Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2391
Peer-review started: August 5, 2015
First decision: September 29, 2015
Revised: October 23, 2015
Accepted: November 24, 2015
Article in press: November 24, 2015
Published online: February 21, 2016
Processing time: 180 Days and 13.7 Hours
We report a case of a 75-year-old man with cystic micropapillary neoplasm of peribiliary glands detected preoperatively by radiologic examination. Enhanced computed tomography showed a low-density mass 2.2 cm in diameter in the right hepatic hilum and a cystic lesion around the common hepatic duct. Under a diagnosis of perihilar cholangiocarcinoma, right hepatectomy with caudate lobectomy and bile duct resection were performed. Pathological examination revealed perihilar cholangiocarcinoma mainly involving the right hepatic duct. The cystic lesion was multilocular and covered by columnar lining epithelia exhibiting increased proliferative activity and p53 nuclear expression; it also contained foci of micropapillary and glandular proliferation. Therefore, the lesion was diagnosed as a cystic micropapillary neoplasm of peribiliary glands and resembled flat branch-type intraductal papillary mucinous neoplasm of the pancreas. Histological examination showed the lesion was discontinuous with the perihilar cholangiocarcinoma. Immunohistochemistry showed the cystic neoplasm was strongly positive for MUC6 and that the cholangiocarcinoma was strongly positive for MUC5AC and S100P. These results suggest these two lesions have different origins. This case warrants further study on whether this type of neoplasm is associated with concomitant cholangiocarcinoma as observed in pancreatic intraductal papillary mucinous neoplasm with concomitant pancreatic duct adenocarcinoma.
Core tip: This report highlights intraductal papillary neoplasm of bile duct (IPNB) arising from peribiliary glands (PBGs) in a case of perihilar cholangiocarcinoma, which is of special interest because to our knowledge this is the first report of IPNB arising from PBGs with concomitant perihilar cholangiocarcinoma occurred separately, and the neoplasm may be regarded as the biliary counterpart of branch duct-type intraductal papillary mucinous neoplasm of pancreas with concomitant pancreatic duct adenocarcinoma.
- Citation: Uchida T, Yamamoto Y, Ito T, Okamura Y, Sugiura T, Uesaka K, Nakanuma Y. Cystic micropapillary neoplasm of peribiliary glands with concomitant perihilar cholangiocarcinoma. World J Gastroenterol 2016; 22(7): 2391-2397
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2391.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2391
Intraductal papillary neoplasm of the bile duct (IPNB) is characterized by exophytic proliferation of neoplastic epithelial cells with fine fibrovascular stalks in the dilated bile duct lumen and mucin hypersecretion[1-3]. IPNB and pancreatic intraductal papillary mucinous neoplasm (IPMN) share some clinicopathological characteristics[1-5], and IPNB can be regarded as a biliary counterpart of IPMN. However, IPNB differs from IPMN in several aspects. In particular, IPMN can be divided into the main pancreatic duct and branch pancreatic duct types[6]. Meanwhile, most previously reported cases of IPNB occurred in the extrahepatic, hilar, and large intrahepatic bile ducts; such IPNB cases can be regarded as counterparts of IPMN, particularly the main duct type[4,6,7]. So far, the IPNB type corresponding to branch duct-type IPMN (BD-IPMN) remains to be established.
Several cases of IPNB arising from peribiliary glands (PBGs) exhibiting grossly visible papillary lesions have recently been reported[8-10]. Nakanuma et al[4,5,11] recently reported that the biliary tract can be regarded as an incomplete pancreas and that PBGs and their own conduits around the large bile ducts may correspond to the branches of the pancreatic duct and exocrine pancreas[12]. In this context, IPNBs arising from PBGs could be regarded as branch duct-type IPNB (BD-IPNB) corresponding to BD-IPMN. Sato et al[13] recently reported an entity of cystic and micropapillary epithelial lesions of PBGs by surveying many autopsy cases; these lesions are characterized by grossly visible multicystic epithelial tumors with foci of micropapillary patterns. After extensive analysis, they concluded these lesions are neoplastic; therefore, the term “cystic micropapillary neoplasm of PBGs” is preferable in this context.
IPNB is a pre-invasive lesion that is eventually followed by invasive carcinoma (i.e., IPNB with an associated invasive carcinoma)[1,2]. IPMN is also a pre-invasive neoplasm that is followed by invasive carcinoma. In addition, IPMN is not uncommonly associated with concomitant pancreatic duct adenocarcinoma (PDAC) (i.e., IPMN with concomitant PDAC)[14-16], while no such cases of IPNB with concomitant cholangiocarcinoma (CCA) have been reported. Herein, we report a case of cystic micropapillary neoplasm of PBGs that was detected preoperatively by radiologic examination. The neoplasm was associated with perihilar CCA, suggesting concomitance similar to BD-IPMN with concomitant PDAC.
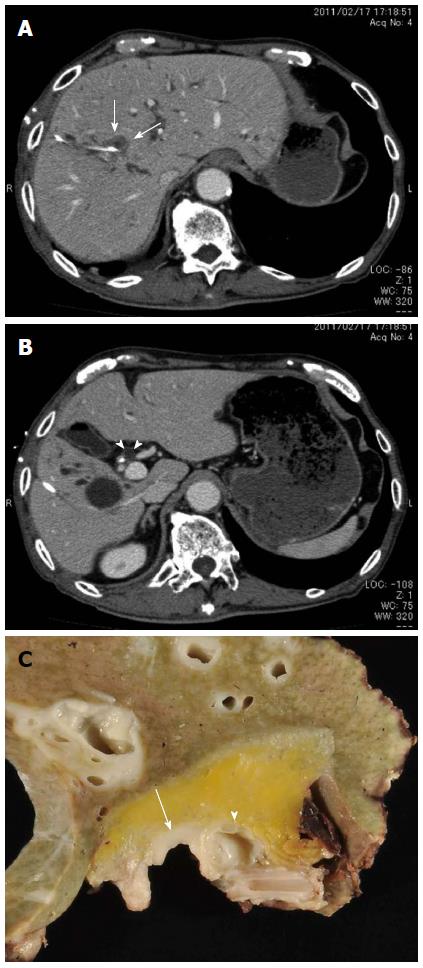
A 75-year-old man complaining of obstructive jaundice was referred to our hospital. He previously underwent endoscopic mucosal dissection for gastric cancer and pharyngolaryngoesophagectomy for hypopharyngeal carcinoma. Laboratory test results on admission were as follows: aspartate aminotransferase, 87 IU/L (normal: 10-40 IU/L); alanine aminotransferase, 84 IU/L (normal: 5-40 IU/L); γ-glutamyl transpeptidase, 309 IU/L (normal: < 70 IU/L); alkaline phosphatase, 909 IU/L (normal: 115-359 IU/L); total bilirubin, 8.6 mg/dL (normal: 0.2-1.0 mg/dL); carcinoembryonic antigen, 2.7 ng/mL (normal: < 5.0 ng/dL); and carbohydrate antigen 19-9, 112 IU/mL (normal: < 37 IU/mL). After hospitalization, serum total bilirubin and liver enzyme levels normalized because of endoscopic nasobiliary drainage and percutaneous transhepatic biliary drainage. Enhanced computed tomography showed a low-density mass 2.2 cm in diameter in the right hepatic hilum (Figure 1A) and a cystic lesion near the common bile duct (Figure 1B); this cyst was retrospectively diagnosed as a peribiliary cyst. After a pathological diagnosis of adenocarcinoma on the basis of cholangioscopic biopsy, right hepatectomy with caudate lobectomy and bile duct resection were performed. The postoperative course was uneventful, and he was discharged on the 17th postoperative day. During follow-up, multiple liver metastases were found, and he died one year postoperatively.
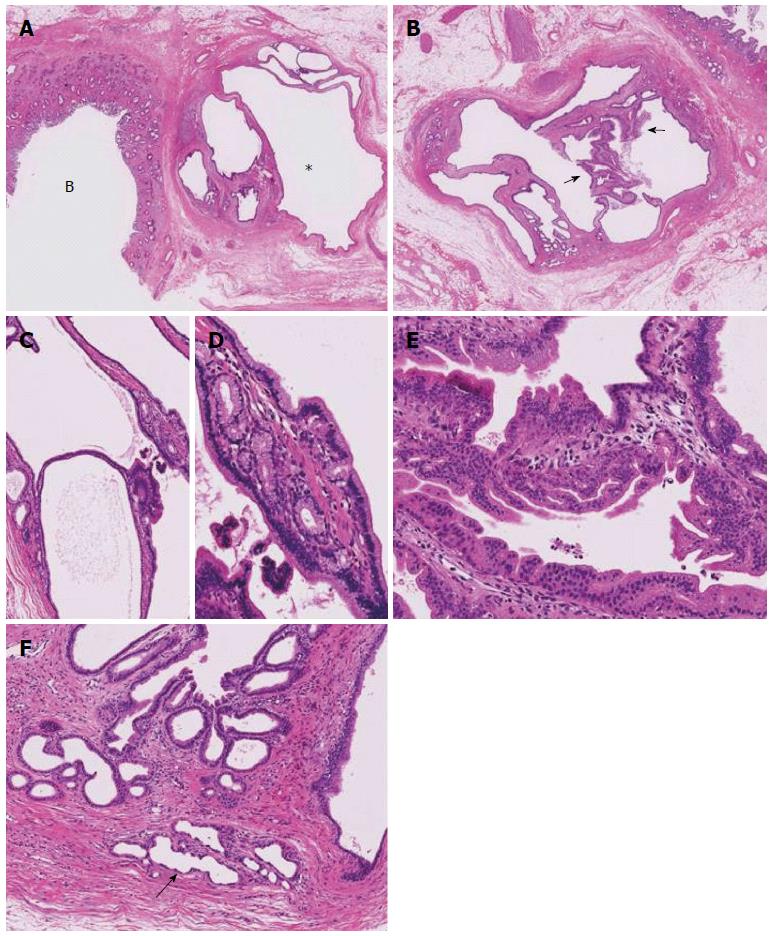
Macroscopic examination revealed the tumor spread from the right hepatic duct to the common hepatic duct. The cystic lesion was 1.0 cm × 1.0 cm and had a multilocular appearance in the hilar connective tissue near the common bile duct (Figure 1C, 2A and 2B).
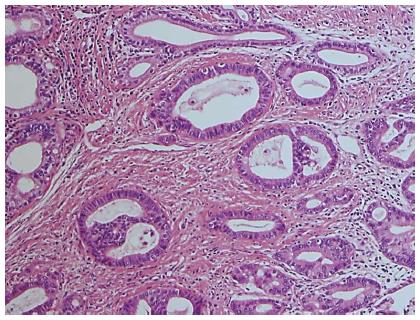
Histologically, the tumor was well-to-moderately differentiated tubular adenocarcinoma of the right hepatic duct extending to the common bile duct (Figure 2A and Figure 3) and was therefore diagnosed as perihilar CCA. The cystic lesion was multilocular with fine fibrous septae. A large part of the inner surfaces of cystic spaces were covered with columnar epithelium resembling gastric epithelia (Figure 2C). Glandular components resembling pyloric glands were focally found under the lining epithelia of cystic spaces (Figure 2D). Micropapillary projections with intermediate-grade intraepithelial neoplasia were focally found (Figure 2E). These cystic lesions were rimmed by non-neoplastic PBGs (Figure 2F). In addition, the lesion was near the common hepatic duct, suggesting it might have originated from PBGs. These findings were compatible with a diagnosis of cystic micropapillary neoplasm of PBGs[13]. The hilar CCA and cystic micropapillary neoplasm were close but not continuous (Figure 2A).
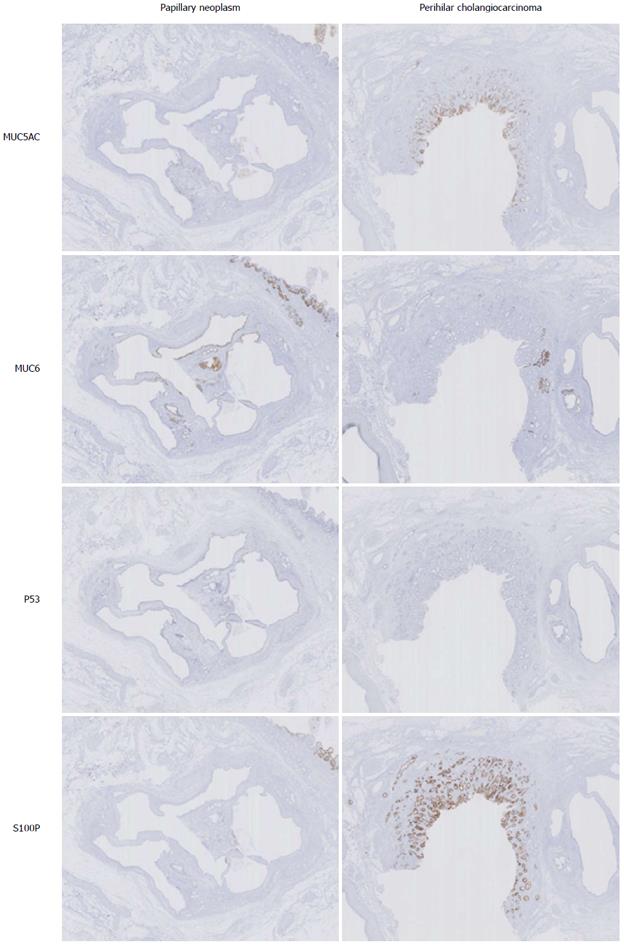
Immunohistochemical staining for MUC1, MUC2, MUC5AC, MUC6, CK7, CK20, S100P, estrogen receptor, progesterone receptor, Ki67, and p53 was performed (Figure 4 and Table 1). No ovarian-like stroma or stromal cells positive for estrogen receptor or progesterone receptor were observed, indicating this cystic neoplasm differed from the hepatic mucinous cystic neoplasm. The perihilar CCA was strongly and diffusely positive for MUC5AC and S100p, while the cystic papillary neoplasm was strongly and diffusely positive for MUC6 and focally positive for MUC5AC. Both were strongly positive for CK7 and focally positive for p53. These findings suggest both neoplasms had different phenotypes.
| Papillary neoplasm | Perihilar cholangiocarcinoma | |
| MUC1 | + | ++ |
| MUC2 | - | - |
| MUC5AC | + | +++ |
| MUC6 | +++ | - |
| p53 | + | + |
| Ki67 | 20% | 30% |
| CK7 | +++ | +++ |
| CK20 | - | - |
| S100P | - | ++ |
| ER | - | - |
| PgR | - | - |
There have been several reports of neoplastic changes of PBGs[8-10,17], showing that CCA[17] and grossly visible papillary neoplasms can arise from PBGs[4,5,10]; the latter are termed BD-IPNB[5,8-10]. However, the clinicopathological characteristics of BD-IPNB remain incompletely understood. Sato et al[13] recently found 9 cases of cystic micropapillary neoplasms of PBGs among 938 consecutive autopsied livers. In the present case, the lesion comprised multilocular cysts covered by columnar epithelial cells with mucinous cytoplasm, increased proliferative activities, and frequent nuclear expression of p53. In addition, the lesion exhibited micropapillary projections. The lesion was located in the hilar connective tissue near the common bile duct and was rimmed by non-neoplastic PBGs. Hence, this lesion was diagnosed as cystic micropapillary neoplasm of PBGs.
The present case differs from previously reported BD-IPNBs in that papillary lesions were not grossly visible; rather, this case histologically resembled flat BD-IPMN[6,15,16,18]. Furthermore, this lesion was positive for MUC6 and focally positive for MUC5AC, suggestive of gastric-type phenotype and phenotypically similar to flat BD-IPMN[6]. In fact, there were several foci of pyloric glands beneath the cyst-lining surface epithelia within the lesion. In this context, the lesion could be also termed branch-type IPNB without a grossly visible papillary component (i.e., flat-type BD-IPNB), such as flat BD-IPMN.
In the pancreas, IPMN is not infrequently associated with PDAC (i.e., IPMN with concomitant PDAC)[15,16,18]. Retrospective studies on BD-IPMN with concomitant PDAC report its incidence among IPMNs to range from 3.3%-9.2%[15,16,19]. The clinical outcome of patients with IPMN with concomitant PDAC is better than that of ordinary PDAC (i.e., without concomitant IPMN); this is because IPMN concomitant with pancreatic cancer is detected at an earlier stage because of the presence of IPMN[16]. Gastric-type BD-IPMN with concomitant PDAC does not exhibit GNAS mutation in contrast to the majority of gastric-type IPMNs[18].
Interestingly, the lesion in the present case was associated with perihilar CCA; they were discontinuous, and their phenotypes were different, suggesting the flat BD-IPNB and hilar CCA occurred separately. Such an association has not been reported in cases of hilar CCA or IPNB; furthermore, our survey of 59 surgically resected perihilar CCAs at our hospital failed to find another case of cystic micropapillary neoplasm of PBGs (i.e., flat BD-IPNB). Therefore, to our knowledge the case presented herein could be the first case of flat BD-IPNB with concomitant perihilar CCA.
Because few cases of flat BD-IPNB have been reported, it is unknown if flat BD-IPNB is associated with a high incidence of CCA. Therefore, the recognition of flat BD-IPNB or cystic micropapillary neoplasm of PBGs as well as analyses of more cases of flat BD-IPNB with or without concomitant CCA are required.
In conclusion, this is the first report of cystic micropapillary neoplasm of PBGs (i.e., flat type BD-IPNB) resembling flat BD-IPMN with concomitant perihilar CCA to our knowledge. Further studies are required to establish the concept of flat BD-IPNB with concomitant perihilar CCA.
We thank Mr. Shogo Fujii and Ms. Asuka Fukushima for their technical assistance.
A 75-year-old man complained of obstructive jaundice.
His skin and palpebral conjunctiva showed jaundice.
Malignant tumors (cholangiocarcinoma, pancreatic cancer, carcinoma of the ampulla of Vater) and benign lesions (common bile duct stone, cholangitis).
He had elevated hematological values for aspartate aminotransferase (87 IU/L) alanine aminotransferase (84 IU/L), γ-glutamyl transpeptidase (309 IU/L), alkaline phosphatase (909 IU/L), total bilirubin (8.6 mg/dL), carcinoembryonic antigen (2.7 ng/mL) and carbohydrate antigen 19-9 (112 IU/mL).
Enhanced computed tomography showed a low-density mass 2.2 cm in diameter in the right hepatic hilum and a cystic lesion near the common bile duct.
Intraductal papillary neoplasm of bile duct (IPNB) arising from peribiliary glands (PBGs) with concomitant perihilar cholangiocarcinoma occurred separately.
Right hepatectomy with caudate lobectomy and bile duct resection were performed.
IPNB is characterized by exophytic proliferation of neoplastic epithelial cells with fine fibrovascular stalks in the dilated bile duct lumen and mucin hypersecretion.
This is the first report of cystic micropapillary neoplasm of PBGs resembling flat branch duct-type (BD)-intraductal papillary mucinous neoplasm (IPMN) with concomitant perihilar concomitant cholangiocarcinoma (CCA) to our knowledge and further studies are required to establish the concept of flat BD-IPNB with concomitant perihilar CCA.
This is a case report presenting a cystic micropapillary neoplasm of peribiliary glands with concomitant perihilar cholangiocarcinoma. The authors conclude that this case warrants further study as it may be correspond to the combination of IPMN with concomitant pancreatic duct adenocarcinoma. This is the first case of such combination of tumours and deserves publication.
P- Reviewer: Cathomas G, Tsolakis A, Xu Z S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Nakanuma Y, Curado MP, Franceschi S, Gores G, Paradis V, Sripa B, Tsui WMS, Wee A. Intrahepatic cholangiocarcinoma. WHO classification of tumours of the digestive system, Fourth Edition. Lyon: IARC Press 2010; 217-224. |
| 2. | Zen Y, Fujii T, Itatsu K, Nakamura K, Minato H, Kasashima S, Kurumaya H, Katayanagi K, Kawashima A, Masuda S. Biliary papillary tumors share pathological features with intraductal papillary mucinous neoplasm of the pancreas. Hepatology. 2006;44:1333-1343. [PubMed] [DOI] [Full Text] |
| 3. | Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, Mitsui T, Asada Y, Miura S, Miyayama S. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19:1243-1254. [PubMed] [DOI] [Full Text] |
| 4. | Nakanuma Y. A novel approach to biliary tract pathology based on similarities to pancreatic counterparts: is the biliary tract an incomplete pancreas? Pathol Int. 2010;60:419-429. [PubMed] [DOI] [Full Text] |
| 5. | Nakanuma Y, Harada K, Sasaki M, Sato Y. Proposal of a new disease concept “biliary diseases with pancreatic counterparts”. Anatomical and pathological bases. Histol Histopathol. 2014;29:1-10. [PubMed] [DOI] [Full Text] |
| 6. | Adsay NV, Fukushima N, Furukawa T, Hruban RH, Klimstra DS, Klöppel G, Offerhaus GJA, Pitman MB, Shimizu M, Zamboni G. Intraductal neoplasms of the pancreas. WHO classification of tumours of the digestive system, Fourth Edition. Lyon: IARC Press 2010; 304-313. |
| 7. | Nakanuma Y, Zen Y, Harada K, Ikeda H, Sato Y, Uehara T, Sasaki M. Tumorigenesis and phenotypic characteristics of mucin-producing bile duct tumors: an immunohistochemical approach. J Hepatobiliary Pancreat Sci. 2010;17:211-222. [PubMed] [DOI] [Full Text] |
| 8. | Nakanishi Y, Zen Y, Hirano S, Tanaka E, Takahashi O, Yonemori A, Doumen H, Kawakami H, Itoh T, Nakanuma Y. Intraductal oncocytic papillary neoplasm of the bile duct: the first case of peribiliary gland origin. J Hepatobiliary Pancreat Surg. 2009;16:869-873. [PubMed] [DOI] [Full Text] |
| 9. | Nakanishi Y, Nakanuma Y, Ohara M, Iwao T, Kimura N, Ishidate T, Kijima H. Intraductal papillary neoplasm arising from peribiliary glands connecting with the inferior branch of the bile duct of the anterior segment of the liver. Pathol Int. 2011;61:773-777. [PubMed] [DOI] [Full Text] |
| 10. | Lim JH, Zen Y, Jang KT, Kim YK, Nakanuma Y. Cyst-forming intraductal papillary neoplasm of the bile ducts: description of imaging and pathologic aspects. AJR Am J Roentgenol. 2011;197:1111-1120. [PubMed] [DOI] [Full Text] |
| 11. | Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38:552-570. [PubMed] |
| 12. | Terada T, Kida T, Nakanuma Y. Extrahepatic peribiliary glands express alpha-amylase isozymes, trypsin and pancreatic lipase: an immunohistochemical analysis. Hepatology. 1993;18:803-808. [PubMed] [DOI] [Full Text] |
| 13. | Sato Y, Harada K, Sasaki M, Nakanuma Y. Cystic and micropapillary epithelial changes of peribiliary glands might represent a precursor lesion of biliary epithelial neoplasms. Virchows Arch. 2014;464:157-163. [PubMed] [DOI] [Full Text] |
| 14. | Tada M, Kawabe T, Arizumi M, Togawa O, Matsubara S, Yamamoto N, Nakai Y, Sasahira N, Hirano K, Tsujino T. Pancreatic cancer in patients with pancreatic cystic lesions: a prospective study in 197 patients. Clin Gastroenterol Hepatol. 2006;4:1265-1270. [PubMed] [DOI] [Full Text] |
| 15. | Yamaguchi K, Ohuchida J, Ohtsuka T, Nakano K, Tanaka M. Intraductal papillary-mucinous tumor of the pancreas concomitant with ductal carcinoma of the pancreas. Pancreatology. 2002;2:484-490. [PubMed] [DOI] [Full Text] |
| 16. | Yamaguchi K, Kanemitsu S, Hatori T, Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda Y. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011;40:571-580. [PubMed] [DOI] [Full Text] |
| 17. | Terada T, Nakanuma Y. Pathological observations of intrahepatic peribiliary glands in 1,000 consecutive autopsy livers. II. A possible source of cholangiocarcinoma. Hepatology. 1990;12:92-97. [PubMed] [DOI] [Full Text] |
| 18. | Ideno N, Ohtsuka T, Matsunaga T, Kimura H, Watanabe Y, Tamura K, Aso T, Aishima S, Miyasaka Y, Ohuchida K. Clinical significance of GNAS mutation in intraductal papillary mucinous neoplasm of the pancreas with concomitant pancreatic ductal adenocarcinoma. Pancreas. 2015;44:311-320. [PubMed] [DOI] [Full Text] |