Published online Feb 7, 2016. doi: 10.3748/wjg.v22.i5.1884
Peer-review started: July 18, 2015
First decision: August 26, 2015
Revised: September 20, 2015
Accepted: November 19, 2015
Article in press: November 19, 2015
Published online: February 7, 2016
Processing time: 188 Days and 19.1 Hours
AIM: To explore the association between serum α-L-fucosidase (AFU) and non-alcoholic fatty liver disease (NAFLD).
METHODS: A total of 16473 individuals (9456 men and 7017 women) were included in the current study, who presented for a health examination at the First Affiliated Hospital of Zhejiang University School of Medicine in 2014. The baseline characteristics of the cohort were compared by NAFLD status. Linear regression analysis and stepwise multiple regression analysis were applied to assess the risk factors for NAFLD. Receiver operating characteristic curve was used to determine the sensitivity and specificity of AFU in the diagnosis of NAFLD.
RESULTS: The prevalence rates of NAFLD and metabolic syndrome (MetS) were 38.0% and 25.4%, respectively. The NAFLD group had significantly higher AFU levels than the non-NAFLD group (28.7 ± 7.9 U/L vs 26.0 ± 7.3 U/L, P < 0.001) and the prevalence rate of NAFLD increased with progressively higher serum AFU levels. AFU was positively correlated with MetS and its five components: central obesity, hypertriglyceridemia, low high-density lipoprotein cholesterol, and elevated blood pressure and fasting glucose. Stepwise multiple logistic regression analysis showed that AFU was associated with an increased risk of NAFLD (OR = 1.009, 95%CI: 1.003-1.014, P < 0.001). The best cut-off value of AFU for the diagnosis of NAFLD was 27.5 U/L. The area under the curve (diagnostic efficacy index) was 0.606. The sensitivity and specificity were 54.6% and 61.8%, respectively.
CONCLUSION: AFU level is significantly associated with NAFLD, and elevated AFU level is an independent risk factor for NAFLD.
Core tip: Alpha-L-fucosidase (AFU) is a well-established marker for hepatocellular carcinoma. This study was the first attempt to investigate the relationship between AFU level and non-alcoholic fatty liver disease (NAFLD) in a large cross-sectional cohort from a southern urban Han Chinese population. It provided evidence that AFU level was significantly associated with NAFLD, and elevated AFU level was an independent risk factor for NAFLD. AFU may be a potential biomarker for the diagnosis of NAFLD.
- Citation: Lu ZY, Cen C, Shao Z, Chen XH, Xu CF, Li YM. Association between serum α-L-fucosidase and non-alcoholic fatty liver disease: Cross-sectional study. World J Gastroenterol 2016; 22(5): 1884-1890
- URL: https://www.wjgnet.com/1007-9327/full/v22/i5/1884.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i5.1884
Non-alcoholic fatty liver disease (NAFLD) has attracted attention for its high prevalence (20%-30%) in developed countries[1,2]. The development of NAFLD is closely associated with central obesity, type 2 diabetes, hypertension, and dyslipidemia, which form a cluster of metabolic disorders that is now recognized as metabolic syndrome (MetS)[3,4]. For this reason, NAFLD is often considered a hepatic manifestation of MetS[3].
Alpha-L-fucosidase (AFU) is a sort of lysosomal enzyme present in all mammalian cells and hydrolyzes sugars containing L-fucose[5]. Deugnier et al[6] first found that AFU is overexpressed in patients with hepatocellular carcinoma (HCC) in 1984. The sensitivity and specificity of AFU for the diagnosis of HCC were about 80% and 70%, respectively, in contrast with 40% and almost 100% for α-fetoprotein (AFP)[7]. A simultaneous determination of both markers can improve the sensitivity to 82%[7]. AFU has been clinically used widely as a supplement to AFP in early detection of HCC.
NAFLD is a clinicopathological syndrome that ranges from simple steatosis to steatohepatitis, fibrosis or cirrhosis of the liver[8], and cirrhosis is the most important risk factor for HCC, regardless of etiology[9]. Thus, NAFLD is often considered a precursor for HCC[10]. Due to the high sensitivity of AFU in early detection of HCC, we hypothesized that AFU could be a biomarker for diagnosis of NAFLD, which is a precursor of HCC.
In this study, we performed a large cross-sectional survey to analyze the association between AFU and NAFLD in a Chinese population.
We conducted a cross-sectional study among adults who presented for their annual health examinations at the First Affiliated Hospital of Zhejiang University School of Medicine in 2014. The analyses were limited to participants who had full records of anthropometric and biochemical data, as well as results of hepatic ultrasonography examination. Exclusion criteria included: (1) those taking antihypertensive or antidiabetic agents, lipid-lowering agents, or uric-acid-lowing agents; (2) those with alcohol consumption > 140 g/wk for men and 70 g/wk for women; (3) those with a history of other known causes of chronic liver disease such as viral hepatitis or autoimmune hepatitis; and (4) those using hepatotoxic medications (e.g., sulfonamides and azithromycin). A total of 16473 participants (9456 men and 7017 women) were included in the final analysis. All participants were informed verbally about the purpose and design of the study. The personal information of each participant was anonymized both at collection and prior to analysis. The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhejiang University School of Medicine.
Study data included four parts: medical history, questionnaire, and anthropometric and biochemical measurements. All medical histories including previous diseases and drug prescription were assessed by examining physicians. Questions about alcohol intake included the frequency of alcohol consumption per week and the usual amount per day. Persons smoking at that time were considered to be current smokers.
The anthropometric measurements involved height, weight, blood pressure and waist circumference (WC). Height and weight were measured while wearing light clothing without shoes. Body mass index (BMI) was calculated as weight (kg) divided by the square of the height (m). Blood pressure, including systolic blood pressure (SBP) and diastolic blood pressure (DBP), was measured on the right arm with participants in a sitting position after a 5-min rest. WC was measured with the measuring tape positioned midway between the lowest rib and the superior border of the iliac crest as the patient exhaled normally.
Biochemical measurements were performed after participants were instructed to complete an overnight fast. Fasting blood samples were obtained from an antecubital vein, and the samples were used for the analysis of biochemical values. The values included triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), γ-glutamyltransferase (GGT), AFU, uric acid (UA), and fasting plasma glucose (FPG). All biochemical values were measured using a Hitachi 7600 clinical analyzer (Hitachi, Tokyo, Japan) and Sysmex XE-2100 auto-analyzer (Sysmex, Kobe, Japan) using standard methods.
Abdominal ultrasonographic examinations were carried out by experienced radiologists who were unaware of the aims of the study and were blinded to the laboratory values, using a Toshiba Nemio 20 sonography machine (Toshiba, Tokyo, Japan) with a 3.5-MHz probe. Images were captured in a standard fashion, with the patient in the supine position, with the right arm raised above the head. Fatty liver disease was diagnosed and its degree was assessed according to the criteria described by the Chinese Liver Disease Association[11].
The diagnosis of MetS was based on the definition recommended by the Asia-Pacific Working Party on NAFLD 2006[12]. MetS was diagnosed if any three or more of the following were present: (1) central obesity: WC > 90 cm for men and > 80 cm for women and/or BMI > 25 kg/m2 in both genders; (2) hypertriglyceridemia: TGs ≥ 1.7 mmol/L; (3) low HDL-C: HDL-C < 1.03 mmol/L for men and < 1.29 mmol/L for women; (4) elevated blood pressure: blood pressure ≥ 130/85 mmHg; and (5) elevated fasting glucose: FPG ≥ 5.6 mmol/L or previously diagnosed type 2 diabetes.
Statistical analyses were performed using SPSS for Windows version 13.0 (SPSS, Chicago, IL, United States). Continuous variables are presented as the mean ± SD or the median and interquartile range (IQR), as appropriate. The Student’s t test or Mann-Whitney U test was used for comparisons of continuous data, while the χ2 test was used for comparisons of categorical variables. Linear regression analysis was used to determine the relationship between AFU level and prevalence of NAFLD and MetS. Stepwise multiple regression analysis (Backward: Wald; Entry: 0.05, Removal: 0.10) was applied to assess the risk factors for NAFLD. P < 0.05 (two-tailed test) was considered statistically significant. The receiver operating characteristic (ROC) curve was used to determine the sensitivity and specificity of AFU in the diagnosis of NAFLD.
Of the 16473 subjects enrolled in this study, 6263 (38.0%) and 4177 (25.4%) fulfilled the diagnostic criteria for NAFLD and MetS, respectively. The prevalence rates of MetS components, including central obesity, hypertriglyceridemia, low HDL-C, elevated blood pressure and elevated FPG, were 46.48%, 29.11%, 36.73%, 33.98% and 12.19%, respectively. Demographic and biochemical characteristics were compared by NAFLD status (Table 1). Patients with NAFLD exhibited higher AFU. Meanwhile, BMI, WC, SBP, DBP, white blood cell count, UA, FBG, TG, TC, LDL, very-low density lipoprotein, alanine aminotransferase (ALT), aspartate aminotransferase (AST), GGT, cholinesterase, alkaline phosphatase, carcinoembryonic antigen and HDL were higher in the NAFLD group.
| Variable | With NAFLD | Without NAFLD | t value | P value |
| Age (yr) | 47.9 (10.3) | 44.3 (11.2) | 20.844 | < 0.001 |
| Gender (male/female, n) | 4616/1647 | 4840/5370 | 1097.9281 | < 0.0011 |
| BMI (kg/m2) | 26.1 (2.9) | 22.5 (2.7) | 80.032 | < 0.001 |
| WC (cm) | 90.7 (8.2) | 79.7 (8.5) | 80.773 | < 0.001 |
| SBP (mmHg) | 134.2 (17.5) | 123.1 (17.1) | 39.998 | < 0.001 |
| DBP (mmHg) | 82.3 (11.1) | 74.6 (11.2) | 42.506 | < 0.001 |
| WBC (109/L) | 6.41 (1.6) | 5.83 (1.5) | 23.672 | < 0.001 |
| UA (mmol/L) | 369.8 (87.8) | 303.3 (81.2) | 49.434 | < 0.001 |
| FBG (mmol/L) | 5.00 (4.66-5.49) | 4.72 (4.46-5.01) | 30.8582 | < 0.0012 |
| TG (mmol/L) | 1.70 (1.20-2.45) | 1.00 (0.73-1.43) | 44.9602 | < 0.0012 |
| TC (mmol/L) | 4.9 (0.9) | 4.62 (0.9) | 22.188 | < 0.001 |
| LDL-C (mmol/L) | 2.7 (0.7) | 2.6 (0.6) | 13.685 | < 0.001 |
| HDL-C (mmol/L) | 1.1 (0.3) | 1.3 (0.3) | 39.894 | < 0.001 |
| AFU (U/L) | 28.7 (7.9) | 26.0 (7.3) | 22.591 | < 0.001 |
| ALT (U/L) | 25 (18-37) | 16 (11-22) | 30.3962 | < 0.0012 |
| AST (U/L) | 22 (18-27) | 19 (16-22) | 16.9162 | < 0.0012 |
| GGT (U/L) | 34 (22-57) | 17 (12-28) | 27.7532 | < 0.0012 |
| Cholinesterase (U/L) | 9537 (1579) | 8315 (1585) | 48.125 | < 0.001 |
| ALP (U/L) | 68 (20.1) | 61 (19.9) | 19.818 | < 0.001 |
| AFP (μg/L) | 2.6 (2.0-3.5) | 2.4 (1.8-3.4) | 0.2102 | < 0.8412 |
In order to have a further understanding of the association between AFU and NAFLD, all 16473 subjects were classified into quartiles by their AFU levels (quartile 1 was defined as AFU ≤ 22 U/L, quartile 2 was 22-27 U/L, quartile 3 was UA 27-31 U/L, and quartile 4 was ≥ 31 U/L). As seen in Table 2, the prevalence rate of NAFLD was significantly and positively correlated with AFU levels. The prevalence rate for NAFLD substantially increased with increasing AFU levels. Compared with individuals in the lowest AFU quartile, those in the highest quartile had a prevalence ratio of 1.85.
| AFU level quartile | Total | NAFLD | χ2 | P value | PR% | PR |
| Quartile 1 | 4119 | 1137 | - | - | 27.60 | 1.00 |
| Quartile 2 | 4118 | 1375 | 32.527 | < 0.001 | 33.39 | 1.21 |
| Quartile 3 | 4118 | 1652 | 143.969 | < 0.001 | 40.11 | 1.45 |
| Quartile 4 | 4118 | 2099 | 471.421 | < 0.001 | 50.97 | 1.85 |
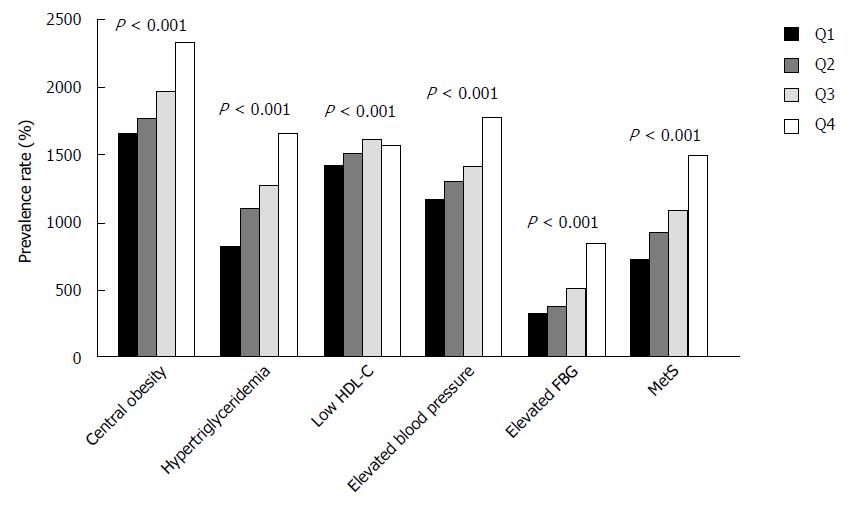
NAFLD is often considered a hepatic manifestation of MetS. To understand better the role of AFU in increasing incidence of NAFLD, we performed another investigation on the association between AFU and MetS. The results showed a significantly higher prevalence rate of MetS with higher AFU levels. In addition, all the five components (central obesity, hypertriglyceridemia, low HDL-C, elevated blood pressure, and elevated FPG) were also seen to be significantly and positively correlated with AFU (Figure 1). It can be inferred that AFU level may be not only an important factor for NAFLD, but also a significant factor for MetS.
To explore the independent risk factors associated with the presence of NAFLD, we performed stepwise multiple regression analysis with a logistic regression model. AFU was found to be a significant independent risk factor for NAFLD (OR = 1.009, 95%CI: 1.003-1.014, P < 0.001). The other risk factors are listed in Table 3, including age, gender, height, weight, BMI, WC, DBP, platelet count, white blood cell count, neutrophil, albumin, UA, FBG, TG, HDL, AFU, ALT, AST, cholinesterase, and AFP.
| Variable | β | SE | Wald χ2 | P value | OR | 95%CI |
| Age | 0.023 | 0.002 | 90.042 | < 0.001 | 1.023 | 1.018-1.028 |
| Male gender | 0.510 | 0.072 | 49.919 | < 0.001 | 1.665 | 1.445-1.918 |
| Height | -0.040 | 0.011 | 13.337 | < 0.001 | 0.961 | 0.941-0.982 |
| Weight | 0.055 | 0.013 | 18.069 | < 0.011 | 1.057 | 1.030-1.084 |
| BMI | 0.087 | 0.034 | 6.456 | < 0.001 | 1.090 | 1.020-1.166 |
| WC | 0.048 | 0.005 | 88.152 | < 0.001 | 1.049 | 1.039-1.060 |
| DBP | 0.010 | 0.002 | 23.193 | < 0.001 | 1.010 | 1.006-1.014 |
| Plt | 0.001 | 0.000 | 3.157 | < 0.001 | 1.001 | 1.000-1.002 |
| WBC | 0.192 | 0.034 | 30.938 | < 0.001 | 1.211 | 1.132-1.296 |
| NEU | -0.202 | 0.043 | 21.986 | < 0.001 | 0.817 | 0.751-0.889 |
| ALB | 0.039 | 0.008 | 26.043 | < 0.001 | 1.039 | 1.024-1.055 |
| UA | 0.003 | 0.000 | 97.787 | < 0.001 | 1.003 | 1.003-1.004 |
| FBG | 0.205 | 0.021 | 93.656 | < 0.001 | 1.227 | 1.177-1.279 |
| TG | 0.226 | 0.022 | 107.298 | < 0.001 | 1.253 | 1.201-1.308 |
| HDL | -0.493 | 0.082 | 36.514 | < 0.002 | 0.611 | 0.520-0.717 |
| AFU | 0.009 | 0.003 | 9.243 | < 0.001 | 1.009 | 1.003-1.014 |
| ALT | 0.022 | 0.002 | 95.540 | < 0.001 | 1.022 | 1.017-1.026 |
| AST | -0.025 | 0.004 | 45.869 | < 0.001 | 0.975 | 0.968-0.982 |
| ChE | 0.000 | 0.000 | 60.140 | < 0.042 | 1.000 | 1.000-1.000 |
| AFP | -0.015 | 0.008 | 4.135 | < 0.001 | 0.985 | 0.970-0.999 |
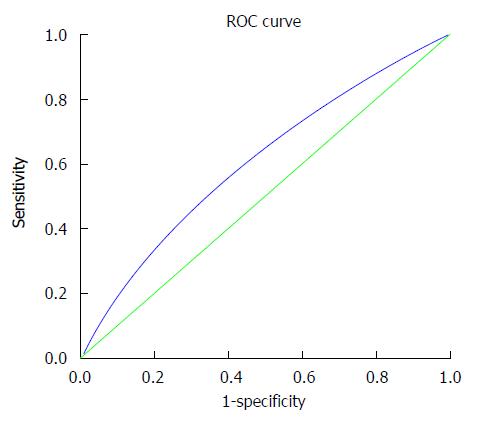
The ROC curve of AFU plotted for the diagnosis of NAFLD is shown in Figure 2. The best cut-off value for AFU was 27.5 U/L, at which the sensitivity was 54.6% and the specificity was 61.8%. The area under the curve (diagnostic efficacy index) was 0.606.
This study may be the first to investigate the relationship between AFU level and NAFLD. The prevalence rates of NAFLD and MetS were 38.0% and 25.4%, respectively, which were comparable with recent studies that investigated the association between NAFLD and MetS in the Chinese population[13-15]. In this study, we provided evidence that AFU level was independently associated with NAFLD. The NAFLD group tended to have elevated AFU levels compared with the non-NAFLD group. In addition, the prevalence rate of NAFLD increased with elevated AFU levels, which means that the subjects with elevated AFU levels had a higher risk of NAFLD. We further analyzed the association between AFU and MetS to confirm indirectly the relationship between AFU and NAFLD. Similarly, the results showed that AFU was positively correlated with MetS and its five components. Logistic regression analysis was performed to screen the risk factors for NAFLD and AFU was found to be an independent risk factor for NAFLD. Finally, the sensitivity was 54.6% and specificity was 61.8% for the diagnosis of NAFLD at the best cut-off value of 27.5 U/L.
However, the physiological mechanism for this association remains unclear. There exist several possible explanations for the relationship. One of the most convincing explanations is that the AFU in the serum comes from lysosomal leakage. The subjects with NAFLD tend to have hypertriglyceridemia and low HDL-C, and lipid peroxidation has been demonstrated to be involved in the formation of NAFLD[16,17]. Lipid peroxidation modifies the functional characteristics not only of the cell membranes, but also membranes of intracellular organelles such as mitochondria and lysosomes[18,19]. The ensuing changes following lysosomal membrane oxidation induce perturbation in this membrane permeability and may result in leakage of lysosomal AFU[20]. According to this explanation, AFU is deemed to be an indicator to monitor the change in membrane permeability.
Another reasonable explanation was related to inflammatory response in NAFLD. It has been demonstrated that development and progression of hepatic inflammation play a key role in the formation and progress of NAFLD[21,22]. As seen in this and previous studies, higher white blood cell counts are known to be associated with the presence of NAFLD[23]. AFU can modulate inflammation by reducing the interaction between fucosylated adhesion molecules, which normally support white blood cell extravasation[24]. Thus, AFU could be seen as a mediator in the NAFLD-associated chronic hepatic inflammation[25].
AFU seems not to be a satisfactory biomarker for the diagnosis of NAFLD with respect to the sensitivity and specificity compared with other biomarkers, such as cytokeratin 18 (AUROC = 0.8)[26,27], HAIR score (hypertension, ALT, insulin resistance, AUROC = 0.9)[28]. But AFU has the advantages of widespread application and convenience of use as a simple biomarker. This is a preliminary study in the investigation of NAFLD biomarkers and further studies are needed to improve the sensitivity and specificity (i.e., setting up a new scoring system including other available biochemical indices).
AFU was established as a tumor marker in a series of studies reporting that its activity increases significantly in the serum of HCC patients[7,29,30]. In the current study, AFU was demonstrated as an independent risk factor for NAFLD. In addition, the link between AFU and NAFLD may provide a potential explanation for why AFU is often elevated in HCC patients. Tumor cell injury, tissue necrosis and mononuclear macrophage accumulation are often present in HCC, thus, elevated AFU level could be an indicator of changes in membrane permeability following cell injury or inflammation response.
There were several limitations to this study. First, the diagnosis of NAFLD was based on ultrasonographic examination. Although liver biopsy is recognized as the gold standard for the diagnosis of NAFLD, invasiveness and complications make it impractical for screening of NAFLD. Ultrasonographic examination has been widely used because of its non-invasiveness and reasonable accuracy, although it is still not sensitive enough to detect mild steatosis. Second, the subjects enrolled in this study were mostly office staff, which is a middle-income group. The prevalence rate of NAFLD and MetS may have been overestimated. Third, it is single-center experience and there was no validation study for AFU. It is hard to confirm the generation of our findings. We are planning to conduct a multi-center large cohort study to investigate the applicability of our findings to the rest of the Chinese population. Moreover, it is still an unresolved question whether elevated AFU is a bystander effect, a cause, or a consequence of NAFLD.
In summary, our large cross-sectional study shows that AFU levels are positively associated with NAFLD and may act as an independent risk factor. AFU may be a potential biomarker for the diagnosis of NAFLD. Further studies are needed to reveal the detailed relationship and the possible mechanisms between serum AFU and NAFLD.
The authors wish to thank the participating department of gastroenterology, the First Affiliated Hospital of Zhejiang University School of Medicine for its expert input and detailed evaluations.
Non-alcoholic fatty liver disease (NAFLD) has attracted attention in recent years because it may progress to hepatocellular carcinoma (HCC). There is no good biomarker to predict NAFLD and this is the first study to investigate the association between serum α-L-fucosidase (AFU) and NAFLD.
Several biomarkers have been investigated to predict NAFLD, but none seems satisfactory.
This study found that AFU levels were positively associated with NAFLD and may act as an independent risk factor. AFU may be a potential biomarker for the diagnosis of NAFLD.
AFU levels are positively associated with NAFLD, which may provide a new promising biomarker to predict NAFLD. It is necessary to monitor serum AFU levels when people undergo health examination.
Changes in permeability of cell membranes and intracellular organelles such as mitochondria and lysosomes are seen in hepatocytes of patients with NAFLD. AFU comes from lysosomal leakage and may act as a predictor of liver function.
It is a preliminary study, which is the first to show AFU levels are positively associated with NAFLD and may act as an independent risk factor. The design is strict and methods are valid. We are inferred that it is necessary to monitor serum AFU to screen NAFLD and the potential risk of HCC.
P- Reviewer: Sertoglu E S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Ma S
| 1. | Jimba S, Nakagami T, Takahashi M, Wakamatsu T, Hirota Y, Iwamoto Y, Wasada T. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22:1141-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 2. | Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37:1202-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1488] [Cited by in RCA: 1478] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 3. | Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844-1850. [PubMed] |
| 4. | Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, Natale S, Vanni E, Villanova N, Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1907] [Cited by in RCA: 1917] [Article Influence: 87.1] [Reference Citation Analysis (0)] |
| 5. | Stefaniuk P, Cianciara J, Wiercinska-Drapalo A. Present and future possibilities for early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2010;16:418-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 124] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 6. | Deugnier Y, David V, Brissot P, Mabo P, Delamaire D, Messner M, Bourel M, Legall JY. Serum alpha-L-fucosidase: a new marker for the diagnosis of primary hepatic carcinoma? Hepatology. 1984;4:889-892. [PubMed] |
| 7. | Tangkijvanich P, Tosukhowong P, Bunyongyod P, Lertmaharit S, Hanvivatvong O, Kullavanijaya P, Poovorawan Y. Alpha-L-fucosidase as a serum marker of hepatocellular carcinoma in Thailand. Southeast Asian J Trop Med Public Health. 1999;30:110-114. [PubMed] |
| 8. | Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3655] [Cited by in RCA: 3718] [Article Influence: 161.7] [Reference Citation Analysis (2)] |
| 9. | Flores A, Marrero JA. Emerging trends in hepatocellular carcinoma: focus on diagnosis and therapeutics. Clin Med Insights Oncol. 2014;8:71-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 10. | Jiang CM, Pu CW, Hou YH, Chen Z, Alanazy M, Hebbard L. Non alcoholic steatohepatitis a precursor for hepatocellular carcinoma development. World J Gastroenterol. 2014;20:16464-16473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Zeng MD, Fan JG, Lu LG, Li YM, Chen CW, Wang BY, Mao YM. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver diseases. J Dig Dis. 2008;9:108-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22:794-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 13. | Zhang T, Zhang Y, Zhang C, Tang F, Li H, Zhang Q, Lin H, Wu S, Liu Y, Xue F. Prediction of metabolic syndrome by non-alcoholic fatty liver disease in northern urban Han Chinese population: a prospective cohort study. PLoS One. 2014;9:e96651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol. 2009;50:1029-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Zhao Y, Xu C, Hong Y, Lu H, Wu J, Chen Y. Association between serum free fatty acid levels and nonalcoholic fatty liver disease: a cross-sectional study. Sci Rep. 2014;4:5832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 16. | Morita M, Ishida N, Uchiyama K, Yamaguchi K, Itoh Y, Shichiri M, Yoshida Y, Hagihara Y, Naito Y, Yoshikawa T. Fatty liver induced by free radicals and lipid peroxidation. Free Radic Res. 2012;46:758-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Bell LN, Molleston JP, Morton MJ, Klipsch A, Saxena R, Vuppalanchi R, Chalasani N. Hepatic lipid peroxidation and cytochrome P-450 2E1 in pediatric nonalcoholic fatty liver disease and its subtypes. J Clin Gastroenterol. 2011;45:800-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Hartnett ME, Stratton RD, Browne RW, Rosner BA, Lanham RJ, Armstrong D. Serum markers of oxidative stress and severity of diabetic retinopathy. Diabetes Care. 2000;23:234-240. [PubMed] |
| 19. | Schleicher E, Nerlich A. The role of hyperglycemia in the development of diabetic complications. Horm Metab Res. 1996;28:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Hickman P, McCollum PT, Belch JJ. Neutrophils may contribute to the morbidity and mortality of claudicants. Br J Surg. 1994;81:790-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med. 2010;363:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1326] [Cited by in RCA: 1485] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 22. | Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52:1836-1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1543] [Cited by in RCA: 1819] [Article Influence: 121.3] [Reference Citation Analysis (0)] |
| 23. | Lee YJ, Lee HR, Shim JY, Moon BS, Lee JH, Kim JK. Relationship between white blood cell count and nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Ali S, Jenkins Y, Kirkley M, Dagkalis A, Manivannan A, Crane IJ, Kirby JA. Leukocyte extravasation: an immunoregulatory role for alpha-L-fucosidase? J Immunol. 2008;181:2407-2413. [PubMed] |
| 25. | Schuck RN, Zha W, Edin ML, Gruzdev A, Vendrov KC, Miller TM, Xu Z, Lih FB, DeGraff LM, Tomer KB. The cytochrome P450 epoxygenase pathway regulates the hepatic inflammatory response in fatty liver disease. PLoS One. 2014;9:e110162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 480] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 27. | Diab DL, Yerian L, Schauer P, Kashyap SR, Lopez R, Hazen SL, Feldstein AE. Cytokeratin 18 fragment levels as a noninvasive biomarker for nonalcoholic steatohepatitis in bariatric surgery patients. Clin Gastroenterol Hepatol. 2008;6:1249-1254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 28. | Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 901] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 29. | Takahashi H, Saibara T, Iwamura S, Tomita A, Maeda T, Onishi S, Yamamoto Y, Enzan H. Serum alpha-L-fucosidase activity and tumor size in hepatocellular carcinoma. Hepatology. 1994;19:1414-1417. [PubMed] |
| 30. | Zhu J, Jiang F, Ni HB, Xiao MB, Chen BY, Ni WK, Lu CH, Ni RZ. Combined analysis of serum γ-glutamyl transferase isoenzyme II, α-L-fucosidase and α-fetoprotein detected using a commercial kit in the diagnosis of hepatocellular carcinoma. Exp Ther Med. 2013;5:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |