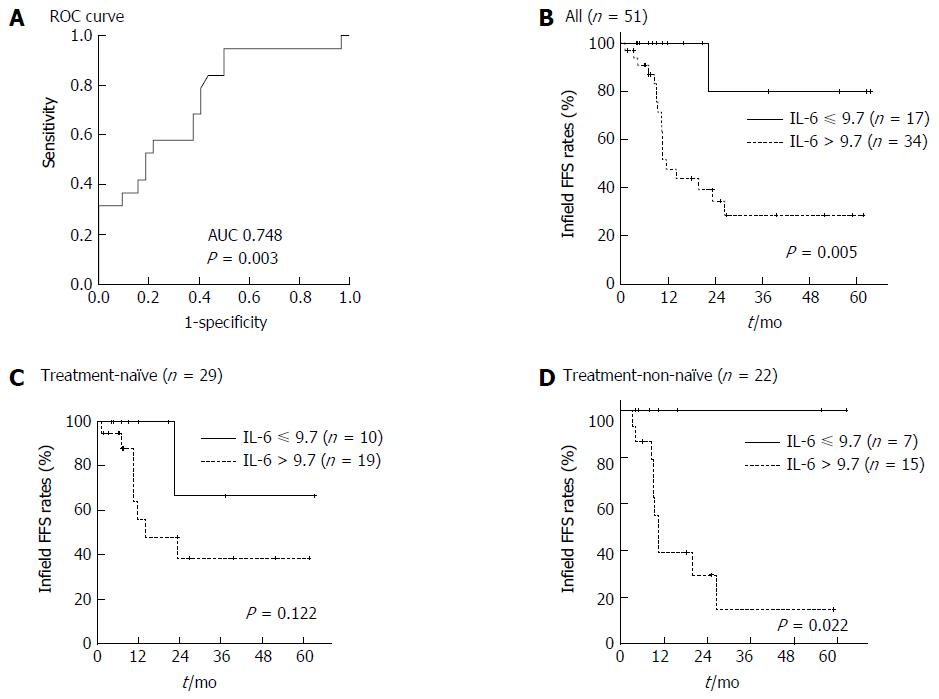
The cut-off value of baseline serum IL-6 level for infield FFS was 9.735 (Figure 2A; AUC 0.748, P = 0.003, 95%CI: 0.607-0.888). Patients with a baseline serum IL-6 level higher than 9.7 pg/mL showed worse infield FFS than those with a baseline serum IL-6 level less than 9.7 pg/mL (Figure 2B; P = 0.005).
As baseline serum IL-6 level significantly differed between treatment-non-naïve and treatment-naïve patients, we performed a subgroup analysis based on pre-RT treatment. The subgroup analysis indicated the significant difference in baseline serum IL-6 level was observed only in treatment-non-naïve patients (Figure 2C and D; P = 0.002).
We also analyzed serum cytokine levels on completion of RT (Table 6). After RT, serum IL-10 level had increased from 4.19 ± 0.41 to 5.83 ± 0.46 (P = 0.002), and serum IL-12 level had decreased from 6.10 ± 1.01 to 4.10 ± 0.59 (P = 0.018). However, these changes were not associated with survival or treatment failure. Variations in the serum levels of other cytokines after RT were not significant.
| Before RT (pg/mL) | After RT (pg/mL) | P value | |
| IL-1 | 6.77 ± 1.18 | 9.13 ± 1.51 | 0.229 |
| IL-6 | 31.63 ± 7.34 | 29.54 ± 6.60 | 0.794 |
| IL-8 | 115.75 ± 19.84 | 159.73 ± 38.96 | 0.211 |
| IL-10 | 4.19 ± 0.41 | 5.83 ± 0.46 | 0.002 |
| IL-12 | 6.10 ± 1.01 | 4.10 ± 0.59 | 0.018 |
| TNF-α | 4.27 ± 0.79 | 3.33 ± 0.40 | 0.130 |
The median value of the mean liver dose was 20.3 Gy (range, 5.7-34.9 Gy), and there were no cases of severe radiation-induced liver disease during RT and until 3 mo after the completion of RT. Grade 3-4 hematologic toxicities including anemia, neutropenia and thrombocytopenia were reported in 6, 12 and 9 patients, respectively. There were also 3 cases of hypoalbuminemia, 8 cases of hyperbilirubinemia, 10 cases of elevated liver enzymes, and 3 cases of gastrointestinal bleeding (> grade 3).
In this study, we investigated whether the serum levels of six cytokines (IL-1, IL-6, IL-8, IL-10, IL-12 and TNF-α) would have clinical significance in patients with HCC who were treated with RT. We demonstrated that patients with a history of pre-RT treatment showed a higher baseline serum IL-6 level than treatment-naïve patients. A high baseline serum IL-6 level was also associated with infield failure. The subgroup analysis revealed that baseline serum IL-6 levels were associated with infield failure only in treatment-non-naïve patients.
IL-6 is a pro-inflammatory cytokine and one of the well-characterized pro-tumorigenic cytokines. It is upregulated in many cancers and is important for tumor cell proliferation, survival, differentiation, migration, invasion, metastasis and angiogenesis[9]. In the liver, IL-6 is involved in acute phase response, liver generation, and regulation of physiological and pathophysiological conditions. Similar to other cancers, IL-6 has been shown to be correlated with HCC development risk, advanced stage and poor survival[10-12]. In this study, serum IL-6 level showed borderline significance between patients with stage II and IV disease, most likely due to the small number of patients with stage II disease (n = 4).
A history of pre-RT treatment was associated with a difference in baseline serum IL-6 level. Most of the treatment-non-naïve patients underwent TACE, and previous studies have demonstrated that serum IL-6 level increases after TACE and is correlated with post-TACE inflammation[13,14]. In these studies, the increase of serum IL-6 level occurred within several days after TACE and was attributed to a protective function of IL-6 against acute liver injury. In this study, the time interval between the last TACE and the start of RT was median 1.5 mo (range, 0.2-17.5 mo) and the baseline serum level was not correlated with the time interval. Therefore, the elevated serum IL-6 level in treatment-non-naïve patients of our study cannot be explained only by a pre-RT history of TACE. After TACE, TACE-induced hepatic tissue injuries around the tumor provoke an inflammatory response and alter the immune system. These inflammatory cytokine responses, including IL-6, may also induce tumor recurrence or progression after TACE.
Among serum cytokines, only baseline serum IL-6 was a significant factor for treatment failure in the RT field in this study. The effect of serum IL-6 on RT and chemoradiotherapy (CRT) response has been reported in many cancers, including lung, prostate, esophagus, stomach, pancreas, and head and neck cancers[15-17]. In previous reports, an elevated serum IL-6 level before RT/CRT was associated with poor treatment outcome. A high serum IL-6 level is known to correlate with tumor aggressiveness and associated tumor factors that affect treatment outcome, such as large size and advanced stage. However, there have been few studies on the effect of serum IL-6 on RT response in HCC. Jang et al[11] reported that IL-6 was a strong inflammatory indicator for predicting the outcome in patients with HCC who received loco-regional therapy (mainly TACE). RT was considered in patients with portal vein thrombosis or extrahepatic metastasis, and only 13 patients received RT after TACE. A higher IL-6 level was correlated with shorter survival. Chen et al[18] investigated the role of IL-6 in the radiation response of liver tumors. IL-6 expression was positively linked to radiation resistance in both in vivo and in vitro experiments. The authors suggested that concurrent treatment with IL-6 inhibitors could be a potential therapeutic strategy for increasing the radiation response of tumors.
In a previous report, we described the results of hepatic arterial concurrent chemoradiotherapy (CCRT) for advanced HCC and found that pre-CCRT treatment history was a predictive risk factor for treatment failure, particularly infield failure[19]. Based on the results of the current study, this difference in treatment outcome might be explained by the fact that treatment-non-naïve patients had higher baseline serum IL-6 levels than treatment-naïve patients. Although application of RT has increased in the treatment of HCC, RT continues to be used as either complementary or salvage treatment after other treatment modalities such as TACE, radiofrequency ablation or surgery, rather than as a primary treatment. Therefore, assessment of baseline serum cytokine levels, particularly IL-6, could be helpful in predicting the treatment outcome in treatment-non-naïve patients.
To determine a useful criterion for predicting treatment outcomes in clinics, we identified the predictive cut-off value of baseline serum IL-6 level by ROC curve analysis. And 9.7 pg/mL as cut-off value was useful to predict infield failure in this study. Further study is needed to verify this cut-off value in many patients with HCC treated with RT.
In this study, lower serum IL-10 level was correlated with worse outfield-intrahepatic FFS. IL-10 is known as a pleiotropic cytokine with dual roles in the immune system, including immune-suppression and immune-stimulation[20]. Many studies reported that serum IL-10 levels were higher in cancer patients than in healthy cohorts and that a higher IL-10 level was associated with worse prognosis[21]. However, other contradictory reports showed that patients with IL-10-negative tumors showed poor prognosis[22,23]. The inhibition of inflammatory cytokines and the stimulation of cytotoxic T cells by IL-10 have been further studied for cancer treatment using recombinant IL-10[24].
After RT, serum levels of IL-10 increased while the levels of IL-12 decreased. Several studies demonstrated changes in cytokine expression resulting from treatment of HCC. Cytokine levels are altered after treatment regimens including surgery or TACE; however, the direction of the change has not always been consistent between studies[25,26]. Although the change in the serum IL-10 or IL-12 level was not significantly associated with treatment failure in this study, these findings indicate that local RT might produce a systemic effect. It has been reported that local RT triggers systemic effects through the recruitment of biological effectors outside the radiation field[27].
The small number of patients was a limitation of this study and this limitation could affect statistical significance. Further large scale studies are needed to confirm our finding and to examine how radiation affects serum cytokine levels in HCC. In addition, this study was performed only in patients treated with RT. Further studies to identify serum cytokine levels in patients with HCC treated with other various treatment modalities might also be useful.
In conclusion, the current findings suggest that an assessment of serum cytokines may be helpful for predicting treatment outcome after RT in patients with HCC. In addition to tumor marker AFP, cytokines seemed to be useful in predicting infield failure (IL-6) and outfield-intrahepatic failure (IL-10). Serum cytokines could be useful biomarkers for predicting RT outcome in HCC.
The role of cytokines in carcinogenesis and treatment response in many cancers has been reported. Serum cytokine levels are easily accessible and may be useful as biomarkers in screening for cancers or predicting outcomes. Although the application of radiotherapy (RT) in the treatment of hepatocellular carcinoma (HCC) has increased, the role of cytokine in the RT response in patients with HCC is not well known.
The study on the identification of significant cytokine in cancer screening or prediction of treatment outcome is an important area. As the application of RT for HCC increases, it has become important to find a useful biomarker to predict RT outcome in patients with HCC.
The association between cytokine levels and treatment response is known in many cancers. However, there are few studies on the role of cytokines in the RT response in patients with HCC. This study aimed to identify the significance of serum cytokines in the treatment outcomes for patients with HCC treated with RT. In this study, the authors collected samples prospectively and analyzed the serum cytokines before and after RT. We demonstrated that patients with a history of pre-RT treatment showed a higher baseline serum IL-6 level than treatment-naïve patients and a high baseline was also shown to be associated with infield failure, especially in treatment-non-naïve patients.
The results of this study suggest that the assessment of pre-RT serum cytokine levels, particularly IL-6, could be helpful in predicting treatment outcome in treatment-non-naïve paints.
IL-6 is a pro-inflammatory cytokine and one of the well-characterized pro-tumorigenic cytokines. A high serum IL-6 level before RT or chemoradiotherapy was associated with poor treatment outcome in many cancers. Infield failure was defined as progression of the tumor within the RT field that covered the planning target volume.
This paper is relevant and adds to the literature. The use of cytokines is an active area of research and this group appears to be at the forefront of this investigation. Although a small number of patients limit the significance of this study, the results are clear and seem to promote further studies.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Kang MK, Lock M, Yin YH S- Editor: Yu J L- Editor: Filipodia E- Editor: Zhang FF
| 1. | Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 239] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 2. | Capone F, Costantini S, Guerriero E, Calemma R, Napolitano M, Scala S, Izzo F, Castello G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur Cytokine Netw. 2010;21:99-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 3. | Ren Y, Poon RT, Tsui HT, Chen WH, Li Z, Lau C, Yu WC, Fan ST. Interleukin-8 serum levels in patients with hepatocellular carcinoma: correlations with clinicopathological features and prognosis. Clin Cancer Res. 2003;9:5996-6001. [PubMed] |
| 4. | Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES, Ng EK, Chan FK, Sung JJ, Chan HL. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer. 2009;124:2766-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | Zhou L, Liu J, Luo F. Serum tumor markers for detection of hepatocellular carcinoma. World J Gastroenterol. 2006;12:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 222] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (3)] |
| 6. | Deorukhkar A, Krishnan S. Targeting inflammatory pathways for tumor radiosensitization. Biochem Pharmacol. 2010;80:1904-1914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 7. | Klein J, Dawson LA. Hepatocellular carcinoma radiation therapy: review of evidence and future opportunities. Int J Radiat Oncol Biol Phys. 2013;87:22-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 8. | Visconti L, Nelissen K, Deckx L, van den Akker M, Adriaensen W, Daniels L, Matheï C, Linsen L, Hellings N, Stinissen P. Prognostic value of circulating cytokines on overall survival and disease-free survival in cancer patients. Biomark Med. 2014;8:297-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Taniguchi K, Karin M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin Immunol. 2014;26:54-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 533] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 10. | Giannitrapani L, Cervello M, Soresi M, Notarbartolo M, La Rosa M, Virruso L, D’Alessandro N, Montalto G. Circulating IL-6 and sIL-6R in patients with hepatocellular carcinoma. Ann N Y Acad Sci. 2002;963:46-52. [PubMed] |
| 11. | Jang JW, Oh BS, Kwon JH, You CR, Chung KW, Kay CS, Jung HS. Serum interleukin-6 and C-reactive protein as a prognostic indicator in hepatocellular carcinoma. Cytokine. 2012;60:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1362] [Cited by in RCA: 1490] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 13. | Chao Y, Wu CY, Kuo CY, Wang JP, Luo JC, Kao CH, Lee RC, Lee WP, Li CP. Cytokines are associated with postembolization fever and survival in hepatocellular carcinoma patients receiving transcatheter arterial chemoembolization. Hepatol Int. 2013;7:883-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Kim MJ, Jang JW, Oh BS, Kwon JH, Chung KW, Jung HS, Jekarl DW, Lee S. Change in inflammatory cytokine profiles after transarterial chemotherapy in patients with hepatocellular carcinoma. Cytokine. 2013;64:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Dehing-Oberije C, Aerts H, Yu S, De Ruysscher D, Menheere P, Hilvo M, van der Weide H, Rao B, Lambin P. Development and validation of a prognostic model using blood biomarker information for prediction of survival of non-small-cell lung cancer patients treated with combined chemotherapy and radiation or radiotherapy alone (NCT00181519, NCT00573040, and NCT00572325). Int J Radiat Oncol Biol Phys. 2011;81:360-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Duffy SA, Taylor JM, Terrell JE, Islam M, Li Y, Fowler KE, Wolf GT, Teknos TN. Interleukin-6 predicts recurrence and survival among head and neck cancer patients. Cancer. 2008;113:750-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Wu CT, Chen MF, Chen WC, Hsieh CC. The role of IL-6 in the radiation response of prostate cancer. Radiat Oncol. 2013;8:159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 18. | Chen MF, Hsieh CC, Chen WC, Lai CH. Role of interleukin-6 in the radiation response of liver tumors. Int J Radiat Oncol Biol Phys. 2012;84:e621-e630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Cha H, Yoon HI, Lee IJ, Koom WS, Han KH, Seong J. Clinical factors related to recurrence after hepatic arterial concurrent chemoradiotherapy for advanced but liver-confined hepatocellular carcinoma. J Radiat Res. 2013;54:1069-1077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Mocellin S, Panelli MC, Wang E, Nagorsen D, Marincola FM. The dual role of IL-10. Trends Immunol. 2003;24:36-43. [PubMed] |
| 21. | Zhao S, Wu D, Wu P, Wang Z, Huang J. Serum IL-10 Predicts Worse Outcome in Cancer Patients: A Meta-Analysis. PLoS One. 2015;10:e0139598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 22. | Kaio E, Tanaka S, Oka S, Hiyama T, Kitadai Y, Haruma K, Chayama K. Clinical significance of thrombospondin-1 expression in relation to vascular endothelial growth factor and interleukin-10 expression at the deepest invasive tumor site of advanced colorectal carcinoma. Int J Oncol. 2003;23:901-911. [PubMed] |
| 23. | Toiyama Y, Miki C, Inoue Y, Minobe S, Urano H, Kusunoki M. Loss of tissue expression of interleukin-10 promotes the disease progression of colorectal carcinoma. Surg Today. 2010;40:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Oft M. IL-10: master switch from tumor-promoting inflammation to antitumor immunity. Cancer Immunol Res. 2014;2:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 25. | Chau GY, Wu CW, Lui WY, Chang TJ, Kao HL, Wu LH, King KL, Loong CC, Hsia CY, Chi CW. Serum interleukin-10 but not interleukin-6 is related to clinical outcome in patients with resectable hepatocellular carcinoma. Ann Surg. 2000;231:552-558. [PubMed] |
| 26. | Kanaoka Y, Yagi T, Sadamori H, Matsukawa H, Matsuda H, Inagaki M, Ishikawa T, Saito S, Iwagaki H, Tanaka N. Analysis of host response to hepatectomy by simultaneous measurement of cytokines in the portal vein, caval vein and radial artery. J Int Med Res. 2002;30:496-505. [PubMed] |
| 27. | Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol. 2009;10:718-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 815] [Cited by in RCA: 770] [Article Influence: 48.1] [Reference Citation Analysis (0)] |