Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10584
Peer-review started: August 24, 2016
First decision: September 28, 2016
Revised: October 10, 2016
Accepted: November 15, 2016
Article in press: November 16, 2016
Published online: December 28, 2016
Processing time: 124 Days and 19.2 Hours
To investigate the pharmacological effect of TongXie-YaoFang (TXYF) formula, a Chinese herbal formula, on Diarrhea-predominant irritable bowel syndrome (D-IBS) rats.
In a neonatal maternal separation plus restraint stress (NMS + RS) model of D-IBS, male Sprague Dawley rats were randomly divided into two groups (NMS + RS group and TXYF-formula group) with no handlings were used as controls (NH group). Starting from postnatal day 60, rats in TXYF-formula group were administered TXYF-formula (4.92 g/100 g bodyweight) orally twice a day for 14 consecutive days while NH group and NMS + RS group were given distilled water. Using short-circuit current technology, we observed 5-HT-induced changes of current across ion channels, such as cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel, epithelial Na+ channel (ENaC), Ca2+-dependent Cl- channel (CACC), Na+-K+-2Cl- co-transporter (NKCC), and Na+-HCO3- co-transporter (NBC), in the colonic epithelium of three groups after exposure to drugs and specific blockers with a Power Lab System (AD Instruments International).
Under basal conditions, the changes of short-circuit current (∆Isc, µA/cm2) induced by 5-HT were similar in NH group and TXYF-formula group, and both higher than NMS + RS group (70.86 µA/cm2 ± 12.32 µA/cm2, 67.67 µA/cm2 ± 11.68 µA/cm2 vs 38.8 µA/cm2 ± 7.25 µA/cm2, P < 0.01, respectively). When CACC was blocked by 4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid, 5-HT-induced ∆Isc was smaller in NMS + RS group than in NH group and TXYF-formula group, respectively (48.41 µA/cm2 ± 13.15 µA/cm2 vs 74.62 µA/cm2 ± 10.73 µA/cm2, 69.22 µA/cm2 ± 11.7 µA/cm2, P < 0.05, respectively). The similar result could be obtained when ENaC was blocked by Amiloride (44.69 µA/cm2 ± 12.58 µA/cm2 vs 62.05 µA/cm2 ± 11.26 µA/cm2, 62.11 µA/cm2 ± 12.01 µA/cm2, P < 0.05, respectively). However, when CFTR Cl- channel was blocked by 1,1-dimethyl piperidinium chloride (DPC), 5-HT-induced ∆Isc did not significantly differ in three groups (42.28 µA/cm2 ± 10.61 µA/cm2 vs 51.48 µA/cm2 ± 6.56 µA/cm2 vs 47.75 µA/cm2 ± 7.99 µA/cm2, P > 0.05, respectively). The similar results could also be obtained in three groups when NBC and NKCC were respectively blocked by their blockers.
TXYF-formula can regulate the Cl- and HCO3- secretion of colonic mucosa via CFTR Cl- channel, Cl-/HCO3- exchanger, NBC and NKCC co-transporters.
Core tip: Diarrhea-predominant irritable bowel syndrome (D-IBS) is a chronic functional gastrointestinal disease. Abnormal ion secretion of colonic mucosal epithelial cells is recognized as one of the pathophysiological factors. In this paper, through the observation of TongXie-YaoFang (TXYF) formula, a Chinese herbal formula, to D-IBS rats obtained by neonatal maternal separation plus restraint stress. The mucosal stripping under a microscope was used for tissue preparation. Short-circuit current technology was used for testing 5-HT-induced changes in the current across ion channels of colonic epithelium. The results indicated that TXYF-formula could regulate the secretion of Cl- and HCO3- in colonic mucosa via cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel, Cl-/HCO3- exchanger, Na+-HCO3- co-transporter and Na+-K+-2Cl- co-transporter co-transporters.
- Citation: Yang C, Xiong Y, Zhang SS, An FM, Sun J, Zhang QL, Zhan Q. Regulating effect of TongXie-YaoFang on colonic epithelial secretion via Cl- and HCO3- channel. World J Gastroenterol 2016; 22(48): 10584-10591
- URL: https://www.wjgnet.com/1007-9327/full/v22/i48/10584.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i48.10584
Diarrhea-predominant irritable bowel syndrome (D-IBS) is a chronic functional gastrointestinal disease. Its clinical manifestations are characterized by diarrhea and abdominal pain or discomfort in the absence of a demonstrable pathology. The diagnosis of D-IBS is based on symptom assessment and the Rome III Diagnostic Criteria[1,2]. According to an epidemiological study, D-IBS mainly affects young adults of 20-40 years old, and the quality of their lives is seriously affected[3].
The pathogenesis of D-IBS has not been fully clarified. Consequently, the usual treatment of the disease in Western medicine involves symptomatic therapy, which is unsatisfactory for patients while simultaneously increasing the use of health-care resources[4-6]. Because traditional Chinese medicine (TCM) can significantly improve patients’ symptoms and quality of life, increasing numbers of patients have begun to seek treatment with TCM[7,8].
A series of randomized, double-blind, placebo-controlled trials had shown that TongXie-YaoFang (TXYF) formula can significantly improve the clinical symptoms, such as diarrhea and abdominal pain or discomfort, of patients with D-IBS and improve the quality of their lives[9,10]. However, the specific mechanism of it has not been completely elaborated. The purpose of this paper is to observe the regulating effects of TXYF-formula on colonic epithelial secretion via relevant ion channels.
Neonatal Sprague Dawley rats, postnatal day 1, were purchased from Vital River Laboratories Animal Technology Co., Ltd. (Beijing, China; Number of qualitative qualification: 11400700015068, 11400700019786), and kept at Dongzhimen Hospital Affiliated with Beijing University of Chinese Medicine [Number of permit: SYXK(Beijing)2009.0028]. In this study, only male litters were used to eliminate the impact of estrogen and hormones on the secretory and sensory responses of the intestine to excitants[11]. Then the pups were randomly assigned to one of the following two rearing conditions: (1) neonatal maternal separation plus restraint stress (NMS + RS); or (2) no handling or separation (NH).
On postnatal days 2-21, the NMS + RS litters were removed from their cages and separated from their dams for 180 min each day, whereas NH pups remained in their home cages[12,13]. Manipulation began at 0900 h ± 30 min each day to minimize the influence of circadian rhythms. During the 180 min period of separation, pups were removed from the nest to stand-alone compartments, where the temperature was maintained at 23 °C ± 0.5 °C in a thermally regulated facility. The compartments contained bedding of 2.5 cm wood chips and were adjacent to their home cages. The litters were returned to their home cages immediately after separation. All the rats were reared on a 12:12 h light-dark cycle (lights on at 0800 h) with access to food and water ad libitum. On day 22, sexes of the pups, including those in NH group, were distinguishable, so the females were removed and the males retained. On day 22, NMS + RS and NH rats were weaned and kept in individual cages with only 3-4 pups per cage. After weaning, pups were weighed once a week until the end of experiment. Manipulations were performed in the morning with the same measuring instrument and at the same location.
On days 50-59, NMS + RS rats were placed in transparent plastic restraint cylinders (4 cm × 4 cm × 18 cm), in which they could move forward and backward but could not turn around[14]. The rats remained in the restraint cylinders for 3 h, with access to food and water ad libitum, in the morning and in the afternoon of each day. Then NMS + RS rats were divided into two groups (NMS + RS group and TXYF-formula group).
All animal care and experimental procedures were conducted according to the institutional ethical guidelines and conformed to the requirements of the Institutional Animal Care and Use Committee of Beijing University of Chinese Medicine and the Animal Ethics Committee of Dongzhimen Hospital Affiliated with Beijing University of Chinese Medicine.
TXYF-formula consisted of the following four Traditional Chinese Herbal Medicines: Bai Zhu (Atractylodesm macrocephala Koidz - Acta Horti Gothoburgensis 12(9): 310 1938)-93.75 g, Shao Yao (Paeonia lactiflora Pall. - Reise Russ. Reich. 3: 286. 1776)-62.5 g, Chen Pi (Citrus reticulata Blanco - Fl. Filip. 610 1837.)-46.875 g and Fang Feng (Saposhnikovia divaricata (Turcz.) Schischk. - Fl. URSS 17: 359 1951.)-31.25 g. It was manufactured by Preparation Room for TCM of Beijing Chinese Medicine Hospital. All formula raw materials were examined according to the quality control criteria in Chinese Pharmacopeia[15].
From postnatal day 60, rats in TXYF-formula group were daily given orally administered TXYF-formula (4.92 g/100 g bodyweight) while NH group and NMS + RS group were treated with distilled water. The delivery volume in three groups was 2 mL/100 g per day, for 14 consecutive days.
After treatment, the rats in three groups were first anesthetized abdominally with 7% chloral hydrate (35 mg/100 g bodyweight). The distal colon (6-7 cm from anus) was then quickly removed and incised longitudinally along the mesenteric border. The mucosa was fixed onto a Petri dish with silica gel in the bottom, with the lumen side down. The Petri dishes were filled with Krebs’ solution under 95% oxygen and 5% carbon dioxide. The mucosal layer was carefully separated from the submucosa, muscularis, and serosal layer with fine tweezers under a microscope. The mucosal layer was then cut into small flat sheets, with areas of about 0.5 cm2, for further analysis. Two sheets could be obtained from one segment of the distal colon.
Six adjacent tissues from the distal colon of each rat were obtained and mounted in Ussing chambers. Krebs’ solution (5 mL) was injected into two small adjacent compartments with circulating 95% oxygen and 5% carbon dioxide while pH was maintained at 7.35-7.45 and temperature at 37 °C. The tissues were left to equilibrate for 60 min to allow the electrical parameters to stabilize. The voltage across tissues was then clamped to zero and the short-circuit current (Isc, μA/cm2) was measured. When the Isc baseline was smooth, 10 µmol/L indomethacin was added to basolateral side of the tissue and maintained for 10 min to block the influence of endogenous prostaglandins[16,17]. The Isc value was recorded at this time. 5-HT (10 µmol/L) was then added to basolateral side and the maximum Isc recorded. The change in Isc (∆Isc) after the addition of 5-HT was then calculated. To measure the transmembrane resistance (Rt, Ω/cm2), electrical stimulation of 1 mV was applied to both sides of the epithelium. Tissue conductance was calculated according to Ohm’s law and expressed in milliSiemens per square centimeter (mS/cm2). The drugs or specific blockers were then applied to the apical or basolateral side of tissue and the ∆Isc of ion channels in colonic epithelium were calculated and recorded for further analysis.
Krebs’ solution of the following composition (in mol/L): 117 NaCl, 4.7 KCl, 1.2 MgCl2, 24.8 NaHCO3, 1.2 KH2PO4, 2.56 CaCl2, and 11.1 glucose; Krebs’ solution without Cl-: sodium gluconate instead of NaCl, potassium gluconate instead of KCl, calcium gluconate instead of CaCl2, and the remaining components were the same; Krebs’ solution without HCO3-: 141.8 NaCl, 5.9 KCl, 1.2 MgSO4∙7 H2O, 2.56 CaCl2, 11.1 glucose, 10 HEPES free acid, and 5.6 Tris. Krebs’ solution without Cl- and HCO3-: sodium gluconate instead of NaCl and NaHCO3, potassium gluconate instead of KCl, calcium gluconate instead of CaCl2, and the remaining components were the same. 5-HT, batch number: 1001156278; Glibenclamide, batch number: 1001068037; Indomethacin, batch number: 1001087688; Bumetanide, batch number: 101016760; Amiloride, batch number: 101093389; SITS (4-acetamido-4′-isothio-cyanato-stilbene-2,2′-disulfonic), batch number: 1001208418; DPC (1,1-dimethyl piperidinium chloride), batch number: 101078880; BaCl2, batch number: 1398; DIDS (4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid), batch number: 1001339605. cystic fibrosis transmembrane conductance regulator (CFTR)(inh)-172, batch number: 6311. All reagents were purchased from Sigma-Aldrich Co.
A multichannel voltage-current clamp (VCC MC6) was purchased from Physiologic Instruments Corporation; the Bridge amplifier (ML228), recording and analysis system for physiological data (Power Lab) was purchased from AD Instruments Corporation.
All experimental data were analyzed by SPSS 17.0 statistical software and expressed as means ± SE. The differences between groups were analyzed with a paired t test. P < 0.05 was considered statistically significant.
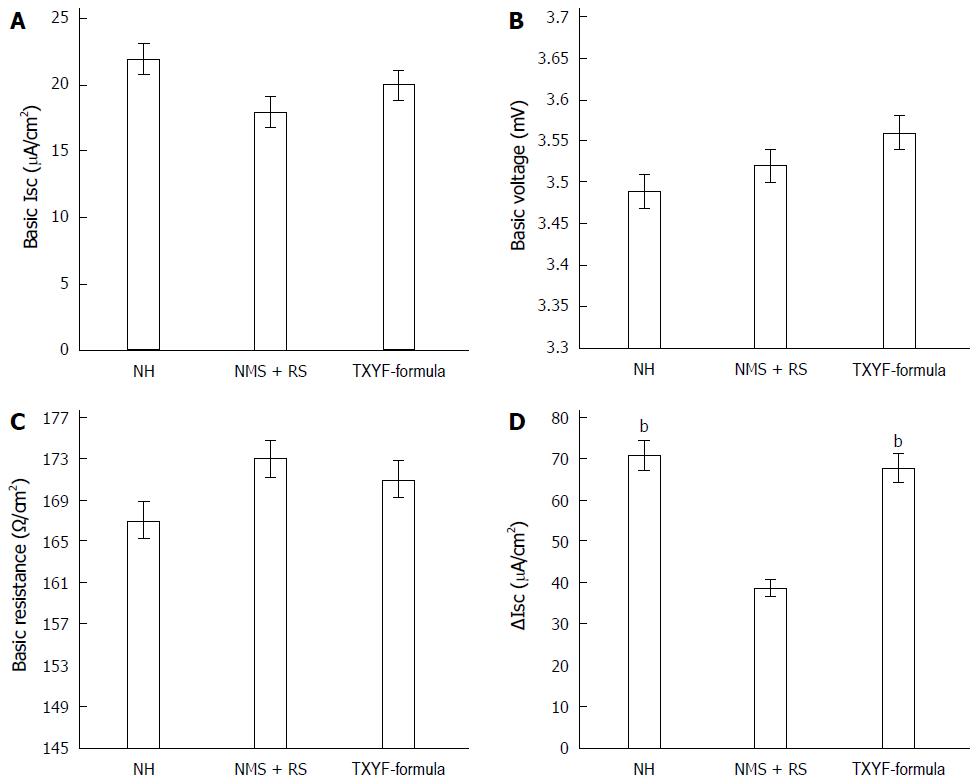
The basic Isc did not differ significantly between three groups (21.02 µA/cm2 ± 2.92 µA/cm2 vs 20.29 µA/cm2 ± 3.58 µA/cm2 vs 20.71 µA/cm2 ± 2.04 µA/cm2, n = 18, P > 0.05, respectively; Figure 1A). The basic voltage in three groups had similar results (3.49 mV ± 0.54 mV vs 3.52 mV ± 0.69 mV vs 3.57 mV ± 0.62 mV, n = 18, P > 0.05, respectively; Figure 1B) as well as the basic resistance (166.8 Ω/cm2 ± 20.11 Ω/cm2 vs 173.66 Ω/cm2 ± 16.39 Ω/cm2 vs 171.94 Ω/cm2 ± 19.03 Ω/cm2, n = 18, P > 0.05, respectively; Figure 1C). The 5-HT-induced change of short-circuit current (∆Isc) in NMS + RS group was respectively smaller than that in NH group and TXYF-formula group (38.8 µA/cm2 ± 7.25 µA/cm2 vs 70.86 µA/cm2 ± 12.32 µA/cm2, 67.67 µA/cm2 ± 11.68 µA/cm2, n = 18, P < 0.01, respectively; Figure 1D).
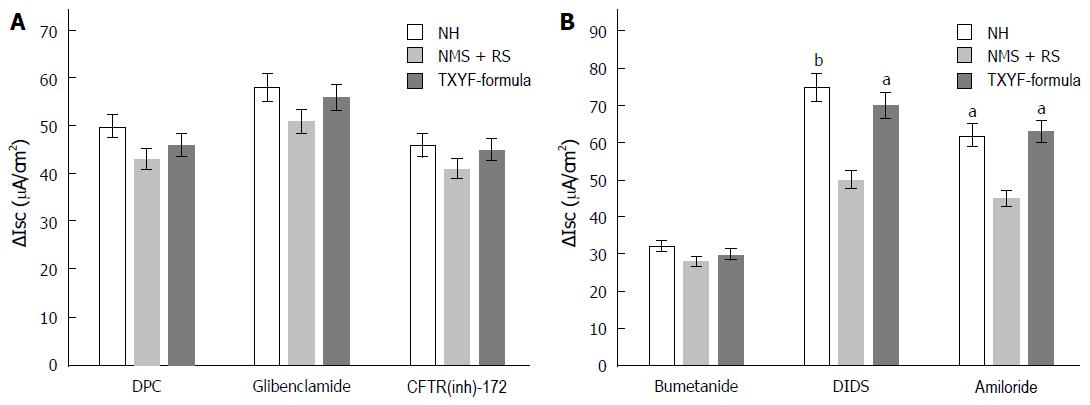
DPC or Glibenclamide with final concentration 1 mmol/L were respectively added to apical side, namely left side of Ussing chamber, of colonic mucosa and equilibrated for 30 min. Then 10 µmol/L 5-HT was added to basolateral side (Equilibrated time and added concentration of 5-HT was same in the following experiment). ∆Isc induced by 5-HT were similar in TXYF-group and NMS + RS group, respectively (47.75 µA/cm2 ± 7.99 µA/cm2 vs 42.28 µA/cm2 ± 10.61 µA/cm2, 57.57 µA/cm2 ± 14.25 µA/cm2 vs 46.78 µA/cm2 ± 11.68 µA/cm2, n = 8, P > 0.05, respectively; Figure 2A). The similar result was obtained when CFTR(inh)-172 was added to apical side with final concentration 100 µmol/L (45.04 µA/cm2 ± 9.18 µA/cm2 vs 36.2 µA/cm2 ± 9.64 µA/cm2, n = 8, P > 0.05; Figure 2A).
5-HT-induced ∆Isc was higher in TXYF-formula group than in NMS + RS group after the effects of Ca2+-dependent Cl- channel (CACC) blocker DIDS (500 µmol/L, added to apical side) or epithelial Na+ channel (ENaC) blocker Amiloride (100 µmol/L, added to apical side), respectively (69.22 µA/cm2 ± 11.7 µA/cm2 vs 48.41 µA/cm2 ± 13.15 µA/cm2, 62.11 µA/cm2 ± 12.01 µA/cm2 vs 44.69 µA/cm2 ± 12.58 µA /cm2, n = 8, P < 0.05, respectively; Figure 2B). There were no statistical differences in three groups when 100 µmol/L Na+-K+-2Cl- co-transporter (NKCC) blocker Bumetanide was added to basolateral (37.64 µA/cm2 ± 10.57 µA/cm2 vs 27.55 µA/cm2 ± 10.94 µA/cm2 vs 29.43 µA/cm2 ± 7.66 µA/cm2, n = 8, P > 0.05; Figure 2B).
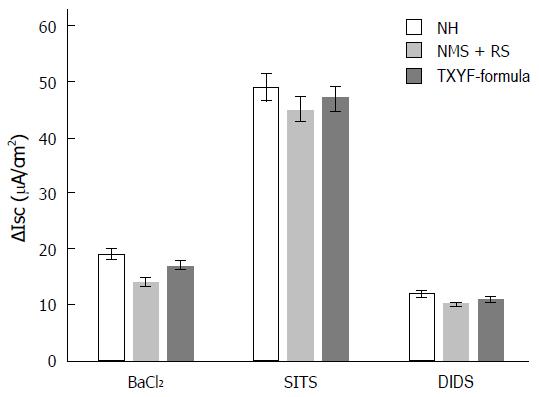
∆Isc induced by 5-HT were similar in three groups when 5 mmol/L K+ channel blocker BaCl2 or 100 µmol/L Cl-/HCO3- exchanger inhibitor SITS was respectively added to basolateral side of colonic mucosa (25.63 µA/cm2 ± 13.69 µA/cm2 vs 13.92 µA/cm2 ± 8.16 µA/cm2 vs 17.03 µA/cm2 ± 9.04 µA/cm2, 49.92 µA/cm2 ± 11.66 µA/cm2 vs 40.41 µA/cm2 ± 14.26 µA/cm2 vs 47.7 µA/cm2 ± 11.43 µA/cm2, n = 8, P > 0.05, respectively; Figure 3). There was also no statistical difference in three groups after the effects of 200 µmol/L Na+-HCO3- co-transporter (NBC) blocker DIDS (500 µmol/L, added to basolateral side) (12.27 µA/cm2 ± 3.6 µA/cm2 vs 10.74 µA/cm2 ± 2.99 µA/cm2 vs 11.88 µA/cm2 ± 3.51 µA/cm2, n = 8, P > 0.05; Figure 3).
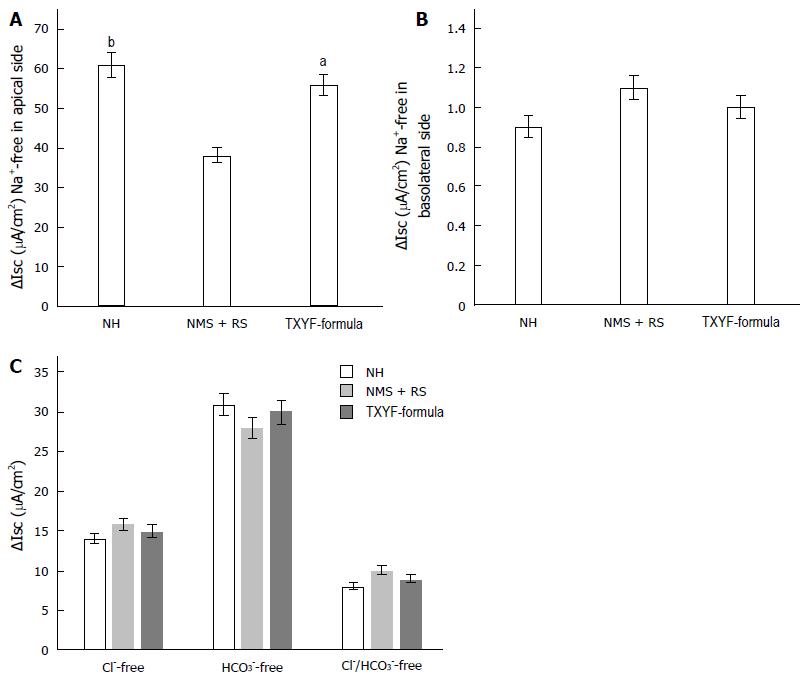
When Na+ applied to apical side of colonic mucosa was substituted with sodium gluconate, 5-HT-induced ∆Isc was higher in TXYF-formula groups than in NMS + RS group (56.86 µA/cm2 ± 11.2 µA/cm2 vs 39.14 µA/cm2 ± 10.83 µA/cm2, n = 8, P < 0.05; Figure 4A). However, when basolateral side Na+ was substituted with sodium gluconate, ∆Isc was similar in three groups (0.87 µA/cm2 ± 0.33 µA/cm2 vs 1.15 µA/cm2 ± 0.5 µA/cm2 vs 1.01 µA/cm2 ± 0.49 µA/cm2, n = 8, P > 0.05, respectively; Figure 4B).
5-HT-induced ∆Isc was similar in three groups when Cl- and HCO3- was respectively removed from basolateral side of colonic mucosa (14.42 µA/cm2 ± 6.07 µA/cm2 vs 15.95 µA/cm2 ± 5.64 µA/cm2 vs 15.09 µA/cm2 ± 4.04 µA/cm2, 33.86 µA/cm2 ± 7.47 µA/cm2 vs 26.54 µA/cm2 ± 8.9 µA/cm2 vs 31.88 µA/cm2 ± 6.07 µA/cm2, n = 8, P > 0.05, respectively; Figure 4C). When both Cl- and HCO3- were simultaneously removed from Krebs’ solution applied to basolateral side, 5-HT-induced ∆Isc was similar in three groups (8.38 µA/cm2 ± 1.15 µA/cm2 vs 9.3 µA/cm2 ± 2.16 µA/cm2 vs 8.51 µA/cm2 ± 1.2 µA/cm2, n = 8, P > 0.05, respectively; Figure 4C).
The etiology and pathogenesis of D-IBS are complex, and its pathophysiological changes predominantly include dynamic gastrointestinal disorder and visceral sensory sensitivity. In TCM, D-IBS is classified as diarrhea or abdominal pain according to its clinical manifestations[18]. Previous studies had shown that TXYF-formula had an inhibitory effect on bowel movement and reduced intestinal peristalsis by regulating 5-HT[19,20]. Pharmacological study showed that Fang Feng could increase intestinal pressure threshold in rats so that to present its analgesic effect[21]. In this study, we used the method of neonatal maternal separation plus restraint stress to establish an animal mode of D-IBS, with the main simultaneous symptoms of diarrhea and high visceral sensitivity. This recapitulates the clinical symptoms of patients with D-IBS.
The secretion activities induced by 5-HT in colonic mucosae of NMS + RS rats were weaker than those in NH rats or TXYF-formula-treated rats in this study. This may be related to increased 5-HT and 5-HT-receptor levels in NMS + RS rats. When 5-HT was added to colonic mucosa from NMS + RS rats, the electrical activities across epithelium declined. The reaction to 5-HT in colonic mucosae of rats was restored by treatment with TXYF-formula, to a level almost the same as that observed in NH rats. This demonstrates that TXYF-formula has specific therapeutic effects on D-IBS.
We found that one of the therapeutic effects of TXYF-formula is achieved by regulating secretion of Cl-. The specific results were as follows: 5-HT-induced ∆Isc in NMS + RS rats differed significantly from that in TXYF-formula group and NH group when extracellular Cl- was not removed. However, when extracellular Cl- was removed, the difference disappeared. When a CFTR Cl- channel blocker was added to apical side or an NKCC co-transporter inhibitor was added to basolateral side of tissue, ∆Isc did not differ statistically in NMS + RS and TXYF-formula-treated rats. In presence of the nonselective K+ channel blocker[22], 5-HT-induced ∆Isc did not differ significantly between NMS + RS group and TXYF-formula group. This indicates that Cl- secretion by mucosal epithelium is dependent on electrochemical gradient across the serosal surface generated by K+ transport.
We also found that TXYF-formula alters HCO3- secretion. When extracellular HCO3- was removed or NBC was inhibited, 5-HT-induced ∆Isc did not differ between NMS + RS group and TXYF-formula group. The same result was obtained when extracellular Cl- and HCO3- were both removed or when Cl-/HCO3- exchange was interrupted. Thus, the therapeutic effect of TXYF-formula is achieved by regulating the secretion of both Cl- and HCO3-. Moreover, when Na+ on apical side of membrane was removed or a Na+ channel blocker was added, ∆Isc still differed between NMS + RS group and TXYF-formula group; and the differences between NMS + RS and NH rats did not disappear. Therefore, the relationship between therapeutic effects of TXYF-formula on Na+ transport and D-IBS is not close. Nevertheless, after Na+ in basilar membrane was removed, the electrical activity across epithelium did not differ between TXYF-formula group and NMS + RS group. Therefore, it can be seen that the regulatory effects of TXYF-formula on Cl- and HCO3- secretion depends on Na+ in basilar membrane.
There are two main types of Cl- channels on apical side of epithelial cells: CFTR and CACC. CFTR is a cAMP-dependent, PKA-activated Cl- channel, and is sensitive to DPC and Glibenclamide[23,24]. After CFTR Cl- channel was blocked by DPC or Glibenclamide, the electrical activity across epithelium induced by 5-HT did not differ obviously between TXYF-formula group and NMS + RS group. However, the electrical activity in two groups differed markedly after the application of CACC blocker DIDS. Therefore, the regulatory effect of TXYF-formula on electrical activity across epithelia of D-IBS rats is associated with CFTR Cl- channel, and has nothing to do with CACC channel.
Our results, combined with preliminary studies[25,26] about secretory mechanisms of epithelial anions in colonic mucosa, allow the following conclusions to be drawn. The regulating effects of TXYF-formula on D-IBS involves the secretion of Cl- and HCO3- in colonic mucosa via CFTR Cl- channel, Cl-/HCO3- exchanger, and NBC and NKCC co-transporters.
Diarrhea-predominant irritable bowel syndrome (D-IBS) is a chronic functional gastrointestinal disease. The pathogenesis of it has not been thoroughly elucidated while colonic abnormal secretory is recognized as one of the pathophysiological factors. The usual treatment in Western medicine, mainly involves symptomatic therapy, is unsatisfactory for patients while simultaneously increasing the use of health-care resources. Traditional Chinese medicine (TCM) can obviously alleviate patients’ clinical symptoms, increasing numbers of them have begun to seek treatment with TCM. A lot of researches have shown that TongXie-YaoFang formula (TXYF-formula), a Chinese herbal formula, can significantly improve D-IBS patients’ clinical symptoms and enhance their quality of lives.
TXYF-formula is a Traditional Chinese classical prescription for clinical treatment on D-IBS. The research hotspot is its effect on colonic abnormal secretory via correlational ion channels, visceral sensitivity and colon movement.
Previous clinical and experimental studies had only shown that TXYF-formula can relieve diarrhea of patients with D-IBS. Its correlational effects might be realized by influencing secretion of colon. However, the specific mechanism and correlative ions are unclear. In this study, the mucosal stripping under a microscope was used for tissue preparation. And short-circuit current technology was applied to observe 5-HT-induced changes in current across ion channels of colonic epithelium so as to reveal the prescription effect of TXYF-formula ion transport in colon.
The key results of this study showed that TXYF-formula can regulate the secretion of Cl- and HCO3- in colonic mucosa of D-IBS rats. And this may be related to cystic fibrosis transmembrane conductance regulator (CFTR) Cl- channel, Cl-/HCO3- exchanger, and Na+-HCO3- co-transporter (NBC) and Na+-K+-2Cl- co-transporter (NKCC) co-transporters.
D-IBS, as a clinically common functional gastrointestinal disease, is closely related to early adverse life events. It seriously affects patient’s quality of jobs and lives. TXYF-formula can regulate the Cl- and HCO3- secretion of colonic mucosa via CFTR Cl- channel, Cl-/HCO3- exchanger, NBC and NKCC co-transporters.
Well written, quite meticulous methodology, nicely executed study.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garg P S- Editor: Qi Y L- Editor: A E- Editor: Liu WX
| 1. | Ford AC, Bercik P, Morgan DG, Bolino C, Pintos-Sanchez MI, Moayyedi P. Validation of the Rome III criteria for the diagnosis of irritable bowel syndrome in secondary care. Gastroenterology. 2013;145:1262-70.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 2. | Shalaby SA, Sayed MM, Ibrahim WA, Abdelhakam SM, Rushdy M. The prevalence of coeliac disease in patients fulfilling Rome III criteria for irritable bowel syndrome. Arab J Gastroenterol. 2016;17:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Marquis P, Lasch KE, Delgado-Herrera L, Kothari S, Lembo A, Lademacher C, Spears G, Nishida A, Tesler WL, Piault E. Qualitative development of a patient-reported outcome symptom measure in diarrhea-predominant irritable bowel syndrome. Clin Transl Gastroenterol. 2014;5:e59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Nee J, Zakari M, Lembo AJ. Current and emerging drug options in the treatment of diarrhea predominant irritable bowel syndrome. Expert Opin Pharmacother. 2015;16:2781-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Lacy BE. Diagnosis and treatment of diarrhea-predominant irritable bowel syndrome. Int J Gen Med. 2016;9:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Chiba T, Yamamoto K, Sato S, Suzuki K. Long-term efficacy and safety of ramosetron in the treatment of diarrhea-predominant irritable bowel syndrome. Clin Exp Gastroenterol. 2013;6:123-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Teschke R, Wolff A, Frenzel C, Eickhoff A, Schulze J. Herbal traditional Chinese medicine and its evidence base in gastrointestinal disorders. World J Gastroenterol. 2015;21:4466-4490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Xiao Y, Liu Y, Huang S, Sun X, Tang Y, Cheng J, Wang T, Li F, Kuang Y, Luo R. The efficacy of Shugan Jianpi Zhixie therapy for diarrhea-predominant irritable bowel syndrome: a meta-analysis of randomized, double-blind, placebo-controlled trials. PLoS One. 2015;10:e0122397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Pan F, Zhang T, Zhang YH, Xu JJ, Chen FM. Effect of Tongxie Yaofang Granule in treating diarrhea-predominate irritable bowel syndrome. Chin J Integr Med. 2009;15:216-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Lai LJ, Han YC, Hui Zhang, Zhu XW, Han XM. The timeliness of Tongxie-Yaofang on patients with D-IBS about improving the clinical symptoms. Shiyong Zhongxiyi Jiehe Linchuang. 2013;13:27-28. [DOI] [Full Text] |
| 11. | Ren TH, Wu J, Yew D, Ziea E, Lao L, Leung WK, Berman B, Hu PJ, Sung JJ. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2007;292:G849-G856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Gareau MG, Jury J, Perdue MH. Neonatal maternal separation of rat pups results in abnormal cholinergic regulation of epithelial permeability. Am J Physiol Gastrointest Liver Physiol. 2007;293:G198-G203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Oines E, Murison R, Mrdalj J, Grønli J, Milde AM. Neonatal maternal separation in male rats increases intestinal permeability and affects behavior after chronic social stress. Physiol Behav. 2012;105:1058-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Irles C, Nava-Kopp AT, Morán J, Zhang L. Neonatal maternal separation up-regulates protein signalling for cell survival in rat hypothalamus. Stress. 2014;17:275-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Pharmacopoeia Commission of the Ministry of Public Health of PRC. Chinese Pharmacopoeia: Chemical Industry Press, 2005. . |
| 16. | Garsed K, Chernova J, Hastings M, Lam C, Marciani L, Singh G, Henry A, Hall I, Whorwell P, Spiller R. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut. 2014;63:1617-1625. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Feng XY, Li Y, Li LS, Li XF, Zheng LF, Zhang XL, Fan RF, Song J, Hong F, Zhang Y. Dopamine D1 receptors mediate dopamine-induced duodenal epithelial ion transport in rats. Transl Res. 2013;161:486-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Li L, Zhang SS. The mechanism research status in Irritable bowel syndrome with traditional Chinese medicine. Zhongguo Zhongxiyi Jiehe Zazhi. 2012;20:466-470. |
| 19. | Rahimi R, Abdollahi M. Herbal medicines for the management of irritable bowel syndrome: a comprehensive review. World J Gastroenterol. 2012;18:589-600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (2)] |
| 20. | Yang C, Zhang SS, Li XL, Wang ZF, Zhao LQ. Inhibitory effect of TongXie-YaoFang formula on colonic contraction in rats. World J Gastroenterol. 2015;21:2912-2917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Hu XG, Liao SL, Wang YF, Gong MJ, Wang Man, Liu SS, Han Bin. Effect of Radix Saposhnikoviae in Tongxie Yaofang on PAR2 mRNA Expression and Inflammatory Mediators in the Colon of Rats with Postinfection Irritable Bowel Syndrome. Zhongyao Linchuang Yaolixue. 2013;24:5-9. |
| 22. | Fujita T, Karaki S, Tateoka T, Kuwahara A. Desacetyl bisacodyl-induced epithelial Cl(-) secretion in rat colon and rectum. Biomed Res. 2016;37:13-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Ao M, Sarathy J, Domingue J, Alrefai WA, Rao MC. Chenodeoxycholic acid stimulates Cl(-) secretion via cAMP signaling and increases cystic fibrosis transmembrane conductance regulator phosphorylation in T84 cells. Am J Physiol Cell Physiol. 2013;305:C447-C456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 24. | Hou Y, Guan X, Yang Z, Li C. Emerging role of cystic fibrosis transmembrane conductance regulator - an epithelial chloride channel in gastrointestinal cancers. World J Gastrointest Oncol. 2016;8:282-288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Dürrnagel S, Falkenburger BH, Gründer S. High Ca(2+) permeability of a peptide-gated DEG/ENaC from Hydra. J Gen Physiol. 2012;140:391-402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, Stanghellini V, Grundy D, Tonini M, De Ponti F. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106:1290-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |