Published online Mar 21, 2017. doi: 10.3748/wjg.v23.i11.1974
Peer-review started: August 5, 2016
First decision: November 21, 2016
Revised: December 2, 2016
Accepted: January 11, 2017
Article in press: January 11, 2017
Published online: March 21, 2017
Processing time: 227 Days and 7.6 Hours
There is currently a pressing need for alternative therapies to liver transplantation. The number of patients waiting for a liver transplant is substantially higher than the number of transplantable donor livers, resulting in a long waiting time and a high waiting list mortality. An extracorporeal liver support system is one possible approach to overcome this problem. However, the ideal cell source for developing bioartificial liver (BAL) support systems has yet to be determined. Recent advancements in stem cell technology allow researchers to generate highly functional hepatocyte-like cells from human pluripotent stem cells (hPSCs). In this mini-review, we summarize previous clinical trials with different BAL systems, and discuss advantages of and potential obstacles to utilizing hPSC-derived hepatic cells in clinical-scale BAL systems.
Core tip: The current lack of transplantable donor livers in the world has led to the development of extracorporeal liver support systems as one possible approach to overcome this problem. Bioartificial liver (BAL) support systems require a cell source to replicate human liver function, yet the ideal cell source for this purpose has yet to be determined. Highly-functional hepatocyte-like cells have recently been generated from human pluripotent stem cells, which show promise as a potential cell source in BAL support systems for the treatment of liver failure in the future.
- Citation: Sakiyama R, Blau BJ, Miki T. Clinical translation of bioartificial liver support systems with human pluripotent stem cell-derived hepatic cells. World J Gastroenterol 2017; 23(11): 1974-1979
- URL: https://www.wjgnet.com/1007-9327/full/v23/i11/1974.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i11.1974
Liver disease is one of the most prevalent medical conditions in the world today, affecting hundreds of millions of people worldwide[1-3]. Many of these diseases, such as end-stage liver diseases and some inherited liver diseases, can only be treated successfully with a liver transplant[4]. Although 11606 patients were added to the liver transplant waiting list in the year 2015, only 7127 patients received a liver transplant in that same year[4]. This discrepancy demonstrates the profound shortage of transplantable donor livers. This shortage of livers resulted in a high waiting list mortality, with 1423 patients dying in 2015 while waiting for a transplant[4]. Therefore, it is imperative that new therapies are developed to provide an alternative to liver transplantation.
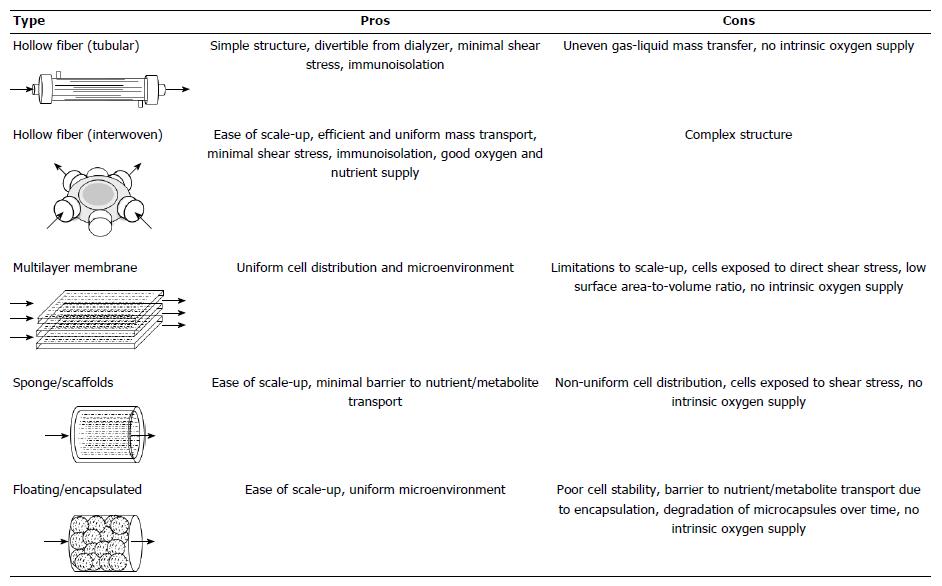
Extracorporeal liver support systems were developed with the aim of stabilizing a patient long enough for his or her own liver to regenerate or for physicians to procure a transplantable liver. Early support systems functioned to supplement liver function by removing toxins from the blood through non-biological hemofiltration[5]. These non-biological type extracorporeal liver support systems have been clinically established and are widely used in countries where liver transplantation is limited[5]. However, it became apparent that non-biological hemofiltration devices were incapable of adequately replicating liver function[5]. In order to overcome the limitations of non-biological devices, live cells that possess liver function were incorporated into the development of bioartificial liver support (BAL) systems[5]. There are several types of BAL systems that have been proposed which differ in their cell housing mechanism, including hollow fiber-based[6-10], multilayer membrane-based[11], sponge/scaffold-based[12-14], and floating/encapsulated-based systems[15] (Figure 1). Although most of these housing mechanisms have successfully cultured cells on the small experimental scale, hollow fiber-based BAL systems are widely used in clinical trials.
Several types of cells may be selected for use in a BAL system. These include primary hepatocytes isolated from human livers, human hepatoblastoma cell lines, and primary animal hepatocytes[16]. Human primary hepatocytes are ideal for the BAL system[16]. However, the low availability and inconsistent quality of primary human hepatocytes prevent their use in clinics[16]. Although human hepatic cancer cell lines and animal liver cells are readily available, they are less metabolically active than primary human hepatocytes[17]. In addition, the risk of zoonoses precludes the use of animal cells. For example, it has been shown that porcine endogenous retroviruses are capable of infecting human cells in vitro[18].
Recent advancements in stem cell research have demonstrated that hepatocyte-like cells can be derived from human pluripotent stem cells (hPSCs)[19]. hPSCs can be generated from a patient’s own cells by introducing several transcription factors[20]. They are capable of differentiating into cells from all three germ layers, including neural cells[21-23], osteogenic cells[24], cardiac cells[25] , adipogenic cells[26], pancreatic cells[27,28], vascular cells[29], hematopoietic cells[30], endothelial cells[30], and hepatocytes[31,32]. hPSC-derived hepatic cells have been shown to express hepatocyte marker genes and proteins[33]. They also demonstrate hepatic functions including albumin secretion, urea synthesis, cytochrome P450 enzyme induction[31], and glycogen storage[34].
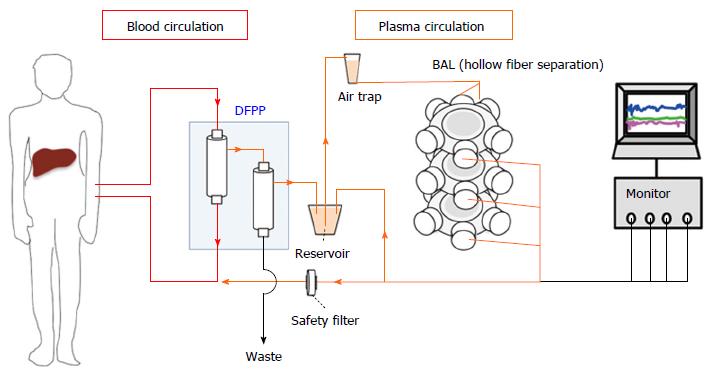
hPSC-derived hepatic cells possess minimal risk when used in a BAL system, but are unsuitable for other applications due to their risk of tumorigenicity. The genetic instability of hPSCs results in an underlying uncertainty of transplanting large quantities of hPSC-derived hepatic cells directly into a patient[35]. On the other hand, the risk of tumorigenicity is minimized in a BAL system, as the hPSC-derived hepatic cells would be isolated from the patient’s blood stream by multiple layers of filter membranes (Figure 2). Therefore, while hPSC-derived hepatic cells may not be ideal for cell transplantation, they are viable candidates for a BAL system.
Several BALs have been evaluated in clinical trials, as previously explored in van de Kerkhove et al[13,14] (Table 1)[6-10,13,14]. The Extracorporeal Liver Assist Device (ELAD) utilizes the human hepatoblastoma cell line HepG2/C3A (100 g) in hollow fiber-based dialysis cartridges. A phase III trial treated 96 patients with alcohol-induced liver decompensation. In subjects age < 50 years, creatinine < 1.3 mg/dL, bilirubin ≥ 16 and international normalized ratio (INR) ≤ 2.5, the 91-d survival rates were 93.9% for ELAD-treated subjects and 68.4% for control subjects (P = 0.006)[10]. A second BAL design, the Modular Extracorporeal Liver Support (MELS) system, consists of interwoven hollow fiber membranes, creating a three-dimensional framework utilizing primary human hepatocytes. In one trial, eight patients (two with acute liver failure, four with acute-on-chronic liver failure, and two with primary nonfunction) were successfully bridged to liver transplantation[8]. Several other trials have yielded similar results regarding degree of effectiveness.
| Bioreactor device | Ref. | Cells | Mass (g)1 | Bioreactor design | Scaffold | Fluid | Separation | Treatment time (h) | Phase | Indication (n) | Effect |
| HepatAssist | Demetriou et al[9] | Cryopreserved porcine hepatocytes | 50-70 | Hollow fiber | Microcarrier + external inoculation | Plasma | 3000 kDa cut-off | 6 | III | ALF (147), PNF (24) | HepatAssist survival of 71.0% vs control survival of 62.0% P = 0.28, (NS) |
| Vitagen ELAD | Reich et al[10] | HepG2/C3A | 200-400 | Hollow fiber | External inoculation | Plasma | 70 kDa cut-off | Up to 168 | III | AILD (96) | ELAD survival of 80.4% vs control survival of 65.2% P = 0.068, (NS) |
| LSS | Mundt et al[7] | Primary porcine hepatocytes | up to 500 | Hollow fiber | External inoculation | Plasma | 300 kDa cut-off | 7-46 | I/II | ALF (8) | Bridged to OLT 8 |
| MELS | Sauer et al[8] | Primary human hepatocytes | up to 600 | Hollow fiber | External inoculation | Plasma | 400 kDa cut-off | 7-74 | I | ALF (2), PNF (2), AOC(4) | Bridged to OLT 6, Survival without OLT 1, Died without OLT 1 |
| Excorp Medical BLSS | Mazariegos et al[6] | Primary porcine hepatocytes | 70-120 | Hollow fiber | Collagen + external inoculation | Whole blood | 100 kDa cut-off | 12 | I | ALF (2), AOC (2) | Bridged to OLT 1, Died without OLT 3 |
| AMC-BAL | van de Kerkhove et al[13,14] | Primary porcine hepatocytes | 100 | Nonwoven | Spiral membrane + polyester matrix | Plasma | None | 24 | I | ALF (12) | Bridged to OLT 11, Survival without OLT 1 |
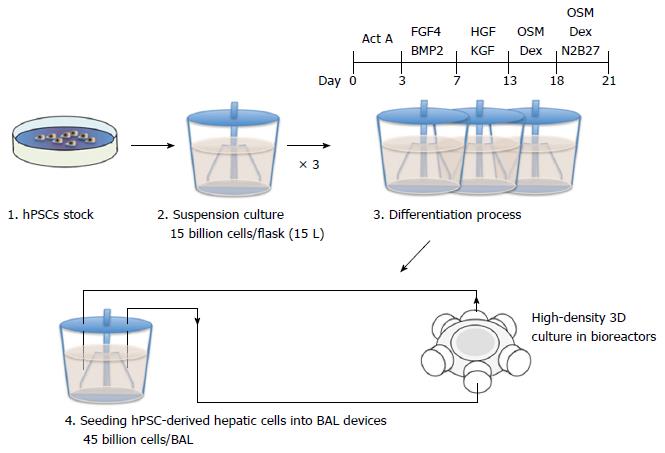
Despite the effectiveness of BAL systems in clinical trials, their translation from the laboratory bench to the patient’s bedside has been hindered by three obstacles. Firstly, it is necessary to prepare a sufficient quantity of hPSC-derived hepatic cells for clinical applications. It has been widely suggested that approximately 30% of the total liver volume is required for survival. Considering that the average mass of a human liver is 1.5 kg, and that 100 million hepatocytes are contained in 1 g of liver tissue, a minimum of 45 billion hPSC-derived hepatic cells would be required to produce a clinical-scale BAL device[36] (Figure 3). Secondly, the operation cost of a BAL device is currently too expensive for widespread clinical use. The process of culturing 45 billion hPSCs and inducing hepatic differentiation consumes large quantities of culture medium and supplements including recombinant growth factors[37]. As the length of treatment increases, the cost of operating a BAL device accumulates significantly. Thirdly, it has not been well investigated whether hPSC-derived hepatic cells maintain their liver functions over a long period of time in BAL devices. The loss of cell viability and functionality throughout the course of treatment may be problematic[38].
The most critical factor for large-scale cell culture is oxygen and nutrient supply. The oxygen and nutrients must be uniformly supplied to a large number of cells. It is well known that the anchorage-dependent hepatocytes easily form aggregates, and if the diameter of the aggregates exceeds 100µm at atmospheric concentrations, central necrosis occurs resulting from lack of oxygen and nutrition[39]. This fact indicates that the organization of the cell culture space in the large-scale BAL system must allow for sufficient oxygen and nutrient penetration of the cell aggregates. A sophisticated controlling system and well-engineered bioreactor will be required to monitor oxygen and nutrient supply. In addition, since hPSCs are sensitive to environmental factors, the shear stress from the culture medium must be minimized[40]. Ideally, the bioreactor should mimic the structure within the liver, which provides appropriate pressure and shear stress similar to the Space of Disse.
BAL systems have demonstrated a potential to treat patients with liver failure by providing temporary support for them to recover their own hepatocytes or to bridge them to liver transplantation. Early BAL systems have encountered significant limitations due to the low functionality and availability of cells for this application. With emerging stem cell technology, hepatocyte-like cells can be differentiated from hPSCs. Due to their functional similarity to primary human hepatocytes and minimal risk of use, these hPSC-derived hepatic cells will be the ideal cell source to develop clinical-grade bioartificial devices. Further clinical translational studies will be required to overcome the obstacles to developing large-scale BAL devices with hPSC-derived hepatic cells. If successful, these readily available and highly functional extracorporeal liver support systems will be a feasible alternative for the treatment of liver failure in the near future.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Fogli L, Huo XL, Inoue K, Sanal MG S- Editor: Gong ZM L- Editor: A E- Editor: Zhang FF
| 1. | Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5322] [Cited by in RCA: 7544] [Article Influence: 838.2] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2508] [Article Influence: 192.9] [Reference Citation Analysis (2)] |
| 3. | Mann RE, Smart RG, Govoni R. The epidemiology of alcoholic liver disease. Alcohol Res Health. 2003;27:209-219. [PubMed] |
| 4. | United Network for Organ Sharing (UNOS). Available from: http://www.unos.org/data/. |
| 5. | Mito M. Hepatic assist: present and future. Artif Organs. 1986;10:214-218. [PubMed] |
| 6. | Mazariegos GV, Patzer JF, Lopez RC, Giraldo M, Devera ME, Grogan TA, Zhu Y, Fulmer ML, Amiot BP, Kramer DJ. First clinical use of a novel bioartificial liver support system (BLSS). Am J Transplant. 2002;2:260-266. [PubMed] |
| 7. | Mundt A, Puhl G, Müller A, Sauer I, Müller C, Richard R, Fotopoulou C, Doll R, Gäbelein G, Höhn W. A method to assess biochemical activity of liver cells during clinical application of extracorporeal hybrid liver support. Int J Artif Organs. 2002;25:542-548. [PubMed] |
| 8. | Sauer IM, Zeilinger K, Obermayer N, Pless G, Grünwald A, Pascher A, Mieder T, Roth S, Goetz M, Kardassis D. Primary human liver cells as source for modular extracorporeal liver support--a preliminary report. Int J Artif Organs. 2002;25:1001-1005. [PubMed] |
| 9. | Demetriou AA, Brown RS, Busuttil RW, Fair J, McGuire BM, Rosenthal P, Am Esch JS, Lerut J, Nyberg SL, Salizzoni M. Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure. Ann Surg. 2004;239:660-667; discussion 667-670. [PubMed] |
| 10. | Reich DJ. The effect of extracorporporeal C3A Cellular Therapy in Severe Alcoholic Hepatitis. The VTI-208 ELAD Trial. 2015. . |
| 11. | Wang L, Sun J, Wang C, Woodman K, Li L, Wu L, Harbour C, Johnston B, Shi L, Horvat M. Analysis of multivariables during porcine liver digestion to improve hepatocyte yield and viability for use in bioartificial liver support systems. Cell Transplant. 2000;9:329-336. [PubMed] |
| 12. | Sakiyama R, Nakazawa K, Ijima H, Mizumoto H, Kajiwara T, Ito M, Ishibashi H, Funatsu K. Recovery of rats with fulminant hepatic failure by using a hybrid artificial liver support system with polyurethane foam/rat hepatocyte spheroids. Int J Artif Organs. 2002;25:1144-1152. [PubMed] |
| 13. | van de Kerkhove MP, Di Florio E, Scuderi V, Mancini A, Belli A, Bracco A, Dauri M, Tisone G, Di Nicuolo G, Amoroso P. Phase I clinical trial with the AMC-bioartificial liver. Int J Artif Organs. 2002;25:950-959. [PubMed] |
| 14. | van de Kerkhove MP, Di Florio E, Scuderi V, Mancini A, Belli A, Bracco A, Scala D, Scala S, Zeuli L, Di Nicuolo G. Bridging a patient with acute liver failure to liver transplantation by the AMC-bioartificial liver. Cell Transplant. 2003;12:563-568. [PubMed] |
| 15. | Sakai Y, Naruse K, Nagashima I, Muto T, Suzuki M. A new bioartificial liver using porcine hepatocyte spheroids in high-cell-density suspension perfusion culture: in vitro performance in synthesized culture medium and in 100% human plasma. Cell Transplant. 1999;8:531-541. [PubMed] |
| 16. | Carpentier B, Gautier A, Legallais C. Artificial and bioartificial liver devices: present and future. Gut. 2009;58:1690-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 17. | Nyberg SL, Remmel RP, Mann HJ, Peshwa MV, Hu WS, Cerra FB. Primary hepatocytes outperform Hep G2 cells as the source of biotransformation functions in a bioartificial liver. Ann Surg. 1994;220:59-67. [PubMed] |
| 18. | Martin U, Kiessig V, Blusch JH, Haverich A, von der Helm K, Herden T, Steinhoff G. Expression of pig endogenous retrovirus by primary porcine endothelial cells and infection of human cells. Lancet. 1998;352:692-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 229] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 19. | Shi XL, Gao Y, Yan Y, Ma H, Sun L, Huang P, Ni X, Zhang L, Zhao X, Ren H. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26:206-216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18205] [Article Influence: 958.2] [Reference Citation Analysis (0)] |
| 21. | Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1445] [Cited by in RCA: 1404] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 22. | Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27:806-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 305] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 23. | Hirami Y, Osakada F, Takahashi K, Okita K, Yamanaka S, Ikeda H, Yoshimura N, Takahashi M. Generation of retinal cells from mouse and human induced pluripotent stem cells. Neurosci Lett. 2009;458:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 300] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 24. | Kärner E, Unger C, Cerny R, Ahrlund-Richter L, Ganss B, Dilber MS, Wendel M. Differentiation of human embryonic stem cells into osteogenic or hematopoietic lineages: a dose-dependent effect of osterix over-expression. J Cell Physiol. 2009;218:323-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Zhang J, Wilson GF, Soerens AG, Koonce CH, Yu J, Palecek SP, Thomson JA, Kamp TJ. Functional cardiomyocytes derived from human induced pluripotent stem cells. Circ Res. 2009;104:e30-e41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1045] [Cited by in RCA: 983] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 26. | Taura D, Noguchi M, Sone M, Hosoda K, Mori E, Okada Y, Takahashi K, Homma K, Oyamada N, Inuzuka M. Adipogenic differentiation of human induced pluripotent stem cells: comparison with that of human embryonic stem cells. FEBS Lett. 2009;583:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 27. | Tateishi K, He J, Taranova O, Liang G, D’Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem. 2008;283:31601-31607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 28. | Zhang D, Jiang W, Liu M, Sui X, Yin X, Chen S, Shi Y, Deng H. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 415] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 29. | Taura D, Sone M, Homma K, Oyamada N, Takahashi K, Tamura N, Yamanaka S, Nakao K. Induction and isolation of vascular cells from human induced pluripotent stem cells--brief report. Arterioscler Thromb Vasc Biol. 2009;29:1100-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 30. | Choi KD, Yu J, Smuga-Otto K, Salvagiotto G, Rehrauer W, Vodyanik M, Thomson J, Slukvin I. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 417] [Cited by in RCA: 311] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 31. | Song Z, Cai J, Liu Y, Zhao D, Yong J, Duo S, Song X, Guo Y, Zhao Y, Qin H. Efficient generation of hepatocyte-like cells from human induced pluripotent stem cells. Cell Res. 2009;19:1233-1242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 366] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 32. | Sullivan GJ, Hay DC, Park IH, Fletcher J, Hannoun Z, Payne CM, Dalgetty D, Black JR, Ross JA, Samuel K. Generation of functional human hepatic endoderm from human induced pluripotent stem cells. Hepatology. 2010;51:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 313] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 33. | Si-Tayeb K, Noto FK, Nagaoka M, Li J, Battle MA, Duris C, North PE, Dalton S, Duncan SA. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 2010;51:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 944] [Cited by in RCA: 953] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 34. | Rashid ST, Corbineau S, Hannan N, Marciniak SJ, Miranda E, Alexander G, Huang-Doran I, Griffin J, Ahrlund-Richter L, Skepper J. Modeling inherited metabolic disorders of the liver using human induced pluripotent stem cells. J Clin Invest. 2010;120:3127-3136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 443] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 35. | Peterson SE, Garitaonandia I, Loring JF. The tumorigenic potential of pluripotent stem cells: What can we do to minimize it? Bioessays. 2016;38 Suppl 1:S86-S95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F. An estimation of the number of cells in the human body. Ann Hum Biol. 2013;40:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 719] [Cited by in RCA: 557] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 37. | Haraguchi Y, Matsuura K, Shimizu T, Yamato M, Okano T. Simple suspension culture system of human iPS cells maintaining their pluripotency for cardiac cell sheet engineering. J Tissue Eng Regen Med. 2015;9:1363-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 38. | De Bruyn T, Chatterjee S, Fattah S, Keemink J, Nicolaï J, Augustijns P, Annaert P. Sandwich-cultured hepatocytes: utility for in vitro exploration of hepatobiliary drug disposition and drug-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2013;9:589-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 39. | Fukuda J, Nakazawa K. Orderly arrangement of hepatocyte spheroids on a microfabricated chip. Tissue Eng. 2005;11:1254-1262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 95] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Rashidi H, Alhaque S, Szkolnicka D, Flint O, Hay DC. Fluid shear stress modulation of hepatocyte-like cell function. Arch Toxicol. 2016;90:1757-1761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |