Published online Feb 21, 2016. doi: 10.3748/wjg.v22.i7.2165
Peer-review started: September 23, 2015
First decision: October 14, 2015
Revised: November 2, 2015
Accepted: December 12, 2015
Article in press: December 12, 2015
Published online: February 21, 2016
Processing time: 132 Days and 6.2 Hours
Diagnostic imaging plays a key role in the diagnosis and management of inflammatory bowel disease (IBD). However due to the relapsing nature of IBD, there is growing concern that IBD patients may be exposed to potentially harmful cumulative levels of ionising radiation in their lifetime, increasing malignant potential in a population already at risk. In this review we explore the proportion of IBD patients exposed to high cumulative radiation doses, the risk factors associated with higher radiation exposures, and we compare conventional diagnostic imaging with newer radiation-free imaging techniques used in the evaluation of patients with IBD. While computed tomography (CT) performs well as an imaging modality for IBD, the effective radiation dose is considerably higher than other abdominal imaging modalities. It is increasingly recognised that CT imaging remains responsible for the majority of diagnostic medical radiation to which IBD patients are exposed. Magnetic resonance imaging (MRI) and small intestine contrast enhanced ultrasonography (SICUS) have now emerged as suitable radiation-free alternatives to CT imaging, with comparable diagnostic accuracy. The routine use of MRI and SICUS for the clinical evaluation of patients with known or suspected small bowel Crohn’s disease is to be encouraged wherever possible. More provision is needed for out-of-hours radiation-free imaging modalities to reduce the need for CT.
Core tip: Due to the chronic and relapsing nature of inflammatory bowel disease (IBD), patients are at risk of exposure to potentially harmful cumulative radiation doses in their lifetime. Computed tomography (CT) imaging remains responsible for the majority of this radiation exposure. As well as new reduced radiation CT imaging techniques, radiation-free alternatives magnetic resonance imaging and small intestine contrast enhanced ultrasonography have emerged, offering comparable diagnostic accuracy. In this review we explore the proportion of IBD patients exposed to high cumulative radiation doses, the factors associated with higher radiation exposures, and we compare conventional imaging with newer radiation-free imaging techniques for the evaluation of patients with IBD.
- Citation: Zakeri N, Pollok RC. Diagnostic imaging and radiation exposure in inflammatory bowel disease. World J Gastroenterol 2016; 22(7): 2165-2178
- URL: https://www.wjgnet.com/1007-9327/full/v22/i7/2165.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i7.2165
Inflammatory bowel disease (IBD), consisting of ulcerative colitis (UC) and Crohn’s disease (CD), is a chronic relapsing-remitting inflammatory disorder of the gastrointestinal tract. The prevalence of IBD is increasing worldwide, with 2.2 million and 1.4 million people affected in Europe and United States respectively[1].
Diagnostic imaging is required to aid the diagnosis of IBD, assess disease extent and severity, detect complications including extra-intestinal manifestations, and monitor response to treatment. Due to the relapsing nature of IBD, multiple imaging studies are often required. Despite this, in clinical practice cumulative exposure to radiation is not routinely monitored.
Patients with IBD have an increased lifetime risk of developing colorectal and small intestinal cancers, irrespective of diagnostic radiation exposure[2,3]. There is growing concern that repeated X-ray based imaging may additionally expose this typically young cohort of patients to harmful cumulative levels of ionising radiation, further increasing their lifetime cancer risk.
In this article we review the proportion of IBD patients exposed to potentially harmful cumulative radiation doses and the risk factors associated with higher radiation exposures. We explore and compare conventional diagnostic imaging and newer radiation-free imaging techniques for the evaluation of patients with IBD.
Extensive study of the atomic bomb survivors from Hiroshima and Nagasaki has formed the basis for quantitative estimates of radiation-induced cancer risk. In this large cohort of survivors, the rates of solid cancer deaths were positively associated with higher radiation doses and younger age of exposure[4,5]. In a 2012 study of the atomic bomb survivors, the relative risk of solid cancers increased by 29% per decade decrease in the initial age of radiation exposure[5]. Younger people appear to be inherently more radiosensitive, and have more remaining life-years during which a cancer may develop[4].
It is estimated that diagnostic medical radiation (DMR) exposure may be responsible for up to 2% of cancers worldwide[6]. Younger patients and females appear to have the greatest radiation-induced cancer risk[7]. Epidemiological data suggests that ionising radiation levels as low as 50 millisieverts (mSv) have been implicated in the development of solid tumours[8]. Potentially harmful radiation exposure is, therefore, commonly defined as cumulative effective dose (CED) > 50 mSv; the equivalent of five computed tomography (CT) abdominal-pelvis scans. A reference table comparing radiation exposure doses of common diagnostic gastrointestinal imaging techniques is included in Table 1[9,10].
| Imaging procedure | Average effective dose (mSv) | Time period for equivalent effective dose from natural background radiation1 |
| Multiphase CT abdomen and pelvis | 31 | 10.3 yr |
| PET/CT | 25 | 8.3 yr |
| CT Abdomen and Pelvis | 10 | 3.3 yr |
| CT Colonography | 10 | 3.3 yr |
| CT Abdomen | 8 | 2.7 yr |
| Barium Enema | 8 | 2.7 yr |
| Small bowel follow-through | 5 | 1.7 yr |
| X-ray abdomen | 0.7 | 2.8 mo |
Several published studies have attempted to quantify the proportion of IBD patients exposed to potentially harmful cumulative levels of ionising radiation (summarised in Table 2). Desmond et al[11] first evaluated DMR exposure in 354 patients with CD in a single tertiary centre in Ireland. CT imaging accounted for 77.2% of the total DMR exposure. The mean CED was 36.1 mSv and exceeded 75 mSv in 15.5% of patients. More recently, a meta-analysis by Chatu et al[12], evaluated six studies including a total of 1704 IBD patients. It reported a pooled estimate of 8.4% of IBD patients receiving high dose radiation exposure (CED > 50 mSv). More patients with CD (11.1%) were exposed to high cumulative radiation doses (CED > 50 mSv) than patients with UC (2%)[12].
| Study | Number of patients (n) | Country | Design | Patient population | Outcome CED≥50 mSv | Mean/Median CED (mSv) | Factors associated with high radiation exposure |
| Newnham et al[59], 2007 | 100 (62 CD, 37 UC, 1 indeterminate colitis) | Australia | Retrospective study, single tertiary centre, patients recruited consecutively from clinic | Adult (16-84 yr) | 11/100 (11%) 9 CD, 2 UC | Median CED 10 mSv | Assessed: age, gender, disease, disease duration, previous surgery, immunomodulator use, referral source Significant: none |
| Desmond et al[11], 2008 | 354 CD | Ireland | Retrospective study, single tertiary centre, patients recruited from IBD database July 1992-June 2007 | Adult and paediatric (8.6-78.3 yr) | CED ≥ 75 mSv in 55/354 patients (15.5%) | Mean CED 36.1 mSv | Assessed: age, gender, smoking, FH, disease distribution, disease behaviour, medication, surgical history Significant: age < 17 at diagnosis, upper GI tract disease, penetrating disease, requirement for IV steroids, infliximab use, multiple surgeries |
| Peloquin et al[18], 2008 | 215 (103 CD, 112 UC) | United States | Retrospective study, population based inception cohort diagnosed between 1991 to 2001 from Olmsted County | Adult and paediatric (1.2-91.4 yr) | N/A | Median CED CD: 26.6 mSv UC: 10.5 mSv | N/A |
| Levi et al[60], 2009 | 324 (199 CD, 125 UC) | Israel | Retrospective study, single tertiary centre, patients diagnosed Jan 1999-Dec 2006, recruited from IBD database | Adult and paediatric ≤ 17 yr (18) > 18 yr (306) | 23/324 (7.1%) | Mean CED CD: 21.1mSv UC: 15.1mSv | Assessed: age, surgery, diagnosis, medical therapy, disease duration, gender Significant: CD, surgery, prednisolone use, disease duration, first year of disease, age |
| Palmer et al[61], 2009 | 1593 (965 CD, 628 UC) | United States | Retrospective study, population based cohort recruited from insurance claims database Jan 2003-December 2004 | Paediatric (2-18 yr) | N/A (34% CD, 23% UC exposed to moderate radiation - at least 1 CT or 3 fluoroscopic procedures) | N/A | Assessed: age, gender, region, hospitalisation, surgery, ED encounter, medication Significant: hospitalisation, inpatient GI surgery, ED encounter, use of steroids |
| Kroeker et al[62], 2011 | 553 (371 CD, 182 UC) | Canada | Retrospective study, single tertiary centre, patients diagnosed 2003-2008, recruited from IBD database | Adult and paediatric (15-84 yr) | 28/553 (5%) 27 CD, 1 UC | Mean CED CD: 14.3 mSv UC: 5.9 mSv | Assessed: age at diagnosis, gender, disease distribution, previous surgery Significant: previous surgery |
| Fuchs et al[63], 2011 | 257 (171 CD, 86 UC) | United States | Retrospective study single tertiary centre, patients reviewed Jan-May 2008 | Paediatric (< 18 yr) | 15/257 (5.8%) 14 CD, 1UC | Mean CED CD: 20.5 mSv UC: 11.7 mSv | Assessed in CD cohort: gender, disease behaviour, previous surgery, disease duration, elevated platelet count at diagnosis Significant: previous surgery, elevated platelet count at diagnosis |
| Sauer et al[64], 2011 | 117 (86 CD, 31 UC) | United States | Retrospective study, single tertiary centre, patients diagnosed 2002-2008 | Paediatric (2-18 yr) | 6/117 (5%) 6 CD | Median CED CD: 15.6 mSv UC: 7.2 mSv | N/A |
| Huang et al[65], 2011 | 105 (61 CD, 32 UC, 12 indeterminate colitis) | United States | Single tertiary paediatric centre, patients identified from medical records | Paediatric cohort (11 mo-18 yr) | 6/105 (6%) | Mean CED 15 mSv | Assessed: surgery, disease type, disease location, racioethnic background, anti TNF agents, use of immunomodulators, hospital admissions, age at diagnosis Significant: CD, small bowel involvement, black ethnicity, number of hospital admissions, previous surgery, anti TNF alpha use |
| Butcher et al[17], 2012 | 280 | United Kingdom | Retrospective study, single tertiary centre, consecutive patients attending IBD clinic | Adult cohort | 6.3% CD | Mean CED 10.17 mSv Median CED 4.12 mSv | Significant: smoking status, disease duration, previous surgery |
| Jung et al[15], 2013 | 2199 (777 CD, 1422 UC) | South Korea | Retrospective study, multicentre conducted at 13 university hospitals in South Korea, patients diagnosed July 1987-Jan 2012 included | Adult cohort (Mean age: CD 29.2 yr; UC 42.2 yr) | 34.7% CD, 8.4% UC | Mean CED CD: 53.6 mSv UC: 16.4 mSv | Assessed: gender, age at diagnosis, disease duration, disease extent, surgery, hospitalisation, 5-ASA use, steroids, immunomodulator use Significant: For CD - longer disease duration, ileocolonic disease, upper GI tract involvement, surgery, hospitalisation, steroids For UC - surgery, hospitalisation, infliximab use |
| Chatu et al[14], 2013 | 415 (217 CD, 198 UC) | United Kingdom | Retrospective study, single tertiary centre, patients consecutively recruited from clinic Jan 2011- June 2011 | Adult cohort (Mean age: CD 30.8 yr; UC 36.9 yr) | 32/415 (8%) 29 CD, 3 UC | Median CED CD: 7.2 mSv UC: 2.8 mSv | Assessed: gender, age at diagnosis, disease type, steroid use within 3 mo diagnosis, use of immunomodulators or biologics, extraintestinal features, IBD related surgery Significant: males, IBD related surgery |
| Estay et al[13], 2015 | 325 (82 CD, 243 UC) | Chile | Retrospective study, patients recruited from IBD Registry 2011-2013 | Adult cohort (16-86 yr) | 22/325 (6.8%): CD 16 (19.5%); UC 6 (2.5%) | Mean CED 11.97 mSv CD: 29.9 mSv UC: 5.92 mSv | Assessed in CD cohort only: age at diagnosis, disease duration, disease location, disease behaviour, perianal disease, surgery, hospitalisation, medications Significant: longer disease duration, ileal involvement, stricturing disease, treatment with steroids and biological agents, CD related hospitalisation or surgery |
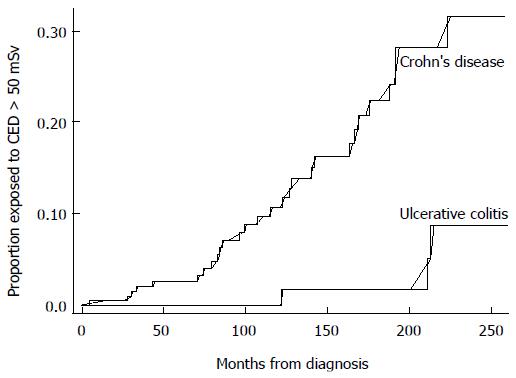
Similar trends have been found in studies following this meta-analysis. A 2015 retrospective review of 325 IBD patients in Chile, reported 19.5% of patients with CD and 2.4% of patients with UC, to be exposed to CED > 50 mSv[13]. A recent United Kingdom retrospective study of 415 patients with IBD referred from primary care, reported a median total CED of 7.2 mSv in CD patients and 2.8 mSv in UC patients, with 8% of IBD patients overall exposed to CED > 50 mSv. Kaplan Meier analysis projected a probability of exposure to CED > 50 mSv of 6% and 14% at 10 years and 15 years from IBD diagnosis respectively (Figure 1)[14].
Concerningly, a retrospective study of IBD patients in South Korea, conducted across 13 university hospitals, reported even higher proportions of patients exposed to potentially harmful radiation levels. Thirty-four point seven percent of patients with CD and 8.4% of patients with UC were exposed to CED > 50 mSv[15]. CT imaging accounted for the vast majority of this radiation exposure (81.6% of the total CED in CD vs 71.2% in UC)[15], indicating that overuse of CT imaging remains a concern worldwide, and may reflect limited availability or lack of awareness of preferable imaging modalities.
Despite the high proportion of IBD patients exposed to high radiation doses, cumulative radiation exposures are not routinely recorded in clinical practice. The creation of IBD radiation diaries has been proposed to log total radiation exposures[16], and improve recognition among physicians where a patient has previously been exposed to ionising radiation.
Risk factors for high radiation exposure in IBD patients have been widely studied[11-15,17]. In a cohort of 354 adult and paediatric patients with CD, Desmond et al[11] identified that patients diagnosed under the age of 17, patients with upper gastrointestinal (GI) disease, penetrating disease, multiple surgeries, or those that required intravenous steroids or infliximab, were at greater risk of receiving high cumulative radiation exposure. Following this, a 2012 meta-analysis of five studies evaluating risk factors in 2627 IBD patients, found a significant association with only previous IBD related surgery and corticosteroid use. The pooled adjusted odds ratios were 5.4 and 2.4, respectively[12].
Across studies, patients with CD consistently appear to receive higher cumulative radiation exposures than patients with UC, possibly due to a greater likelihood of extraluminal complications commonly examined by CT. After adjusting for time since symptom onset, a retrospective study by Peloquin et al[18] (2008) found patients with CD to be exposed to 2.46 times more diagnostic radiation than patients with UC (median CED 26.6 mSv in CD vs 10.5 mSv in UC).
A summary of outcomes from studies investigating predictive factors for high radiation exposure in IBD patients is provided in Table 2. While there are discrepancies regarding the significance of some associations, the majority of the risk factors described are surrogate markers of disease activity and severity. It is therefore apparent that patients with more severe disease, who are more likely to receive corticosteroids and require surgery, undergo more diagnostic imaging including greater use of CT imaging, to guide further management.
A 2011 survey revealed small bowel follow-through (SBFT) to be the most frequently performed investigation in the United Kingdom for the assessment of small bowel CD[19]. CT was predominantly performed for suspected extra-luminal complications or obstruction[19]. SBFT and small bowel enteroclysis (SBE) have, for many years, been the routine first-line imaging modalities to evaluate small bowel involvement in patients with suspected or confirmed CD. Both SBFT and SBE have similar sensitivities (85%-95%) and specificities (89%-94%) for detecting radiological features of CD[20]. SBFT is usually preferred for patient tolerance, since nasal or oral intubation is not required. However, these techniques both employ ionising radiation and appear to have lower diagnostic accuracy compared to newer cross-sectional imaging modalities[21,22].
In a 2005 United States study, SBFT had a lower diagnostic yield for mild to moderate CD compared to CT enterography, video capsule endoscopy and ileoscopy[21]. A 2009 Korean study of 30 patients with CD, found a significantly lower sensitivity of SBFT for the detection of extra-enteric complications (P < 0.01), although no significant difference in the detection of active terminal ileitis, compared to CT and magnetic resonance enterography (MRE)[22]. Barium based studies may still have a role to play in the evaluation of small bowel CD, but are increasingly being replaced by alternative imaging modalities such as CT, MRE and small bowel ultrasound.
In the United States, CT has largely superseded SBFT as the preferred first-line imaging modality for CD. Between 2002 and 2007, there was a reported 840% increase in the use of CT enterography in IBD patients in Minnesota, United States[6]. Similarly, a 310% increase in use of abdominal CT imaging was reported in a United Kingdom study of IBD patients between 1990 and 2010[14]. CT imaging offers the advantages of widespread availability, rapid acquisition of images, high sensitivity and specificity for the detection of intramural and extra-intestinal disease, as well as being well tolerated by patients[4]. The effective radiation dose is, however, considerably higher than other abdominal imaging modalities (Table 1)[9,10]. The United States National Research Council estimates that one out of every 1000 patients undergoing a 10 mSv CT scan will develop a radiation-induced cancer in their lifetime [23].
Conventional CT abdominal-pelvis imaging is typically used for the detection of extra-intestinal complications of IBD, such as abscesses, fistula, bowel obstruction or perforation. It may have a limited role in the assessment of colonic disease activity. A small study by Patel et al[24] of 23 patients with UC (2012), identified positive correlation of contrast-enhanced CT features (bowel wall thickening, mucosal hyper-enhancement and mural stratification), compared with clinical assessment (P < 0.05) and colonoscopy (P < 0.0001) in evaluating UC disease severity. However, only increasing bowel wall thickness on CT correlated with histological disease severity[24].
Conventional CT is limited in its assessment of small bowel inflammation due to artefact produced from collapsed bowel loops. CT enterography (CTE) is a newer imaging technique, combining high resolution CT scanning with multiplanar reconstructions after administration of an oral and parenteral contrast which acts to promote bowel loop distension. This improves visualisation of the small bowel mucosa, enabling more accurate assessment of small bowel disease activity[25]. High correlation has been shown between quantitative measures of bowel wall thickness and terminal ileal mural attenuation at CTE compared with ileocolonoscopy and histological analysis in active CD[26]. Furthermore, CTE may be a useful adjunct to ileocolonoscopy. In a 2012 study of 153 patients with CD in the United States, CTE detected active small bowel disease in 36 of the 67 patients (54%) with normal ileoscopy appearances. The negative ileoscopy results were largely due to disease “skipping” of the terminal ileum, or confinement to intramural or mesenteric distal ileum. CTE also detected extra-colonic CD in 26% of patients[27].
Data for the benefit of CTE in assessing colonic disease is limited. A small study analysing CTE in 35 patients with inflammatory colitis, identified a sensitivity of 93% and specificity of 91% for the detection of moderate to severe disease in well-distended colons. However, there was a tendency for CTE to underestimate the full extent and severity of colonic disease[28].
CT colonography (CTC) is an emerging imaging technique developed for colonic evaluation. While colonoscopy remains standard practice for the assessment of colonic disease, CTC may offer advantages where colonoscopy is incomplete or contra-indicated. The majority of data comparing CTC and colonoscopy has been obtained from studies detecting colorectal cancer[25]. Only a few studies have investigated the efficacy of CTC in IBD, hence its role is not clearly defined. A small German prospective study of 21 IBD patients suggested sensitivities of 63.6% and 100% for the identification of acute and chronic IBD by CTC, with a specificity of 75% and 100% respectively[29]. CTC requires full bowel preparation, as well as air or carbon dioxide insufflation for colonic distension, and therefore is not always well tolerated. There have been reported cases of CTC-induced bowel perforation as well. Although the perforation rate is low, at around 0.04%, CTC is generally avoided in the acute phase of IBD[30].
Unfortunately while CT performs very well as an imaging modality there is an emerging recognition that it is responsible for the majority of the total radiation dose to which IBD patients are exposed[11-13,15]. Indeed in a recent study from Chile, abdominal-pelvic CT and CT enteroclysis accounted for 93.6% of the total CED exposure[13]. Excessive use of CT imaging in IBD patients presenting to the emergency department (ED) has also raised concern. In a study from the United States, no significant findings were observed in 32.8% of CT imaging studies carried out in IBD patients in the ED[31]. Preliminary algorithms to avoid inappropriate use of CT imaging in IBD patients presenting to the ED have been proposed and require validation[31,32].
Due to concerns regarding high radiation exposure from CT imaging, recent developments in technology have paved the way for strategies to reduce the radiation dose associated with CT imaging, without compromising diagnostic imaging quality. These techniques include tube current (mA) modulation, lowering tube potential modulation (kV), and minimising the number of dynamic CT phases[33]. Multiphase CT abdomen and pelvis imaging exposes a patient to around 31 mSv, equivalent to over three times the radiation dose of standard CT abdominal-pelvis imaging[9]. Single-phase CTE is in most cases believed to be sufficient to evaluate small bowel CD[33]. Reduced radiation CT techniques may help to lessen cumulative radiation exposures and bridge the gap in situations where radiation-free imaging is not widely available.
Technetium-99-m hexamethyl-propyleneamine oxime (99mTc-HMPAO) labeled white blood cell scintigraphy is an imaging technique that employs radioactive isotopes to detect active inflammation[34]. It may be used in IBD to assess disease activity, but due to limited availability and high cost, it is not routinely performed. 99mTc-HMPAO white cell scintigraphy can visualise the entire GI tract and emits a lower radiation dose than CT (2-4 mSv)[35]. Reported uses include evaluating responses to treatment and differentiating between disease relapse and fibrotic tissue post surgery[36]. It also has a role in assessing disease extent in acute severe colitis, where colonoscopy is usually contra-indicated. A United Kingdom study by Subramanian et al[37] of 135 patients with UC, noted substantial correlation (k = 0.7) between 99mTc-HMPAO white cell scintigraphy and histological assessment of the proximal extent of disease involvement in patients with UC. Scintigraphy performed better than colonoscopy (P = 0.02) in assessing patients with more extensive colitis, while colonoscopy predicted disease extent more accurately in patients with limited colitis (P = 0.002)[37].
Positron emission tomography (PET) is a non-invasive nuclear imaging technique that provides three dimensional, quantitative imaging. It is primarily used for tumour staging, though preliminary data has shown it may have some value in the diagnosis of IBD[38,39]. PET imaging is expensive and its availability is limited to certain centres. Data on the potential role of Fluorine-18-Fluorodeoxyglucose/PET (18F-FDG/PET) and PET/CT in IBD is limited and requires further review. Routine use of PET/CT for IBD assessment is unlikely due to the high doses of radiation involved (Table 1)[9,10].
In view of the concerns over cumulative radiation exposure in IBD patients, alternative radiation-free imaging strategies have emerged as a focus of interest, and are increasingly being favoured in clinical practice. Studies comparing the diagnostic accuracies for radiation-free imaging vs conventional imaging modalities in small bowel CD are summarised in Table 3.
| Study | Country | Number of patients (n) | Design | Imaging compared | Study findings |
| Low et al[66], 2000 | United States | 26 CD | Prospective study, single centre | Contrast enhanced MR with single phase CT using findings from surgery, barium studies, endoscopy and histology as reference standard | Side-by-side comparison: MR imaging superior than helical CT in depiction of normal bowel wall, mural thickening or enhancement and overall GI tract evaluation MR images showed 55 (85%) and 52 (80%) of 65 abnormal bowel segments for the two observers, compared with helical CT which showed 39 (60%) and 43 (65%) of bowel segments affected by CD (P < 0.001, P < 0.05) |
| Maconi et al[67], 2003 | Italy | 128 CD | Prospective study, consecutive CD patients who underwent surgery immediately after diagnostic work-up | US, barium studies, CT to detect internal fistulae and intra-abdominal abscesses compared to intraoperative findings | Detecting internal fistula: comparable diagnostic accuracy of US (85.2%) and barium X-ray (84.8%) studies Sensitivity US (71.4%), X-ray (69.6%), Specificity US (95.8%), X-ray (95.8%) Detection of abscesses: US (90.9%), CT (86.4%) Overall diagnostic accuracy higher with CT than US (91.8% vs 86.9%) due to false positives with US |
| Parente et al[49], 2004 | Italy | 102 CD | Prospective study, consecutive patients with proven CD by BE and ileocolonoscopy enrolled from IBD clinic Dec 2002-July 2003 Adult cohort (≥ 18 yr) | Conventional US vs oral contrast enhanced US, compared to BE and ileocolonoscopy as gold standard | Per segment analysis: Superior diagnostic accuracy of contrast US in detecting small bowel CD. Sensitivity: conventional US 91.4%, contrast US 96.1% Good correlation of disease extent measurements with BE: US (r = 0.83), contrast US (r = 0.94) Higher sensitivity and specificity with contrast US in detecting ≥ 1 small bowel strictures: Sensitivity: US (74%), contrast US (88.8%) Specificity: US (93.3%), contrast Us (97.3%) US and contrast US more accurate in detecting internal fistulas than BE, but no significant difference in diagnostic accuracy between US and contrast US. US (80%), contrast US (86%), BE (67%) Significantly improved interobserver variability between sonographers with contrast US for detecting bowel wall thickness and disease location |
| Calabrese et al[55], 2005 | Italy | 28 CD | Prospective study, consecutive patients recruited from IBD clinic Adult cohort (age range 21-60 yr) | SICUS (performed by a sonologist of 1 yr experience) vs TUS (performed by an experienced sonologist of 10 yr experience), compared to SBE as gold standard | Sensitivity for detection of small bowel lesions: 96% TUS, 100% SICUS Greater correlation of extension of lesions between SICUS and SBE (r = 0.88) vs TUS and SBE (r = 0.64) Sensitivity for detection of ≥ 1 stricture: 76% TUS, 94% SICUS Sensitivity and specificity for assessing prestenotic dilatation: 50% and 100% for TUS, vs 100% and 90% for SICUS |
| Horsthuis et al[40], 2007 | Amsterdam | 1735 (sample size 15-440) | Meta-analysis of 33 prospective studies published between Jan 1993- Feb 2006 Adult and paediatric cohort (age range 2-86 yr) | US, MRI, scintigraphy, CT US evaluated in 11 studies, MRI in 11, scintigraphy in 9 and CT in 7 studies | Per-patient analysis: Significantly lower specificity for scintigraphy vs US. No significant difference between mean sensitivities for diagnosis of IBD Sensitivities: 89.7% US, 93% MRI, 87.8% scintigraphy, 84.3% CT Specificities: 95.6% US, 92.8% MR, 84.5% scintigraphy, 95.1% CT Per bowel segment analysis: Significantly lower sensitivity and specificity for CT compared to scintigraphy and MRI. Sensitivities: 73.5% US, 70.4% MRI, 77.3% scintigraphy, 67.4% CT. Specificities: 92.9% US, 94% MRI, 90.3% scintigraphy, 90.2% CT |
| Lee et al[22], 2009 | South Korea | 30 CD | Prospective study, single centre, consecutive patients with known or suspected CD enrolled Adult cohort (age range 18-44 yr) | MRE, CT, SBFT for detection of active small bowel inflammation and extra enteric complications with ileocolonoscopy as reference standard | No significant difference between CTE, MRE and SBFT for the detection of active terminal ileitis. Sensitivity CTE (89%), MRE (83%), SBFT (67%-72%) Significantly higher sensitivity for MRE (100%) and CTE (100%) compared to SBFT (32% reader 1, 37% reader 2) for the detection of extra enteric complications |
| Siddiki et al[68], 2009 | United States | 33 CD | Prospective blinded study, single centre, consecutive patients with suspected active small bowel CD April 2005-May 2008 Adult cohort (age range 20-63 yr) | MRE, CTE compared with ileocolonoscopy | No significant difference between sensitivity of MRE (90.5%) and CTE (95.2%) in detecting active small bowel CD In 8 cases (24%) MRE and CTE identified active small bowel inflammation not detected at ileocolonoscopy MRE significantly lower image quality score than CTE |
| Ippolito et al[69], 2009 | Italy | 29 CD | Prospective study, Single centre, symptomatic patients with proven CD and suspected relapse, recruited from outpatient clinic Adult and paediatric cohort (age range 14-70 yr) Mean age 43.8 yr | Contrast MRE and contrast multi-detector CTE | Complete agreement between MRE and CTE in classification of disease activity (k = 1) Good level of agreement between MRE and CTE for wall thickening and mucosal hyperenhancement (k = 1), comb (k = 0.9) and halo signs (k = 0.86) CTE superior to MRE in detecting fibrofatty proliferation (P = 0.045) MRE depicted higher number of fistulas than CTE but non-significant (P = 0.083) |
| Schreyer et al[70], 2010 | Germany | 53 CD | Retrospective study, Single centre, Patients with advanced CD and acute abdominal pain attending the emergency department Adult cohort | Conventional CT, MRE | No significant difference in image quality between CT and MRE No significant difference in diagnosis of small bowel inflammation between CT (69.4%) and MRE (71.4%) CT detection of lymph nodes significantly higher than MRE No significant difference in detection of fistulae (CT n = 25, MRE n = 27) or abscesses (CT n = 32, MRE n = 32) |
| Panés et al[41], 2011 | Spain | N/A | Systematic review of 68 prospective studies, minimum 15 patients per study | US, CT, MRI for diagnosis of CD, assessment of disease extent and activity, detection of complications | Sensitivity for diagnosis of suspected CD and evaluation of disease activity: US 84%, MRI 93% Specificity for diagnosis of suspected CD and evaluation of disease activity: US 92%, MRI 90% CT similar accuracy to MRI for assessment of disease activity and extension. US accuracy lower for disease proximal to terminal ileum US, CT, MRI all high accuracy for detection of fistulas, abscesses, stenosis. US higher false positive for abscesses |
| Fiorino et al[43], 2011 | Italy | 44 CD | Prospective study, Single centre, consecutive patients with ileocolonic CD requiring endoscopic or radiological evaluation Enrolled 2006-2009 Adult cohort (> 18 yr) Mean age 44 yr | CTE and MRE to assess disease activity and complications in ileocolonic CD, using ileocolonoscopy as reference standard | MRE significantly superior to CTE in detecting internal strictures: sensitivity (92% vs 85%), accuracy (95% vs 91%), specificity (90% vs 51%) Overall no significant difference in sensitivity and specificity of MRE and CTE in localising CD, bowel wall thickening, bowel wall enhancement, enteroenteric fistulas, detection of abdominal nodes, perivisceral fat enhancement Per segment analysis, MRE significantly superior to CTE in detecting ileal wall enhancement, with higher sensitivity (93% vs 81%) and accuracy (88% vs 81%), but lower specificity (72% vs 81%). MRE significantly superior in localising rectal disease, with higher accuracy (93% vs 85%), specificity (100% vs 50,9%) but lower sensitivity (72% vs 81%) |
| Jensen et al[71], 2011 | Denmark | 50 CD | Prospective, multicentre study, patients with symptomatic pre-existing CD requiring small bowel imaging for treatment decisions | MRE and CTE compared with gold standard of ileoscopy or surgery | No significant difference between MRE and CTE for detection of small bowel CD MRE: sensitivity 74%, specificity 80% CTE: sensitivity 83%, specificity 70% No significant difference for detection of small bowel stenosis. MRE: sensitivity 55%, specificity 92%. CTE: sensitivity 70%, specificity 92% |
| Chatu et al[50], 2012 | United Kingdom | 143 CD | Retrospective study, single tertiary centre, all symptomatic patients with known or suspected CD who underwent SICUS retrospectively were reviewed June 2007-Dec 2010 Adult cohort Mean age 36 yr | SICUS compared with SBFT, CT, histological findings from ileocolonoscopy or surgery, and CRP, using final diagnosis as the reference standard | Sensitivity of SICUS in detecting active small bowel CD in known or suspected cases 93%, specificity 99%, positive predictive value 98%, negative predictive value 95% Agreement between SICUS with SBFT (k = 0.88), CT (k = 0.91), histological findings (k = 0.62), CRP (k = 0.07) |
| Pallotta et al[51], 2012 | Italy | 49 CD | Prospective study, consecutive patients, adult and paediatric CD who underwent resective bowel surgery Jan 2000-Oct 2010 Mean age 37.7 yr (Age range 12-78 yr) | Conventional transabdominal US and SICUS compared to intraoperative and histological findings to assess CD complications | SICUS ability to: Detect at least one stricture: Sensitivity 97.5%, specificity 100%, k = 0.93 Detect two or more strictures: Sensitivity 75%, specificity 100%, k = 0.78 Detect fistulas: Sensitivity 96%, specificity 90.5%, k = 0.88 Detect intra-abdominal abscesses: Sensitivity 100%, specificity 95%, k = 0.89 |
| Qiu et al[44], 2014 | China | 290 CD | Systematic review with meta-analysis including six studies, all prospective with enrollment of consecutive CD patients | MRE and CTE in detecting active small bowel CD and complications | Pooled sensitivity MRE in detecting active small bowel CD: 87.9%, specificity 81.2% Pooled sensitivity CTE in detecting active small bowel CD 85.8%, specificity 83.6% No significant difference between MRE and CTE in detecting fistula, stenosis and abscesses. |
| Kumar et al[52], 2015 | United Kingdom | 67 CD | Retrospective study, Single tertiary centre. Adult cohort (age 18.8-68.9 yr) CD patients requiring resective bowel surgery within 6 mo of SICUS/MRE investigation being performed June 2007-December 2012 | SICUS and MRE compared to intraoperative findings | Sensitivity of SICUS and MRE in detecting: Strictures: 87.5%, 100% Fistulae: 87.7%, 66.7 Abscesses: 100%, 100% Bowel dilatation: 100%, 66.7% Bowel wall thickening: 94.7% and 81.8% Compared with surgery, high level of agreement of SICUS, MRE in: Localising strictures: k = 0.75, 0.88 Fistulae: k = 0.82, 0.79 Abscesses k = 0.87, 0.77 High level of agreement between SICUS and MRE in identifying stricturing disease (k = 0.84), number and location of strictures (k = 0.85), fistulae (k = 0.65), mucosal thickening (k = 0.61) |
| Aloi et al[53], 2015 | Italy | 25 CD | Single tertiary centre for paediatric IBD Paediatric cohort with known or suspected small bowel CD | MRE, SICUS, CE for diagnosis of small bowel CD | Jejunum: Specificity CE significantly lower (61%) than MRE. No significant difference in sensitivity: SICUS 92%, CE 92%, MRE (75%) Proximal and mid-ileum: Specificity CE significantly lower. No significant difference in sensitivity: MRE 100%, CE 100%, SICUS 80% Terminal ileum: Sensitivity of SICUS and MRE (94%, 94%) higher than CE (81%), CE more specific |
A 2008 meta-analysis by Horsthuis et al[40] comparing magnetic resonance imaging (MRI), ultrasonography (US), scintigraphy and CT across 33 studies, showed high per-patient sensitivity for the diagnosis of IBD with no significant differences between imaging modalities. Mean sensitivity estimates were 93%, 90%, 88% and 84% for MRI, US, white cell scintigraphy and CT respectively. Per-patient specificity was also high and comparable across imaging modalities: 93%, 96%, 85% and 95% for MRI, US, scintigraphy and CT respectively. The only significant difference was a lower specificity for scintigraphy compared to US (P = 0.009)[40]. Mean per-bowel-segment sensitivity estimates were lower across all imaging modalities (70%, 74%, 77% and 68% for MRI, US, scintigraphy and CT respectively). Per-bowel-segment analysis showed CT to be significantly less sensitive and specific compared to MRI (P = 0.037) and scintigraphy (P = 0.006)[40]. More recently, a 2011 systematic review by Panés et al[41] also compared US, MRI and CT for the assessment of disease location and extension in CD. Overall, US had superior diagnostic accuracy for the detection of disease localised to the terminal ileum and colon, while MRI performed better than US for the detection of CD lesions in the jejunum and proximal ileum. CT and MRI demonstrated similar diagnostic accuracy for the assessment of CD extension and activity[41].
MRE is a non-invasive technique used to obtain cross-sectional imaging of the small bowel without exposure to diagnostic medical radiation. MRE provides superior soft tissue contrast resolution compared to CTE, allowing detailed visualisation of inflammatory and fibrotic bowel wall[42]. A 2011 Italian prospective study by Fiorino et al[43] compared MRE and CTE in 44 patients with ileocolonic CD. They found comparable accuracy between MRE and CTE in localising CD, assessing bowel wall thickening, bowel wall enhancement and enteroenteric fistula. However, MRE was superior to CTE in detecting strictures (P = 0.04) and ileal wall enhancement (P = 0.02). A 2014 meta-analysis by Qiu et al[44] of 290 CD patients across six studies, found no significant difference between the diagnostic accuracy of MRE and CTE in detecting active small bowel CD and its complications including fistula, stenosis and abscess formation.
Given its proven diagnostic accuracy, updated guidelines by the European Crohn’s and Colitis Organisation and the European Society of Gastrointestinal and Abdominal Radiology, advocate increased routine usage of MRI for the assessment of small bowel CD, to reduce radiation exposure in this cohort of patients[45].
Recently, diagnostic indices from MRE have been developed to attempt to quantify disease severity. The magnetic resonance index of activity score has demonstrated a significant correlation with the CD endoscopic index of severity[42]. In perianal CD, MRI remains the preferred imaging modality, permitting accurate diagnosis and staging of perianal fistula[42]. Drawbacks of MR imaging, however, include higher procedure costs, lengthy acquisition times and limited availability, particularly out of routine working hours.
The efficacy of MR Colonography (MRC) for the evaluation of colonic disease activity in IBD is less well defined. A 2005 study comparing MRC using contrast gadolinium enemas, to standard colonoscopy in 22 patients with suspected or known IBD revealed disappointing results, with a per-segment sensitivity of 58.8% and 31.6% for identifying colonic inflammation in UC and CD respectively[46]. Other studies have since produced more promising results. A German study of 23 patients with suspected IBD, comparing MRC using water enemas to colonoscopy findings, identified a sensitivity of 87% and specificity of 100% for detecting colonic inflammatory changes[47]. Recent studies have supported the reliability of diffusion weighted imaging MRC (DWI-MRC) for detecting colonic inflammation in UC, without the need for bowel preparation[48]. Advances in contrast media and DWI-MRI may increase the sensitivity and role of MRC in evaluating colonic inflammation in IBD, particularly in patients intolerant to colonoscopy. However, larger scale comparative data is still required.
Trans-abdominal US has increasingly been favoured as a non-invasive imaging tool useful for the diagnosis of small bowel CD. It has advantages over SBFT in detecting extra-intestinal disease, is more cost effective and better tolerated than MRI, and avoids the radiation exposure of CT imaging. However, conventional trans-abdominal US is often limited by the presence of endoluminal gas and collapsed bowel walls, which may obscure pathology[49]. Administering oral contrast prior to performing US promotes bowel loop distension, improving bowel wall visualisation. As a consequence, small intestine contrast-enhanced US (SICUS) has emerged as a more accurate alternative to conventional US for the diagnosis and monitoring of small bowel CD[45,49].
A prospective Italian study by Parente et al[49] of 102 patients with CD, compared conventional US with SICUS for the diagnosis of CD and its intraluminal complications. Per-segment analysis revealed a superior diagnostic accuracy of SICUS in detecting small bowel CD. Indeed use of an oral anechoic contrast agent resulted in an increase in sensitivity from 91.4% to 96.1%[49]. SICUS was also more accurate than conventional US in detecting strictures and measuring the extent of small bowel involvement. Both conventional US and SICUS had a higher diagnostic accuracy than SBE in detecting fistulas, using intra-operative findings as the gold standard[49]. More recently, a United Kingdom-based study of 143 patients with suspected or known CD, found SICUS to have a similar diagnostic yield compared to SBFT and CT (k coefficient 0.88 and 0.91 respectively) for the detection of features of small bowel CD in routine clinical practice[50]. The sensitivity and specificity of SICUS for the detection of active small bowel CD was 93% and 99% respectively, with a positive predictive value of 98% and a negative predictive value of 95%. Furthermore, there was substantial agreement between SICUS and histology obtained at ileocolonoscopy or surgery (k = 0.62)[50].
SICUS may also have a role to play in the pre-operative assessment of CD. A prospective study by Pallotta et al[51] of 49 patients with CD, compared SICUS with intra-operative findings for the detection of small intestinal complications of CD. SICUS demonstrated high sensitivity and specificity for the detection of small bowel strictures (97.5% sensitivity, 100% specificity, k = 0.78), fistulas (96% sensitivity, 90.5% specificity, k = 0.88), and abscesses (100% sensitivity, 95% specificity, k = 0.89)[51]. Similarly, Kumar et al[52] compared SICUS and MRE in routine clinical practice with intra-operative findings in patients with CD requiring surgery. Correlating SICUS and MRE with surgery, there was a high level of agreement in localising strictures (k = 0.75, k = 0.88), fistulae (k = 0.82, 0.79) and abscesses (k = 0.87, 0.77)[52].
SICUS may be particularly well suited to investigating small bowel CD in children, where routine additional challenges include poorer tolerance to ileocolonoscopy (IC) requiring general anaesthetic, difficulty lying still for a time-consuming MRI, and increased sensitivity to ionising radiation. A recent prospective study by Aloi et al[53] compared MRE, SICUS and video capsule endoscopy in the evaluation of 25 children with suspected or known CD. Overall there was no significant difference among the three imaging modalities for the detection of active small bowel CD. Combining diagnostic imaging improved collective sensitivities, and combining SICUS with the serological marker C-reactive protein increased the specificity for the detection of CD from 89% to 100% in the jejunum, and from 79% to 100% in the distal ileum (P < 0.05)[53].
Preliminary data has suggested a role for power Doppler imaging in enhancing the diagnostic accuracy of conventional US and SICUS. Power Doppler US allows assessment of bowel wall vascularity, which has been shown to correlate well with disease activity in CD[54]. It can also aid in distinguishing between inflammatory and fibrotic stenosis[50].
Overall, SICUS has emerged as an accurate, well tolerated, radiation-free imaging tool for the assessment of small bowel CD. Limitations include inter-observer variability and difficulty interpreting and comparing images retrospectively given that it is a dynamic procedure. The diagnostic accuracy of SICUS is operator dependent and often thought to be dependent on experience. Although, in a 2005 Italian study SICUS performed by a inexperienced sonographer achieved superior diagnostic accuracy for assessing small bowel CD lesions compared to conventional trans-abdominal US performed by an experienced sonographer[55]. The results of a large multi-centre prospective study comparing MRE with US in CD patients are keenly awaited[56].
Contrast enhanced ultrasonography (CEUS) is a new technique that involves the administration of an intravenous contrast agent, real-time, during ultrasonography. It allows more accurate evaluation of bowel wall vascularisation. Mural hyper-enhancement following contrast in CEUS has been shown to correlate well with bowel inflammation and allows grading of CD activity[57,58]. CEUS also has the potential additional benefit of better distinguishing between inflammatory and fibro-stenotic lesions, which can be difficult with conventional ultrasound[58]. CEUS does not require oral preparation, therefore it is well tolerated by patients and can be repeatedly performed to monitor disease activity. Limitations include the need for specific software, and increased procedure time. Current studies suggest a role for CEUS in monitoring treatment response in CD, but further prospective studies are required to quantify how well CEUS correlates with endoscopic changes and SICUS[57].
Increased awareness of the cumulative exposure of IBD patients to diagnostic medical radiation is warranted, particularly given the potential for an increased risk of radiation-induced malignancy in patients exposed at a younger age. Creation of radiation diaries is a useful consideration to log total radiation exposures. MRI and SICUS are alternative, radiation-free imaging modalities, with proven diagnostic accuracy, and should be routinely considered for the diagnosis and evaluation of patients with small bowel CD wherever possible. More provision is needed for out-of-hours radiation-free imaging modalities to reduce the need for CT.
P- Reviewer: Ierardi E, Tandon R S- Editor: Gong ZM L- Editor: A E- Editor: Liu XM
| 1. | Loftus EV. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2154] [Article Influence: 102.6] [Reference Citation Analysis (1)] |
| 2. | Jess T, Gamborg M, Matzen P, Munkholm P, Sørensen TI. Increased risk of intestinal cancer in Crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol. 2005;100:2724-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 403] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 3. | Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;14:378-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 215] [Cited by in RCA: 225] [Article Influence: 13.2] [Reference Citation Analysis (1)] |
| 4. | Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277-2284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6212] [Cited by in RCA: 5799] [Article Influence: 322.2] [Reference Citation Analysis (3)] |
| 5. | Ozasa K, Shimizu Y, Suyama A, Kasagi F, Soda M, Grant EJ, Sakata R, Sugiyama H, Kodama K. Studies of the mortality of atomic bomb survivors, Report 14, 1950-2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177:229-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 585] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 6. | Flasar M, Patil S. Radiating disparity in IBD. Dig Dis Sci. 2014;59:504-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, Berrington de González A, Miglioretti DL. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169:2078-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1844] [Cited by in RCA: 1715] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 8. | Brenner DJ, Doll R, Goodhead DT, Hall EJ, Land CE, Little JB, Lubin JH, Preston DL, Preston RJ, Puskin JS. Cancer risks attributable to low doses of ionizing radiation: assessing what we really know. Proc Natl Acad Sci USA. 2003;100:13761-13766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1180] [Cited by in RCA: 1127] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 9. | Radiological Society of North America (RSNA), American college of Radiology (ACR). RadiologyInfo.org. Radiation dose in X-ray and CT exams. Accessed 2015-04. Available from: http://www.radiologyinfo.org/en/pdf/sfty_xray.pdf. |
| 10. | Mettler FA, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1364] [Article Influence: 80.2] [Reference Citation Analysis (0)] |
| 11. | Desmond AN, O’Regan K, Curran C, McWilliams S, Fitzgerald T, Maher MM, Shanahan F. Crohn’s disease: factors associated with exposure to high levels of diagnostic radiation. Gut. 2008;57:1524-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 265] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 12. | Chatu S, Subramanian V, Pollok RC. Meta-analysis: diagnostic medical radiation exposure in inflammatory bowel disease. Aliment Pharmacol Ther. 2012;35:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 13. | Estay C, Simian D, Lubascher J, Figueroa C, O’Brien A, Quera R. Ionizing radiation exposure in patients with inflammatory bowel disease: are we overexposing our patients? J Dig Dis. 2015;16:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Chatu S, Poullis A, Holmes R, Greenhalgh R, Pollok RC. Temporal trends in imaging and associated radiation exposure in inflammatory bowel disease. Int J Clin Pract. 2013;67:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Jung YS, Park DI, Kim ER, Kim YH, Lee CK, Lee SH, Kim JH, Chan Huh K, Jung SA, Yoon SM. Quantifying exposure to diagnostic radiation and factors associated with exposure to high levels of radiation in Korean patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1852-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Herfarth H, Palmer L. Risk of radiation and choice of imaging. Dig Dis. 2009;27:278-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Butcher RO, Nixon E, Sapundzieski M, Filobbos R, Limdi JK. Radiation exposure in patients with inflammatory bowel disease--primum non nocere? Scand J Gastroenterol. 2012;47:1192-1199. [PubMed] |
| 18. | Peloquin JM, Pardi DS, Sandborn WJ, Fletcher JG, McCollough CH, Schueler BA, Kofler JA, Enders FT, Achenbach SJ, Loftus EV. Diagnostic ionizing radiation exposure in a population-based cohort of patients with inflammatory bowel disease. Am J Gastroenterol. 2008;103:2015-2022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 19. | Hafeez R, Greenhalgh R, Rajan J, Bloom S, McCartney S, Halligan S, Taylor SA. Use of small bowel imaging for the diagnosis and staging of Crohn’s disease--a survey of current UK practice. Br J Radiol. 2011;84:508-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Saibeni S, Rondonotti E, Iozzelli A, Spina L, Tontini GE, Cavallaro F, Ciscato C, de Franchis R, Sardanelli F, Vecchi M. Imaging of the small bowel in Crohn’s disease: a review of old and new techniques. World J Gastroenterol. 2007;13:3279-3287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Hara AK, Leighton JA, Heigh RI, Sharma VK, Silva AC, De Petris G, Hentz JG, Fleischer DE. Crohn disease of the small bowel: preliminary comparison among CT enterography, capsule endoscopy, small-bowel follow-through, and ileoscopy. Radiology. 2006;238:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 189] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 22. | Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, Ha HK. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009;251:751-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 23. | Council NR. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: The National Academies Press 2006; . |
| 24. | Patel B, Mottola J, Sahni VA, Cantisani V, Ertruk M, Friedman S, Bellizzi AM, Marcantonio A, Mortele KJ. MDCT assessment of ulcerative colitis: radiologic analysis with clinical, endoscopic, and pathologic correlation. Abdom Imaging. 2012;37:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Laghi A. Computed tomography colonography in 2014: an update on technique and indications. World J Gastroenterol. 2014;20:16858-16867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Bodily KD, Fletcher JG, Solem CA, Johnson CD, Fidler JL, Barlow JM, Bruesewitz MR, McCollough CH, Sandborn WJ, Loftus EV. Crohn Disease: mural attenuation and thickness at contrast-enhanced CT Enterography--correlation with endoscopic and histologic findings of inflammation. Radiology. 2006;238:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 260] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 27. | Samuel S, Bruining DH, Loftus EV, Becker B, Fletcher JG, Mandrekar JN, Zinsmeister AR, Sandborn WJ. Endoscopic skipping of the distal terminal ileum in Crohn’s disease can lead to negative results from ileocolonoscopy. Clin Gastroenterol Hepatol. 2012;10:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 166] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 28. | Johnson KT, Hara AK, Johnson CD. Evaluation of colitis: usefulness of CT enterography technique. Emerg Radiol. 2009;16:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Andersen K, Vogt C, Blondin D, Beck A, Heinen W, Aurich V, Häussinger D, Mödder U, Cohnen M. Multi-detector CT-colonography in inflammatory bowel disease: prospective analysis of CT-findings to high-resolution video colonoscopy. Eur J Radiol. 2006;58:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Bellini D, Rengo M, De Cecco CN, Iafrate F, Hassan C, Laghi A. Perforation rate in CT colonography: a systematic review of the literature and meta-analysis. Eur Radiol. 2014;24:1487-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Govani SM, Guentner AS, Waljee AK, Higgins PD. Risk stratification of emergency department patients with Crohn’s disease could reduce computed tomography use by nearly half. Clin Gastroenterol Hepatol. 2014;12:1702-1707.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Yarur AJ, Mandalia AB, Dauer RM, Czul F, Deshpande AR, Kerman DH, Abreu MT, Sussman DA. Predictive factors for clinically actionable computed tomography findings in inflammatory bowel disease patients seen in the emergency department with acute gastrointestinal symptoms. J Crohns Colitis. 2014;8:504-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Park MJ, Lim JS. Computed tomography enterography for evaluation of inflammatory bowel disease. Clin Endosc. 2013;46:327-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Deepak P, Bruining DH. Radiographical evaluation of ulcerative colitis. Gastroenterol Rep (Oxf). 2014;2:169-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Bennink RJ, Peeters M, Rutgeerts P, Mortelmans L. Evaluation of early treatment response and predicting the need for colectomy in active ulcerative colitis with 99mTc-HMPAO white blood cell scintigraphy. J Nucl Med. 2004;45:1698-1704. [PubMed] |
| 36. | Biancone L, Scopinaro F, Ierardi M, Paoluzi P, Marcheggiano A, Di Paolo MC, Porowska B, Colella AC, Pallone F. 99mTc-HMPAO granulocyte scintigraphy in the early detection of postoperative asymptomatic recurrence in Crohn’s disease. Dig Dis Sci. 1997;42:1549-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Subramanian V, Banerjee A, Beharry N, Farthing MJ, Pollok RC. Determining the proximal extent of ulcerative colitis: white cell scan correlates well with histological assessment. Aliment Pharmacol Ther. 2007;25:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Treglia G, Quartuccio N, Sadeghi R, Farchione A, Caldarella C, Bertagna F, Fania P, Cistaro A. Diagnostic performance of Fluorine-18-Fluorodeoxyglucose positron emission tomography in patients with chronic inflammatory bowel disease: a systematic review and a meta-analysis. J Crohns Colitis. 2013;7:345-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Perlman SB, Hall BS, Reichelderfer M. PET/CT imaging of inflammatory bowel disease. Semin Nucl Med. 2013;43:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 40. | Horsthuis K, Bipat S, Bennink RJ, Stoker J. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology. 2008;247:64-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 432] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 41. | Panés J, Bouzas R, Chaparro M, García-Sánchez V, Gisbert JP, Martínez de Guereñu B, Mendoza JL, Paredes JM, Quiroga S, Ripollés T. Systematic review: the use of ultrasonography, computed tomography and magnetic resonance imaging for the diagnosis, assessment of activity and abdominal complications of Crohn’s disease. Aliment Pharmacol Ther. 2011;34:125-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 475] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 42. | Amitai MM, Ben-Horin S, Eliakim R, Kopylov U. Magnetic resonance enterography in Crohn’s disease: a guide to common imaging manifestations for the IBD physician. J Crohns Colitis. 2013;7:603-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, Fries W, Balzarini L, Montorsi M, Malesci A. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn’s disease. Inflamm Bowel Dis. 2011;17:1073-1080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 175] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 44. | Qiu Y, Mao R, Chen BL, Li XH, He Y, Zeng ZR, Li ZP, Chen MH. Systematic review with meta-analysis: magnetic resonance enterography vs. computed tomography enterography for evaluating disease activity in small bowel Crohn’s disease. Aliment Pharmacol Ther. 2014;40:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 45. | Panes J, Bouhnik Y, Reinisch W, Stoker J, Taylor SA, Baumgart DC, Danese S, Halligan S, Marincek B, Matos C. Imaging techniques for assessment of inflammatory bowel disease: joint ECCO and ESGAR evidence-based consensus guidelines. J Crohns Colitis. 2013;7:556-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 539] [Cited by in RCA: 478] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 46. | Schreyer AG, Rath HC, Kikinis R, Völk M, Schölmerich J, Feuerbach S, Rogler G, Seitz J, Herfarth H. Comparison of magnetic resonance imaging colonography with conventional colonoscopy for the assessment of intestinal inflammation in patients with inflammatory bowel disease: a feasibility study. Gut. 2005;54:250-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Ajaj WM, Lauenstein TC, Pelster G, Gerken G, Ruehm SG, Debatin JF, Goehde SC. Magnetic resonance colonography for the detection of inflammatory diseases of the large bowel: quantifying the inflammatory activity. Gut. 2005;54:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 48. | Oussalah A, Laurent V, Bruot O, Bressenot A, Bigard MA, Régent D, Peyrin-Biroulet L. Diffusion-weighted magnetic resonance without bowel preparation for detecting colonic inflammation in inflammatory bowel disease. Gut. 2010;59:1056-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 49. | Parente F, Greco S, Molteni M, Anderloni A, Sampietro GM, Danelli PG, Bianco R, Gallus S, Bianchi Porro G. Oral contrast enhanced bowel ultrasonography in the assessment of small intestine Crohn’s disease. A prospective comparison with conventional ultrasound, x ray studies, and ileocolonoscopy. Gut. 2004;53:1652-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | Chatu S, Pilcher J, Saxena SK, Fry DH, Pollok RC. Diagnostic accuracy of small intestine ultrasonography using an oral contrast agent in Crohn’s disease: comparative study from the UK. Clin Radiol. 2012;67:553-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Pallotta N, Vincoli G, Montesani C, Chirletti P, Pronio A, Caronna R, Ciccantelli B, Romeo E, Marcheggiano A, Corazziari E. Small intestine contrast ultrasonography (SICUS) for the detection of small bowel complications in crohn’s disease: a prospective comparative study versus intraoperative findings. Inflamm Bowel Dis. 2012;18:74-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | Kumar S, Hakim A, Alexakis C, Chhaya V, Tzias D, Pilcher J, Vlahos J, Pollok R. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn’s disease: correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol. 2015;30:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 53. | Aloi M, Di Nardo G, Romano G, Casciani E, Civitelli F, Oliva S, Viola F, Maccioni F, Gualdi G, Cucchiara S. Magnetic resonance enterography, small-intestine contrast US, and capsule endoscopy to evaluate the small bowel in pediatric Crohn’s disease: a prospective, blinded, comparison study. Gastrointest Endosc. 2015;81:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Drews BH, Barth TF, Hänle MM, Akinli AS, Mason RA, Muche R, Thiel R, Pauls S, Klaus J, von Boyen G. Comparison of sonographically measured bowel wall vascularity, histology, and disease activity in Crohn’s disease. Eur Radiol. 2009;19:1379-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | Calabrese E, La Seta F, Buccellato A, Virdone R, Pallotta N, Corazziari E, Cottone M. Crohn’s disease: a comparative prospective study of transabdominal ultrasonography, small intestine contrast ultrasonography, and small bowel enema. Inflamm Bowel Dis. 2005;11:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 56. | Taylor S, Mallett S, Bhatnagar G, Bloom S, Gupta A, Halligan S, Hamlin J, Hart A, Higginson A, Jacobs I. METRIC (MREnterography or ulTRasound in Crohn’s disease): a study protocol for a multicentre, non-randomised, single-arm, prospective comparison study of magnetic resonance enterography and small bowel ultrasound compared to a reference standard in those aged 16 and over. BMC Gastroenterol. 2014;14:142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Ripollés T, Martínez-Pérez MJ, Blanc E, Delgado F, Vizuete J, Paredes JM, Vilar J. Contrast-enhanced ultrasound (CEUS) in Crohn’s disease: technique, image interpretation and clinical applications. Insights Imaging. 2011;2:639-652. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 58. | Quaia E. Contrast-enhanced ultrasound of the small bowel in Crohn’s disease. Abdom Imaging. 2013;38:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Newnham E, Hawkes E, Surender A, James SL, Gearry R, Gibson PR. Quantifying exposure to diagnostic medical radiation in patients with inflammatory bowel disease: are we contributing to malignancy? Aliment Pharmacol Ther. 2007;26:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 60. | Levi Z, Fraser E, Krongrad R, Hazazi R, benjaminov O, meyerovitch J, Tal OB, Choen A, Niv Y, Fraser G. Factors associated with radiation exposure in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;30:1128-1136. [PubMed] |
| 61. | Palmer L, Herfarth H, Porter CQ, Fordham LA, Sandler RS, Kappelman MD. Diagnostic ionizing radiation exposure in a population-based sample of children with inflammatory bowel diseases. Am J Gastroenterol. 2009;104:2816-2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 62. | Kroeker KI, Lam S, Birchall I, Fedorak RN. Patients with IBD are exposed to high levels of ionizing radiation through CT scan diagnostic imaging: a five-year study. J Clin Gastroenterol. 2011;45:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 63. | Fuchs Y, Markowitz J, Weinstein T, Kohn N, Choi-Rosen J, Levine J. Pediatric inflammatory bowel disease and imaging-related radiation: are we increasing the likelihood of malignancy? J Pediatr Gastroenterol Nutr. 2011;52:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Sauer CG, Kugathasan S, Martin DR, Applegate KE. Medical radiation exposure in children with inflammatory bowel disease estimates high cumulative doses. Inflamm Bowel Dis. 2011;17:2326-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Huang JS, Tobin A, Harvey L, Nelson TR. Diagnostic medical radiation in pediatric patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2011;53:502-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Low RN, Francis IR, Politoske D, Bennett M. Crohn’s disease evaluation: comparison of contrast-enhanced MR imaging and single-phase helical CT scanning. J Magn Reson Imaging. 2000;11:127-135. [PubMed] |
| 67. | Maconi G, Sampietro GM, Parente F, Pompili G, Russo A, Cristaldi M, Arborio G, Ardizzone S, Matacena G, Taschieri AM. Contrast radiology, computed tomography and ultrasonography in detecting internal fistulas and intra-abdominal abscesses in Crohn’s disease: a prospective comparative study. Am J Gastroenterol. 2003;98:1545-1555. [PubMed] |
| 68. | Siddiki HA, Fidler JL, Fletcher JG, Burton SS, Huprich JE, Hough DM, Johnson CD, Bruining DH, Loftus EV, Sandborn WJ. Prospective comparison of state-of-the-art MR enterography and CT enterography in small-bowel Crohn’s disease. AJR Am J Roentgenol. 2009;193:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 277] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 69. | Ippolito D, Invernizzi F, Galimberti S, Panelli MR, Sironi S. MR enterography with polyethylene glycol as oral contrast medium in the follow-up of patients with Crohn disease: comparison with CT enterography. Abdom Imaging. 2010;35:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Schreyer AG, Hoffstetter P, Daneschnejad M, Jung EM, Pawlik M, Friedrich C, Fellner C, Strauch U, Klebl F, Herfarth H. Comparison of conventional abdominal CT with MR-enterography in patients with active Crohn’s disease and acute abdominal pain. Acad Radiol. 2010;17:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Jensen MD, Kjeldsen J, Rafaelsen SR, Nathan T. Diagnostic accuracies of MR enterography and CT enterography in symptomatic Crohn’s disease. Scand J Gastroenterol. 2011;46:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |