Published online Jan 7, 2017. doi: 10.3748/wjg.v23.i1.141
Peer-review started: September 30, 2016
First decision: December 2, 2016
Revised: December 9, 2016
Accepted: December 21, 2016
Article in press: December 21, 2016
Published online: January 7, 2017
Processing time: 98 Days and 6.3 Hours
To evaluate the effect of sitagliptin vs placebo on histologic and non-histologic parameters of non-alcoholic steatohepatitis (NASH).
Twelve patients with biopsy-proven NASH were randomized to sitagliptin (100 mg daily) (n = 6) or placebo (n = 6) for 24 wk. The primary outcome was improvement in liver fibrosis after 24 wk. Secondary outcomes included evaluation of changes in NAFLD activity score (NAS), individual components of NAS (hepatocyte ballooning, lobular inflammation, and steatosis), glycemic control and insulin resistance [including measurements of glycated hemoglobin (HbA1C) and adipocytokines], lipid profile including free fatty acids, adipose distribution measured using magnetic resonance imaging (MRI), and thrombosis markers (platelet aggregation and plasminogen activator inhibitor 1 levels). We also sought to determine the correlation between changes in hepatic fat fraction (%) [as measured using the Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation (IDEAL) MRI technique] and changes in hepatic steatosis on liver biopsy.
Sitagliptin was not significantly better than placebo at reducing liver fibrosis score as measured on liver biopsy (mean difference between sitagliptin and placebo arms, 0.40, P = 0.82). There were no significant improvements evident with the use of sitagliptin vs placebo for the secondary histologic outcomes of NAS total score as well as for the individual components of NAS. Compared to baseline, those patients who received sitagliptin demonstrated improved HbA1C (6.7% ± 0.4% vs 7.9% ± 1.0%, P = 0.02), and trended towards improved adiponectin levels (4.7 ± 3.5 μg/mL vs 3.9 ± 2.7 μg/mL, P = 0.06) and triglyceride levels (1.26 ± 0.43 mmol/L vs 2.80 ± 1.64 mmol/L, P = 0.08). However, when compared with placebo, sitagliptin did not cause a statistically significant improvement in HbA1C (mean difference, -0.7%, P = 0.19) nor triglyceride levels (mean difference -1.10 mmol/L, P = 0.19) but did trend towards improved adiponectin levels only (mean difference, 0.60 μg/mL, P = 0.095). No significant changes in anthropometrics, liver enzymes, other adipocytokines, lipid profile, thrombosis parameters, or adipose distribution were demonstrated. The MRI IDEAL procedure correlated well with steatosis scores obtained on liver biopsy in both groups at baseline and post-treatment, and the Spearman correlation coefficients ranged from r = 0.819 (baseline) to r = 0.878 (post-treatment), P = 0.002.
Sitagliptin does not improve fibrosis score or NAS after 24 wk of therapy. The MRI IDEAL technique may be useful for non-invasive measurement of hepatic steatosis.
Core tip: Presently, there is no approved medical therapy for non-alcoholic steatohepatitis (NASH). In this randomized placebo-controlled trial, the effect of sitagliptin on liver fibrosis in patients with NASH after 24 wk was evaluated. There was no significant improvement with the use of sitagliptin on liver fibrosis, total non-alcoholic fatty liver disease activity score or its individual components. Similarly, there were no significant improvements in liver enzymes, adipocytokines, lipid profile, thrombosis parameters, or adipose distribution. There was a strong correlation between hepatic fat % measured using the MRI IDEAL technique and hepatic steatosis on liver biopsy.
- Citation: Joy TR, McKenzie CA, Tirona RG, Summers K, Seney S, Chakrabarti S, Malhotra N, Beaton MD. Sitagliptin in patients with non-alcoholic steatohepatitis: A randomized, placebo-controlled trial. World J Gastroenterol 2017; 23(1): 141-150
- URL: https://www.wjgnet.com/1007-9327/full/v23/i1/141.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i1.141
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease in the United States, affecting approximately 95 million adults[1-3]. The spectrum of disease ranges from simple steatosis to steatohepatitis with or without fibrosis (NASH)[4]. The pathogenesis of NASH has been associated with not only insulin resistance, metabolic syndrome, and diabetes but also oxidative stress and lipotoxicity[3]. Approximately 50%-70% of patients with type 2 diabetes (DM2) have hepatic steatosis[5,6]. More importantly, those with DM2 and/or insulin resistance are at a greater risk and have a greater likelihood for progression of NASH[7]. Although lifestyle modification is the mainstay of treatment, achievement and/or maintenance of dietary goals and weight loss is often difficult[3,8].
Currently, there is no approved pharmacologic agent for the management of NASH. Given the importance of insulin resistance, several anti-diabetic agents have been investigated in NASH but have yielded variable outcomes[9-14]. Sitagliptin is an oral antidiabetic agent that inhibits dipeptidyl peptidase IV (DPP-IV), a naturally occurring enzyme that degrades the incretins - glucagon-like peptide 1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP). GLP-1 and GIP are hormones secreted from the gastrointestinal system in response to food intake and cause increased insulin secretion and suppressed glucagon secretion, resulting in improved serum glucose levels[15]. There are two available incretin-based anti-diabetic classes - GLP-1 analogues and DPP-IV inhibitors, both of which are being actively investigated for NASH.
DPP-IV activity correlates with hepatic steatosis and NASH grading as well as with markers of liver damage such as gamma glutamyl transferase (GGT) and alanine aminotransferase (ALT) levels[16,17]. In rodents, reduced DPP-IV levels are associated with reduced lipogenesis and decreased hepatic steatosis[18,19]. Thus, DPP-IV itself may be important to the pathogenesis of NASH. Use of the DPP-IV inhibitor, sitagliptin, improves lipid metabolism, and attenuates the progression of hepatic fibrosis in mice with NASH, as well as decreases platelet aggregation in vitro[20,21]. Thus, sitagliptin may be an attractive therapeutic option for NASH, especially given its low risk of hypoglycemia, weight-neutrality, and demonstrated safety profile in individuals with moderate hepatic insufficiency[22,23].
In uncontrolled human studies, sitagliptin improved serum ALT, aspartate aminotransferase (AST), and GGT levels in patients with DM2 and NASH[24] as well as significantly decreased hepatocyte ballooning and NASH scores[25]. Recently, a randomized placebo-controlled trial[26] examining 24 wk of sitagliptin therapy in patients with non-alcoholic fatty liver disease demonstrated no significant improvement in liver fat as measured using magnetic resonance imaging (MRI). However, liver biopsies were not performed in that trial.
Thus, the aim of this randomized placebo-controlled trial was to evaluate the efficacy of sitagliptin vs placebo in patients with DM2 and biopsy-proven NASH in reducing liver fibrosis histologically using paired liver biopsies. Additionally, we evaluated the effects of sitagliptin on NAFLD activity score (NAS), mediators of insulin resistance (adipocytokines and adipose distribution), lipid profile and thrombosis parameters. We also aimed to examine the use of MRI-derived hepatic fat fraction as a surrogate for histologic assessment of hepatic steatosis.
We conducted an investigator-initiated, randomized, double-blinded, allocation-concealed, placebo-controlled clinical trial of 24 wk’ duration of sitagliptin 100 mg daily versus placebo in patients with DM2 and biopsy-confirmed NASH. Participants were recruited from the Endocrinology and Gastroenterology outpatient clinics at St. Joseph’s Hospital and London Health Sciences Centre, respectively, at Western University. The trial was registered at http://www.clinicaltrials.gov (registration number: NCT01260246). The clinical trial was approved by the Western University Research Ethics Board (REB No. 17389), and all patients provided written informed consent.
Inclusion criteria included age 18 years or older, established diagnosis of type 2 diabetes on lifestyle management alone or with approved treatment (see exclusions below) with a hemoglobin A1C (HbA1C) of 7.1%-8.9%, and a diagnosis of NASH based on the American Association for the Study of Liver Disease criteria[3] including histological evidence of NASH on the basis of pre-randomization liver biopsy. Patients were excluded for substantial alcohol consumption (> 20 g/d for women or > 30 g/d for men); prior exposure to DPP-IV inhibitor, GLP-1 analogue, or thiazolidinedione; Child’s class B or C cirrhosis; any contraindication for MRI; any contraindication for liver biopsy; current or prior use of medications that can induce steatohepatitis; participation in another clinical trial; prior history of pancreatitis; pregnancy, breastfeeding, or intention to become pregnant.
The St. Joseph’s Hospital clinical trial pharmacy team randomized patients into either sitagliptin or placebo groups 1:1, stratified by gender, using computer-generated numbers. Blinding and allocation concealment was maintained by use of identical-looking bottles and capsules, in which sitagliptin or placebo were compounded by the hospital pharmacy. Physicians and all other study personnel were also blinded to drug allocation. Unblinding of treatment allocation was done only after all study procedures were completed in all study patients.
After screening, patients underwent a baseline assessment (Visit 1) including medical history; physical examination; documentation of anthropometric measures [weight, waist-to-hip ratio, and body mass index (BMI)]; blood work for glycemic control (HbA1C), markers of insulin resistance [homeostasis model of assessment for insulin resistance (HOMA-IR)] (which used fasting glucose and insulin levels) and adipocytokines [adiponectin, adipsin, leptin, resistin, visfatin, interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), free fatty acids (FFA), lipid profile [total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), liver parameters [AST, ALT, alkaline phosphatase (Alk Phos), GGT] markers of thrombosis [platelet aggregation and plasminogen activator inhibitor 1 (PAI-1)]; and MRI assessment of adipose distribution[% fat in the liver, visceral adipose tissue (VAT), subcutaneous abdominal adipose tissue (SAAT), and subcutaneous peripheral adipose tissue (SPAT) of the left thigh].
After randomization, patients returned for study visits at weeks 12 (Visit 2) and 24 (Visit 3). At visit 2, adverse effects were noted, and compliance was assessed using pill count. Anthropometric measures and blood work for HbA1C, fasting glucose and insulin, FFA, lipid profile, and liver parameters were measured. Visit 3 study procedures included documentation of adverse effects and compliance as well as all study procedures as visit 1, including a repeat liver biopsy.
Histologic evaluation: Ultrasonography-guided percutaneous liver biopsies were obtained from all subjects prior to initiation of therapy and at completion of the study. All biopsy specimens were placed in formalin solution for fixation and embedded in paraffin blocks. An independent liver histopathologist (SC) who was blinded to study treatment allocation and clinical or laboratory information assessed baseline and end-of-treatment liver biopsies. The grade and stage of liver disease severity was assessed according to the scoring system proposed by the NASH Clinical Research Network[27]. This was recorded as the sum of the scores for steatosis (0-3), lobular inflammation (0-3) and ballooning (0-2). Fibrosis (0-4) was scored separately. For the purposes of analysis, fibrosis stages 1a, 1b, and 1c were considered as stage 1. A diagnosis of NASH required the presence of steatohepatitis (NAS ≥ 3) with a hepatocyte ballooning score of ≥ 1 and fibrosis score of ≥ 1.
MRI: MRI examinations were performed using 3 Tesla Discovery MR750 MRI (General Electric). Quantification of adiposity in the hepatic, VAT, SAAT, and thigh SPAT depots used the Iterative Decomposition of water and fat with Echo Asymmetry and Least-squares estimation (IDEAL) procedure[28], a computer-based quantification method separating fat and water signals in MRI images. Excellent correlation (r2 = 0.99, slope = 1.00 ± 0.01) for hepatic fat quantification using IDEAL vs the gold standard method, magnetic resonance spectroscopy (MRS) has been previously established[29-32]. Regions of interest (ROIs) were placed in vessel free regions of the lower right lobe of the liver to obtain water-only and fat-only images. Then, hepatic fat-fraction (HFF) was obtained [HFF (%) = fat/(water + fat) × 100] and mean fat fraction calculated[32].
Left thigh SPAT (%) was quantified using ImageJ version 1.34 n image analysis software, specifically utilizing the Connected Threshold Grower and Voxel Counter tools. Percent adipose tissue was calculated by dividing the total voxels determined for fat intensity signals connected to the subcutaneous adipose seed point by the total voxels for the slice. Similarly, single slices (1 cm) at the L4 region were obtained to quantify VAT and SAAT using these techniques, as previously published by our group[33,34].
Biochemical parameters: Blood work for fasting glucose, insulin, HbA1C, FFA, AST, ALT, Alk Phos, GGT, and lipid profile were collected and analyzed per standard hospital procedures within our hospital core laboratory. The analytes (adiponectin, adipsin, resistin, leptin, visfatin IL-6, TNF-α, PAI-1) were measured from venous blood samples collected in BDTM P800 EDTA tubes pre-coated with general protease inhibitors (to allow for accurate measurement). Samples were centrifuged and stored at -80 °C until thawing for grouped analysis at the completion of the study. They were analyzed using the Human Diabetes Bio-Plex Panel and a Bio-PlexTM 200 readout System (Bio-Rad Laboratories, CA, United States), which utilizes Luminex® xMAPTM multiplexed immunoassay technology (Luminex Corp., TX, United States). Levels of analytes were automatically calculated from standard curves using Bio-Plex Manager software (v.4.1.1, Bio-Rad). VerifyNow-P2Y12 (Accumetrics, CA, United States) is a rapid platelet-function cartridge-based assay that was used to directly measure platelet aggregation[35]. The data are expressed as platelet reaction units (PRU) (ref. range 194-418).
The primary outcome was improvement in liver fibrosis on histology from baseline to end of treatment. Secondary histological outcomes included changes in overall NAS and individual components of NAS (steatosis, hepatocyte ballooning, and lobular inflammation)[27]. Other secondary outcome measures included changes from baseline to 24 wk in serum liver enzyme concentrations, fasting lipid and FFA concentrations, measures of insulin resistance (adipocytokines and HOMA-IR), glycemic control (HbA1C), thrombosis (platelet aggregation and PAI-1 levels), and adipose distribution.
Statistical review of the study was completed by a biomedical statistician (LS). The sample size was calculated based on a mean difference in fibrosis score[27] between pre and post conditions of -0.55, or a decrease of slightly more than one half score on 4 point scale (SD of difference = 0.68). Using the methods of Cohen[36], a sample size of 16 patients would provide 80% power to detect at least this difference with alpha (2-tailed) = 5%. We anticipated a 25% drop out rate, and thus our recruitment target was increased to 20 individuals.
All evaluable patients who underwent an end-of-treatment biopsy at week 24 were included in the modified intent-to-treat analysis. All data are expressed as mean ± SD (standard deviation) for normally distributed data and median (interquartile range) for non-normally distributed data. Comparisons between sitagliptin and placebo were made using unpaired t-tests or Wilcoxon two sample tests for continuous variables and χ2 tests or, where expected counts were less than five, Fisher’s exact test for categorical variables. The association between liver steatosis and MRI assessment for hepatic fat was evaluated using Spearman rank correlations. A two-tailed P value ≤ 0.05 was considered statistically significant. Statistical analysis was performed using SAS 9.4 (Cary NC, United States).
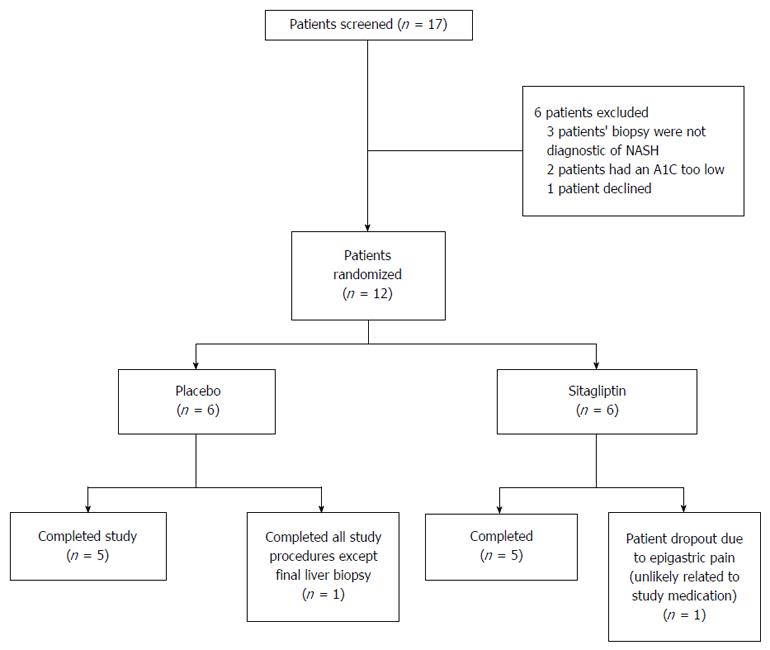
Between September 2011 and August 2014, we randomly assigned 12 patients with histologically confirmed NASH to receive sitagliptin (n = 6) or placebo (n = 6) (Figure 1). One patient from the sitagliptin arm withdrew from the study due to epigastric pain (see below), and one patient from the placebo arm completed all end of study procedures except the liver biopsy. In the participants who completed the trial, compliance was above 95% in both arms. Participants were predominantly female (66% vs 50%, sitagliptin vs placebo, P = 1.00), with similar durations of diabetes and NASH, and similar fibrosis score and NAS (Table 1).
| Placebo (n = 6) | Sitagliptin (n = 6) | |
| Demographics | ||
| Age (yr) | 54.7 ± 9.8 | 56.7 ± 9.9 |
| Gender - male, n (%) | 2 (33) | 3 (50) |
| White, n (% | 6 (100 | 5 (83) |
| Weight (kg) | 105.8 ± 23.5 | 100.4 ± 28.7 |
| BMI (kg/m2) | 37.4 ± 4.7 | 35.9 ± 6.6 |
| Diabetes duration (yr)1 | 6.0 (5.0, 6.0) | 6.5 (5.0, 23.0) |
| NASH Duration (yr)1 | 4.0 (1.0, 4.0) | 1.3 (0.1, 2.0) |
| Biochemical profile | ||
| HbA1C (%) | 8.2 ± 0.9 | 7.9 ± 1.0 |
| HOMA-IR | 3.5 ± 2.2 | 3.4 ± 1.2 |
| Fasting glucose (mmol/L) | 11.3 ± 4.9 | 8.1 ± 3.1 |
| Fasting insulin (pmol/L) | 165 ± 134 | 168 ± 63 |
| AST (IU/L) | 39 ± 19 | 44 ± 22 |
| ALT (IU/L) | 46 ± 36 | 72 ± 50 |
| Alkaline phosphatase (IU/L) | 85 ± 34 | 93 ± 41 |
| GGT (IU/L) | 100 ± 98 | 164 ± 182 |
| Triglycerides (mmol/L) | 2.33 ± 2.00 | 2.80 ± 1.64 |
| HDL-C (mmol/L) | 1.10 ± 0.34 | 1.12 ± 0.46 |
| LDL-C (mmol/L) | 1.61 ± 0.71 | 1.42 ± 0.97 |
| Free fatty acids (μmol/L) | 645 ± 195 | 559 ± 158 |
| Platelet aggregation (PRU) | 292 ± 37 | 236 ± 28 |
| Histologic profile | ||
| Fibrosis | 2.2 ± 0.8 | 2.2 ± 1.0 |
| NAS | 4.2 ± 1.5 | 3.8 ± 0.8 |
| Steatosis | 1.8 ± 0.8 | 1.8 ± 0.8 |
| Lobular Inflammation | 1.2 ± 0.4 | 1.0 ± 0 |
| Ballooning | 1.2 ± 0.4 | 1.0 ± 0 |
| MRI | ||
| Hepatic fat (%) | 21.9 ± 10.6 | 19.0 ± 9.7 |
| SAAT (%) | 40.5 ± 7.1 | 38.0 ± 22.0 |
| VAT (%) | 30.5 ± 3.4 | 26.8 ± 11.6 |
| Left thigh fat (%) | 37.8 ± 9.9 | 39.5 ± 15.8 |
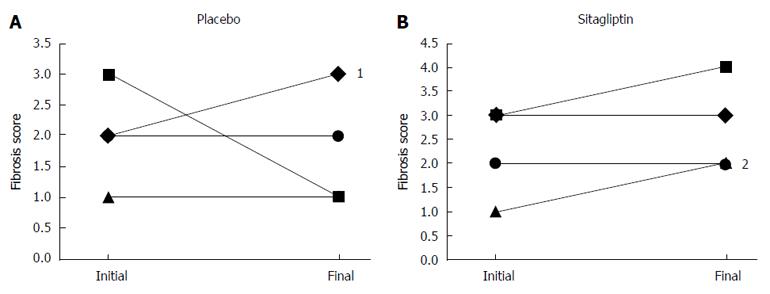
Sitagliptin was not significantly better than placebo at reducing liver fibrosis score as measured on liver biopsy (mean difference between sitagliptin and placebo arms, 0.40, P = 0.82) (Table 2). Individual patient data on changes in liver fibrosis stratified by treatment group are shown in Figure 2. There were no significant improvements evident with the use of sitagliptin vs placebo for the secondary histologic outcomes of NAS total score as well as for the individual components of NAS (Table 2).
| Placebo (n = 6) | Sitagliptin (n = 6) | Difference | ||||||
| Baseline | Post-treatment1 | P value | Baseline | Post-treatment1 | P value | (95%CI) | P value | |
| Primary outcome | ||||||||
| Fibrosis | 2.2 ± 0.8 | 2.0 ± 1.0 | 0.85 | 2.2 ± 1.0 | 2.4 ± 1.1 | 0.85 | 0.4 (-0.98, 1.78) | 0.82 |
| Secondary outcomes | ||||||||
| NAS | 4.2 ± 1.5 | 3.8 ± 1.9 | 0.50 | 3.8 ± 0.8 | 3.4 ± 1.5 | 0.85 | 0.2 (-1.62, 2.02) | 1.00 |
| Steatosis | 1.8 ± 0.8 | 1.6 ± 0.9 | 0.62 | 1.8 ± 0.8 | 1.4 ± 0.9 | 0.55 | 0 (-1.08, 1.08) | 0.91 |
| Hepatocyte ballooning | 1.2 ± 0.4 | 1.4 ± 0.9 | 0.89 | 1.0 ± 0 | 0.8 ± 0.4 | 0.36 | -0.40 (-1.05, 0.25) | 0.23 |
| Lobular inflammation | 1.2 ± 0.4 | 0.8 ± 0.4 | 0.22 | 1.0 ± 0 | 1.2 ± 0.4 | 0.36 | 0.60 (-0.13, 1.33) | 0.12 |
Compared to baseline, those patients who received sitagliptin demonstrated improved glycemic control (HbA1C) (6.7% ± 0.4% vs 7.9% ± 1.0%, P = 0.02), and trended towards improved adiponectin levels (4.7 ± 3.5 μg/mL vs 3.9 ± 2.7 μg/mL, P = 0.06) and triglyceride levels (1.26 ± 0.43 mmol/L vs 2.80 ± 1.64 mmol/L, P = 0.08) (Table 3). However, when compared with placebo, sitagliptin did not cause a statistically significant improvement in HbA1C (mean difference, -0.7%, P = 0.19) nor triglyceride levels (mean difference -1.10 mmol/L, P = 0.19) but did trend towards improved adiponectin levels (mean difference, 0.60 μg/mL, P = 0.095). No significant changes in anthropometrics, liver enzymes, adipocytokines, lipid parameters, thrombosis, or adipose distribution were demonstrated (Table 3).
| Placebo (n = 6) | Sitagliptin (n = 6) | Difference | |||
| Baseline | Post-treatment | Baseline | Post-treatment1 | (95%CI) | |
| Anthropometric and biochemical parameters | |||||
| Weight (kg) | 105.8 ± 23.5 | 104.7 ± 23.7 | 100.4 ± 28.7 | 101.1 ± 32.4 | 0.8 (-6.3, 8.0) |
| BMI (kg/m2) | 37.4 ± 4.7 | 36.8 ± 4.6 | 35.9 ± 6.6 | 35.8 ± 8.0 | 0.6 (-2.8, 4.0) |
| HbA1C (%) | 8.2 ± 0.9 | 8.0 ± 1.7 | 7.9 ± 1.0 | 6.7 ± 0.4a | -0.7 (-1.7, 0.4) |
| HOMA-IR | 3.5 ± 2.2 | 2.73 ± 1.631 | 3.4 ± 1.2 | 3.5 ± 2.9 | -0.36 (-3.3,2.6) |
| Triglycerides (mmol/L) | 2.33 ± 2.00 | 2.29 ± 1.40 | 2.80 ± 1.64 | 1.26 ± 0.43b | -1.10 (-2.88, 0.66) |
| HDL-C (mmol/L) | 1.10 ± 0.34 | 1.11 ± 0.29 | 1.12 ± 0.46 | 1.12 ± 0.40 | -0.05 (-0.32, 0.22) |
| LDL-C (mmol/L) | 1.61 ± 0.71 | 1.42 ± 0.34 | 1.42 ± 0.97 | 1.54 ± 0.51 | 0.30 (-0.17, 0.77) |
| Free fatty acids (μmol/L) | 645 ± 195 | 914 ± 411 | 559 ± 158 | 488 ± 270 | -143 (-788, 503) |
| Platelet aggregation (PRU) | 292 ± 37 | 266 ± 63 | 236 ± 28 | 251 ± 74 | -11 (-92, 70) |
| AST (IU/L) | 39 ± 19 | 42 ± 23 | 44 ± 22 | 35 ± 9 | -5 (-36, 26) |
| ALT (IU/L) | 46 ± 36 | 48 ± 28 | 72 ± 50 | 51 ± 15 | -5 (-51, 41) |
| Alk phos (IU/L) | 85 ± 34 | 97 ± 43 | 93 ± 41 | 76 ± 28 | -14 (-36, 7) |
| GGT (IU/L) | 100 ± 98 | 153 ± 176 | 164 ± 182 | 90 ± 76 | -62 (-164, 39) |
| Adiponectin (μg/mL) | 2.01 ± 1.30 | 2.09 ± 1.14 | 3.93 ± 2.65 | 4.70 ± 3.46b | 0.60 (-0.13, 1.32)c |
| Adipsin (μg/mL) | 0.62 ± 0.26 | 0.61 ± 0.26 | 0.66 ± 0.14 | 0.62 ± 0.07 | -0.07 (-0.26, 0.13) |
| Visfatin (ng/mL) | 3.21 ± 3.64 | 3.94 ± 3.59 | 1.82 ± 2.33 | 1.70 ± 1.08 | -0.58 (-3.26, 2.09) |
| Leptin (ng/mL) | 14.62 ± 12.52 | 17.64 ± 16.20 | 9.61 ± 3.29 | 8.87 ± 5.54 | -3.5 (-8.6, 1.6) |
| Resistin (ng/mL) | 3.66 ± 1.01 | 4.31 ± 1.66 | 7.19 ± 8.66 | 4.55 ± 1.73 | -4.05 (-11.33, 2.34) |
| TNF-α (pg/mL) | 3.69 ± 1.22 | 4.95 ± 4.79 | 2.85 ± 0.66 | 5.14 ± 3.20 | 0.95 (-4.70, 6.61) |
| IL-6 (pg/mL) | 5.89 ± 1.37 | 6.19 ± 2.37 | 7.15 ± 6.22 | 6.40 ± 3.41 | -1.44 (-7.76, 4.87) |
| MRI parameters | |||||
| Hepatic fat (%) | 21.9 ± 10.6 | 19.1 ± 9.6 | 19.0 ± 9.7 | 16.1 ± 12.9 | 2.0 (-7.3, 11.2) |
| SAAT (%) | 40.5 ± 7.1 | 39.8 ± 7.7 | 38.0 ± 22.0 | 34.1 ± 20.1 | 0.7 (-2.3, 3.7) |
| VAT (%) | 30.5 ± 3.4 | 30.3 ± 4.4 | 26.8 ± 11.6 | 27.6 ± 13.4 | 0 (-6.4, 6.4) |
The MRI IDEAL technique correlated well with steatosis scores obtained on liver biopsy in both groups at baseline and post-treatment, and the Spearman correlation coefficients ranged from r = 0.819 (baseline) to r = 0.878 (post-treatment), P = 0.002.
Treatment with sitagliptin was well tolerated. A total of 3 patients had adverse events. Two weeks after randomization, one patient developed a serious adverse event of a right subdural hemorrhage, manifest as left leg weakness and numbness, most likely caused by treatment with his blood thinner (warfarin) for atrial fibrillation. He elected to continue in the trial with blinded medication and was deemed to have had an event unrelated to study medication. A second patient had a non-serious adverse event of low back pain and right foot pain, diagnosed as lumbar spondylolysis, which resolved within 48 h of oral analgesic use. The adverse event was deemed unrelated to study medication and did not result in discontinuation of study medication as well. The third patient developed epigastric pain approximately 1 month after starting sitagliptin. She was hospitalized for her symptoms; pancreatitis was ruled out. The final discharge diagnosis was possible gastritis. She had a history of epigastric pain occurring 1 to 3 times per year for the 4 years prior to her study entry. She withdrew from the trial at the time of her hospitalization, and had another bout of epigastric discomfort 1 mo following her hospitalization. Although she was classified as having a serious adverse event, it was deemed by the investigators that it was unlikely related to the study medication.
In this randomized double-blinded placebo-controlled trial, sitagliptin did not significantly improve liver fibrosis or any parameter of NAS after 24 wk of therapy. Those receiving sitagliptin trended towards having improved adiponectin levels, but no other improvements in parameters of insulin resistance, adipose distribution, thrombosis, liver enzymes, or lipid profile. This trial however did show a strong correlation between hepatic steatosis on liver biopsy and hepatic fat fraction measured using MRI with the IDEAL technique, thus providing further data regarding the validity of this non-invasive technique in assessing and monitoring changes in hepatic steatosis in patients with NASH.
This study has a number of strengths. This is the first randomized, placebo-controlled trial to report the effect of a DPP-IV inhibitor on liver histology in patients with NASH. We utilized a single, expert histopathologist who remained blinded to study treatment as well as laboratory data throughout the study. Thus, he was able to provide unbiased determinations of histologic features. Second, all patients included in this trial had histologically-proven NASH, were extensively phenotyped and well-matched for baseline features. Third, to the best of our knowledge this is the first trial to document the validity of the IDEAL technique for MRI in relation to paired liver biopsies assessing histologic changes in hepatic steatosis. And finally, the trial was conducted by experienced investigators from multidisciplinary backgrounds which allowed for us to examine the effect of sitagliptin on a number of important outcomes related to the pathogenesis of NASH (insulin resistance, lipid parameters) in a rigidly executed placebo-controlled trial.
In rodent models, sitagliptin use has resulted in improved features of NASH[20,37,38]. In humans, randomized controlled trials examining the effect of sitagliptin on liver histology has not previously been examined. Several studies have instead examined changes in liver enzymes as a marker of NASH but effects have not been consistent[24,26,39,40] Recently, a randomized double-blinded placebo-controlled trial of 50 patients by Cui et al[26] demonstrated no improvement in hepatic steatosis using the MRI-based biomarker of proton density-fat fraction (MRI-PDFF), following 24 wk of sitagliptin therapy. Our results further extend these findings by demonstrating no improvement in the histologic features of NASH with sitagliptin in patients with histologically-proven NASH. These results are in contrast with those of Yilmaz et al[25], where sitagliptin demonstrated improved NAS and hepatocyte ballooning with a trend towards improved hepatic steatosis, after 1 year of therapy in 15 patients. However, the latter trial was open-label without a comparator arm.
Our data are similar to a larger randomized controlled trial examining the effects of another incretin-based antidiabetic agent, the GLP-1 analogue liraglutide. Armstrong et al[14] randomized 52 patients to liraglutide or placebo for 48 wk to determine the effects on liver histology, liver enzymes, FFA, lipid parameters. Their primary endpoint was improvement in liver histology (defined as disappearance of hepatocyte ballooning without worsening of fibrosis) and their secondary histologic endpoints were changes in NAS and fibrosis score. Although they demonstrated improvement in the primary endpoint, they did not demonstrate any improvements in NAS, fibrosis score, HOMA-IR, FFA, or liver enzymes (apart from GGT). Thus, improvement in liver histology or liver enzymes may be difficult to achieve with incretin-based agents, although it is possible that a longer treatment period beyond 48 wk may be required to document an effect.
While the HbA1C mean difference between sitagliptin vs placebo of -0.7% did not reach statistical significance, the result is consistent with the expected drop in HbA1C following the addition of sitagliptin to patients with DM2 having a mean baseline HbA1C of 8.0%[41]. This demonstrates that sitagliptin did indeed improve glycemic control to the expected degree from published data. Furthermore, this effect was accompanied by a trend towards improved adiponectin levels, an adipocytokine associated with insulin sensitivity, suggesting that improvement in insulin resistance is still possible with sitagliptin even in patients with NASH. Since high levels of VAT and low levels of SPAT correlate with insulin resistance, we examined adipose distribution as one of our secondary outcomes. Our results demonstrating no improvement in VAT or SPAT with sitagliptin are in contrast to those by Lima-Martínez et al[42], who demonstrated decreases in VAT of 12% after 24 wk of sitagliptin therapy. However, the latter trial was open-label without a comparator arm, and utilized a less precise technique (bioimpedance analyzer) compared to MRI to document VAT.
The hepatic fat % did not improve significantly with the use of sitagliptin vs placebo, as evident also in the trial by Cui et al[26]. However, the MRI IDEAL technique did have high correlation with hepatic steatosis on histology. This technique, while having been previously validated with MRS[29-32], is certainly much more feasible than MRS. This trial therefore supports that hepatic fat fraction measurements through the IDEAL technique may be an accurate non-invasive method to longitudinally monitor hepatic steatosis changes in patients with NASH.
Our study has some important limitations. Firstly, our sample size was small. This may have affected our ability to assess for changes in the outcomes measured as well as the validity of MRI IDEAL technique. For the primary histologic outcome, the mean potentially detectable difference in fibrosis score with our attained sample size was 0.8. However, given that our negative findings were supported by the trials by Cui et al[26] and Armstrong et al[14], it is unlikely that a true histologic improvement with 24 wk of sitagliptin therapy has been missed. Certainly, a larger sample size and longer duration of therapy would be helpful to address this limitation. Secondly, this study enrolled patients with milder NASH compared to other studies. Our baseline NAS in the sitagliptin arm was 3.8 ± 0.8, slightly lower than the mean scores in the studies by Armstrong et al[14] and the observational study by Yilmaz et al[25], where the mean baseline scores were 4.9 ± 0.9 and 5.6 ± 1.6, respectively. Thus, whether sitagliptin would have impacted histologic and non-histologic outcomes differently in patients with more advanced histologic NASH severity remains uncertain. Although our results lend support to the use of MRI as a non-invasive technique in patients with NASH, additional multicenter trials are required to assess the utility of the IDEAL technique for measuring longitudinal changes in hepatic steatosis. Regardless, the current data allow our centre to use the IDEAL technique for future research in NASH patients to monitor hepatic steatosis. With the emergence of additional novel MR technologies, such as MR Elastography to assess fibrosis, it is likely that in coming years non-invasive means of assessing disease severity and response to therapy may supplant liver biopsy in this condition.
Our trial therefore demonstrates that sitagliptin 100 mg daily for 24 wk, compared to placebo, does not improve histologic features of NASH significantly. Sitagliptin was well-tolerated in patients with NASH and demonstrated the expected improved in glycemic control, as measured through HbA1C. Importantly, we did demonstrate the feasibility and validity of the MRI IDEAL technique for non-invasive measurement of hepatic steatosis longitudinally. Future studies of longer duration, larger sample size and in patients with worse severity of NASH may demonstrate different results and should be considered.
We would like to sincerely thank Leanne Vanderhaeghe and Christopher Reynaert of the Pharmacy Department at St. Joseph’s Hospital for their help with the conduct of this trial, particularly with regards to generation of randomization schemes, purchase and compounding of active and placebo capsules, and dispensing of study medications. Similarly, the invaluable efforts of our research assistant, Patricia Rosas-Arellanos, as well as participation of the patients are greatly appreciated.
This study was undertaken to evaluate the efficacy of an established and effective antidiabetic medication, sitagliptin, on features of nonalcoholic fatty liver disease (NAFLD). As NAFLD and type 2 diabetes share many pathophysiologic mechanisms, including insulin resistance, it was theorized that Sitagliptin may also improve liver disease in NAFLD.
There are presently no approved medical therapies for NAFLD. As such the identification of potential drug therapy for this condition is very important; given it is a very common disorder with the potential to cause significant liver injury.
In this study sitagliptin did not improve liver fibrosis in a small group of NAFLD patients treated for 24 wk. There was a trend toward improvement in adiponectin level, an important hormone in the regulation of glucose regulation and fatty acid oxidation.
Sitagliptin was safe and well tolerated in this population with known liver disease. It is possible that further studies, of larger size and longer duration, may be needed to accurately assess whether a treatment effect may be evident.
NAFLD the presence of hepatic steatosis in the absence of other causes for secondary fat accumulation; Non-alcoholic steatohepatitis - the presence of hepatic steatosis in addition to inflammation and hepatocyte injury with or without fibrosis.
As the authors pointed out sitagliptin did not significantly improve liver fibrosis or any parameter of NAFLD activity score after 24 wk of therapy, and this result is in agreement with previous literature. The major concern, however, from the clinical applicability’s point of view is the size of the sample, which was too small and could have limited the ability to assess for changes in the outcomes measured and broaden the information in relation to identify appropriate surrogates for histologic assessment of hepatic steatosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Lee HC, Ramos-Gomez M, Thompson JR S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Federico A, Zulli C, de Sio I, Del Prete A, Dallio M, Masarone M, Loguercio C. Focus on emerging drugs for the treatment of patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2014;20:16841-16857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1330] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 3. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 4. | Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Creutzfeldt W, Frerichs H, Sickinger K. Liver diseases and diabetes mellitus. Prog Liver Dis. 1970;3:371-407. [PubMed] |
| 6. | Leite NC, Salles GF, Araujo AL, Villela-Nogueira CA, Cardoso CR. Prevalence and associated factors of non-alcoholic fatty liver disease in patients with type-2 diabetes mellitus. Liver Int. 2009;29:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 368] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 7. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2292] [Article Influence: 163.7] [Reference Citation Analysis (0)] |
| 8. | Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 9. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2467] [Article Influence: 164.5] [Reference Citation Analysis (2)] |
| 10. | Loomba R, Lutchman G, Kleiner DE, Ricks M, Feld JJ, Borg BB, Modi A, Nagabhyru P, Sumner AE, Liang TJ. Clinical trial: pilot study of metformin for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2009;29:172-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 222] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 11. | Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 12. | Ratziu V, Charlotte F, Bernhardt C, Giral P, Halbron M, Lenaour G, Hartmann-Heurtier A, Bruckert E, Poynard T. Long-term efficacy of rosiglitazone in nonalcoholic steatohepatitis: results of the fatty liver improvement by rosiglitazone therapy (FLIRT 2) extension trial. Hepatology. 2010;51:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 292] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 13. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1328] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 14. | Armstrong MJ, Gaunt P, Aithal GP, Barton D, Hull D, Parker R, Hazlehurst JM, Guo K, Abouda G, Aldersley MA. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387:679-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1464] [Article Influence: 162.7] [Reference Citation Analysis (1)] |
| 15. | Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17:819-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 918] [Cited by in RCA: 1057] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 16. | Balaban YH, Korkusuz P, Simsek H, Gokcan H, Gedikoglu G, Pinar A, Hascelik G, Asan E, Hamaloglu E, Tatar G. Dipeptidyl peptidase IV (DDP IV) in NASH patients. Ann Hepatol. 2007;6:242-250. [PubMed] |
| 17. | Firneisz G, Varga T, Lengyel G, Fehér J, Ghyczy D, Wichmann B, Selmeci L, Tulassay Z, Rácz K, Somogyi A. Serum dipeptidyl peptidase-4 activity in insulin resistant patients with non-alcoholic fatty liver disease: a novel liver disease biomarker. PLoS One. 2010;5:e12226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 18. | Ben-Shlomo S, Zvibel I, Shnell M, Shlomai A, Chepurko E, Halpern Z, Barzilai N, Oren R, Fishman S. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol. 2011;54:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 19. | Conarello SL, Li Z, Ronan J, Roy RS, Zhu L, Jiang G, Liu F, Woods J, Zycband E, Moller DE. Mice lacking dipeptidyl peptidase IV are protected against obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:6825-6830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 266] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 20. | Onoyama T, Koda M, Okamoto T, Kishina M, Matono T, Sugihara T, Murawaki Y. Therapeutic effects of the dipeptidyl peptidase-IV inhibitor, sitagliptin, on non-alcoholic steatohepatitis in FLS-ob/ob male mice. Mol Med Rep. 2015;12:6895-6902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Gupta AK, Verma AK, Kailashiya J, Singh SK, Kumar N. Sitagliptin: anti-platelet effect in diabetes and healthy volunteers. Platelets. 2012;23:565-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Williams-Herman D, Round E, Swern AS, Musser B, Davies MJ, Stein PP, Kaufman KD, Amatruda JM. Safety and tolerability of sitagliptin in patients with type 2 diabetes: a pooled analysis. BMC Endocr Disord. 2008;8:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Migoya EM, Stevens CH, Bergman AJ, Luo WL, Lasseter KC, Dilzer SC, Davies MJ, Wagner JA, Herman GA. Effect of moderate hepatic insufficiency on the pharmacokinetics of sitagliptin. Can J Clin Pharmacol. 2009;16:e165-e170. [PubMed] |
| 24. | Iwasaki T, Yoneda M, Inamori M, Shirakawa J, Higurashi T, Maeda S, Terauchi Y, Nakajima A. Sitagliptin as a novel treatment agent for non-alcoholic Fatty liver disease patients with type 2 diabetes mellitus. Hepatogastroenterology. 2011;58:2103-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 25. | Yilmaz Y, Yonal O, Deyneli O, Celikel CA, Kalayci C, Duman DG. Effects of sitagliptin in diabetic patients with nonalcoholic steatohepatitis. Acta Gastroenterol Belg. 2012;75:240-244. [PubMed] |
| 26. | Cui J, Philo L, Nguyen P, Hofflich H, Hernandez C, Bettencourt R, Richards L, Salotti J, Bhatt A, Hooker J. Sitagliptin vs. placebo for non-alcoholic fatty liver disease: A randomized controlled trial. J Hepatol. 2016;65:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 27. | Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6807] [Cited by in RCA: 8228] [Article Influence: 411.4] [Reference Citation Analysis (5)] |
| 28. | Reeder SB, Pineda AR, Wen Z, Shimakawa A, Yu H, Brittain JH, Gold GE, Beaulieu CH, Pelc NJ. Iterative decomposition of water and fat with echo asymmetry and least-squares estimation (IDEAL): application with fast spin-echo imaging. Magn Reson Med. 2005;54:636-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 556] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 29. | Reeder SB, Robson PM, Yu H, Shimakawa A, Hines CD, McKenzie CA, Brittain JH. Quantification of hepatic steatosis with MRI: the effects of accurate fat spectral modeling. J Magn Reson Imaging. 2009;29:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 30. | Hines CD, Yu H, Shimakawa A, McKenzie CA, Brittain JH, Reeder SB. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: validation in a fat-water-SPIO phantom. J Magn Reson Imaging. 2009;30:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 178] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 31. | Hines CD, Yu H, Shimakawa A, McKenzie CA, Warner TF, Brittain JH, Reeder SB. Quantification of hepatic steatosis with 3-T MR imaging: validation in ob/ob mice. Radiology. 2010;254:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Meisamy S, Hines CD, Hamilton G, Sirlin CB, McKenzie CA, Yu H, Brittain JH, Reeder SB. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258:767-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 33. | Al-Attar SA, Pollex RL, Robinson JF, Miskie BA, Walcarius R, Little CH, Rutt BK, Hegele RA. Quantitative and qualitative differences in subcutaneous adipose tissue stores across lipodystrophy types shown by magnetic resonance imaging. BMC Med Imaging. 2007;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Alabousi A, Al-Attar S, Joy TR, Hegele RA, McKenzie CA. Evaluation of adipose tissue volume quantification with IDEAL fat-water separation. J Magn Reson Imaging. 2011;34:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Malinin A, Pokov A, Spergling M, Defranco A, Schwartz K, Schwartz D, Mahmud E, Atar D, Serebruany V. Monitoring platelet inhibition after clopidogrel with the VerifyNow-P2Y12(R) rapid analyzer: the VERIfy Thrombosis risk ASsessment (VERITAS) study. Thromb Res. 2007;119:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Cohen J. Statistical Power Analysis for the Behavioural Sciences. New Jersey: Lawrence Erlbaum Associates Inc 1988; . |
| 37. | Akaslan SB, Degertekin CK, Yilmaz G, Cakir N, Arslan M, Toruner FB. Effects of sitagliptin on nonalcoholic fatty liver disease in diet-induced obese rats. Metab Syndr Relat Disord. 2013;11:243-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 38. | Maiztegui B, Borelli MI, Madrid VG, Del Zotto H, Raschia MA, Francini F, Massa ML, Flores LE, Rebolledo OR, Gagliardino JJ. Sitagliptin prevents the development of metabolic and hormonal disturbances, increased β-cell apoptosis and liver steatosis induced by a fructose-rich diet in normal rats. Clin Sci (Lond). 2011;120:73-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Fukuhara T, Hyogo H, Ochi H, Fujino H, Kan H, Naeshiro N, Honda Y, Miyaki D, Kawaoka T, Tsuge M. Efficacy and safety of sitagliptin for the treatment of nonalcoholic fatty liver disease with type 2 diabetes mellitus. Hepatogastroenterology. 2014;61:323-328. [PubMed] |
| 40. | Arase Y, Kawamura Y, Seko Y, Kobayashi M, Suzuki F, Suzuki Y, Akuta N, Kobayashi M, Sezaki H, Saito S. Efficacy and safety in sitagliptin therapy for diabetes complicated by non-alcoholic fatty liver disease. Hepatol Res. 2013;43:1163-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 41. | Charbonnel B, Karasik A, Liu J, Wu M, Meininger G. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 555] [Cited by in RCA: 533] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 42. | Lima-Martínez MM, Paoli M, Rodney M, Balladares N, Contreras M, D’Marco L, Iacobellis G. Effect of sitagliptin on epicardial fat thickness in subjects with type 2 diabetes and obesity: a pilot study. Endocrine. 2016;51:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |