Published online Dec 28, 2016. doi: 10.3748/wjg.v22.i48.10566
Peer-review started: June 29, 2016
First decision: August 19, 2016
Revised: September 5, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: December 28, 2016
Processing time: 180 Days and 23.2 Hours
To study the impact on cleavage of tumor necrosis factor receptor-associated factor 1 (TRAF1) regulated by Helicobacter pylori (H. pylori).
Cleavage of TRAF1 was detected by western blotting in the human gastric cancer cell line AGS following treatment with an apoptosis inducer. Cleavage of TRAF1 mediated by caspase was examined in vitro using specific caspase inhibitors. The effect of the COOH-terminal TRAF1 fragment on gastric cell apoptosis during H. pylori infection was measured using flow cytometry. The impact of H. pylori infection on TRAF1 cleavage was detected in the presence of apoptosis inducer. The roles of H. pylori virulence factors that may regulate TRAF1 cleavage were analyzed using isogenic cagA-, vacA- and cagE-null mutants.
TRAF1 was found to be cleaved in AGS cells treated with the apoptosis inducer, and caspase-8 was the major caspase involved in the cleavage of TRAF1. The COOH-terminal TRAF1 fragment significantly induced cell apoptosis (P < 0.05) as well as promoted H. pylori-induced cell apoptosis (P < 0.05). H. pylori infection was found to significantly inhibit the cleavage of TRAF1 and to inhibit the activation of caspase-8 in the presence of the apoptosis inducer at specific infection times and different cell/bacteria ratios. We also found that the effects of cagE- and cagA-null mutants on the inhibition of TRAF1 cleavage and activation of caspase-8 were significantly attenuated, compared with wild-type H. pylori, in the presence of the apoptosis inducer, showing that the virulence factor CagA was mainly involved in the inhibition of TRAF1 cleavage.
H. pylori infection significantly inhibits the cleavage of TRAF1 via a CagA-dependent mechanism, which would increase the relative amounts of full-length TRAF1 and exert an antiapoptotic effect on H. pylori-infected cells.
Core tip: We report our first results from a study of Helicobacter pylori (H. pylori)-mediated tumor necrosis factor receptor-associated factor 1 (TRAF1) cleavage. The impact of H. pylori infection and its virulence factors on TRAF1 cleavage were detected in the presence of an apoptosis inducer. This study demonstrates, for the first time, that the cleavage of TRAF1 and the activation of caspase-8 were significantly inhibited by H. pylori infection in the presence of an apoptosis inducer. In addition, the virulence factor CagA was mainly involved in the inhibition of TRAF1 cleavage.
- Citation: Wan XK, Yuan SL, Wang YC, Tao HX, Jiang W, Guan ZY, Cao C, Liu CJ. Helicobacter pylori inhibits the cleavage of TRAF1 via a CagA-dependent mechanism. World J Gastroenterol 2016; 22(48): 10566-10574
- URL: https://www.wjgnet.com/1007-9327/full/v22/i48/10566.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i48.10566
Gastric cancer is one of the most common malignant tumors and the third leading cause of cancer-related deaths worldwide[1]. Helicobacter pylori (H. pylori) is a gram-negative, spiral shaped pathogen that successfully colonizes human gastric mucosa and is a strong risk factor for chronic gastritis, peptic ulcer, mucosa-associated lymphoid tissue lymphoma and gastric cancer[2-4]. H. pylori pathogenesis is determined largely by interactions between bacterial factors and host cells. The best-characterized virulence determinants of H. pylori are the cytotoxin-associated gene pathogenicity island (cag PAI) and the vacuolating cytotoxin A (vacA). The cag PAI encodes a type IV secretion system (T4SS), which is responsible for injecting virulence factors, such as CagA protein, directly into host cells. Once injected into cells, the CagA protein will induce complex cell changes involving various host signaling pathways[5,6]. Though numerous studies have identified the association between H. pylori infection and the development of gastric carcinoma, the mechanisms underlying the carcinogenic potential of H. pylori are still not completely understood.
TRAF1 is a member of the TRAF family and is characterized by its diverse biological functions, acting through direct or indirect interactions with multiple tumor necrosis factor receptor (TNFR) family members and intracellular proteins[7]. Several studies have demonstrated that TRAF1 might exert an antiapoptotic role in lymphoma cells through regulation of the activation of NF-κB[8-10]. Wang et al[11] reported that TRAF1 expression was up-regulated in the human gastric mucosal samples infected with H. pylori in a clinical immunohistochemical analysis. Moreover, the up-regulation of TRAF1 was increased as the gastric disease progressed from chronic gastritis to gastric cancer.
Our previous study showed that the expression of TRAF1 is up-regulated by H. pylori infection in both gastric epithelial cells and mice. The up-regulation of TRAF1 inhibited cell apoptosis as well as increased the viability of infected cells, which suggested that TRAF1 is an important protein that contributes to the pathogenesis of H. pylori-related gastric cancer[12]. Interestingly, several studies have shown that TRAF1 could be transformed into a pro-apoptotic form after cleavage by caspase-8 in the presence of Fas ligand or TNF-α-induced apoptosis[13-15]. Caspase-8 cleaves TRAF1 into two fragments and overexpression of the COOH-terminal fragment could enhance Fas or TNF-α mediated apoptosis. However, whether the cleavage of TRAF1 could be influenced by H. pylori and the mechanisms are not known.
The aim of our work was to elucidate the effect on TRAF1 cleavage regulated by H. pylori and the roles of H. pylori virulence factors regulating TRAF1 cleavage. To gain a better understanding of the role of H. pylori infection related to TRAF1 cleavage, in the present study we detected the cleavage of TRAF1 in AGS cells co-cultured with H. pylori or sterile saline alone in the presence of an apoptosis inducer. We also analyzed the roles of H. pylori virulence factors that may regulate TRAF1 cleavage using isogenic cagA-, vacA- and cagE-null mutants.
The human gastric cancer cell line AGS was obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). AGS cells were grown in F-12 medium (Invitrogen, United States) supplemented with 10% (vol/vol) fetal bovine serum and were cultured at 37 °C in a humidified 5% CO2 atmosphere. H. pylori strain NCTC11637, obtained from Chinese Center for Disease Control and Prevention (Beijing, China) was grown on Columbia agar plates with 5% sheep blood. The plates were incubated in a mixed atmosphere of 10% CO2, 5% O2, and 85% N2, at 37 °C for 24 h, after which they were harvested by centrifugation at 1000 ×g for 5 min at 4 °C, washed 3 times with sterile saline, resuspended in sterile saline and added to gastric cells as indicated. Isogenic cagA-, cagE- and vacA-null mutants of H. pylori strain NCTC11637 were constructed by insertional mutagenesis using homologous recombination and selected with 25 μg/mL kanamycin.
The following primary antibodies were used: mouse anti-HA tag from Zhongshan Golden Bridge Biotechnology (Beijing, China); rabbit anti-GAPDH from Bioworld Technology (United States); rabbit anti-caspase-8 from Beyotime Biotechnology (Jiangsu, China). The following secondary antibodies were used: peroxidase-conjugated goat anti-mouse IgG (H + L) and peroxidase-conjugated goat anti-rabbit IgG (H + L) from Zhongshan Golden Bridge Biotechnology.
The pharmacological inhibitors were as follows: caspase-8 inhibitor Z-IETD-FMK from BioVision (United States); pan caspase inhibitor Z-VAD-FMK and caspase-3 inhibitor Ac-DEVD-CHO from Beyotime Biotechnology. The apoptosis inducer cycloheximide (CHX) and TNF-α were purchased from BioVision and Peprotech (United States) respectively.
The plasmids pcDNA-TRAF1-HA, pcDNA-TRAF1(D163A)-HA, pcDNA- TRAF1-N-HA, pcDNA-TRAF1-C-HA were constructed by inserting the specific cDNA and HA-tag sequences into pcDNA3.1 (+) (conserved in our laboratory) respectively.
In brief, AGS cells were seeded in a 24-well plate at a density of 1 × 105 cells/well and cultured for 24 h, with a target of 60%-80% confluency at the time of transfection. Cells were transfected with 0.5 μg of plasmids using jetPRIME Transfection Reagent (Polyplus, France) according to the manufacturer’s protocol. After 24 h of transfection, cells were co-cultured with H. pylori for the indicated experiments.
Proteins were extracted from the cultured cells and homogenized in a lysis buffer containing a protease inhibitor cocktail (CWBiotech, Beijing, China) according to the instructions. Then, the proteins were separated on 12% SDS-PAGE gels and transferred to nitrocellulose membranes (GE Healthcare Life Sciences, United States). The membranes were blocked in phosphate-buffered saline plus Tween-20 (PBST) containing 5% skim milk powder for 2 h at 37 °C, and then incubated with the primary antibodies for 1.5 h at 37 °C. Next, the membranes were incubated with peroxidase-conjugated secondary antibodies for 1 h at 37 °C. The membranes were treated with ECL reagents (Engreen Biosystem, Beijing, China) and visualized using a Minichemi Lane1D imager (Sagecreation, Beijing, China), according to the manufacturer’s instructions.
Cell apoptosis was measured according to the instructions of the Annexin V-FITC Apoptosis Detection Kit purchased from Beyotime Biotechnology. Briefly, AGS cells were transfected with the respective plasmids for 24 h and followed by co-culture with H. pylori for 24 h. Then, the cells were collected, washed in sterile PBS, and resuspended at a density of 1 × 106 cells/mL. After that, the cells were stained with Annexin V-FITC and propidium iodide (PI) for 10 min prior to the analysis using a FACScan flow cytometer (Bio-Rad, United States).
Statistical analyses in our paper were performed using the software GraphPad Prism 5. The results are presented as mean ± SD. Differences among groups were compared using variance analysis, and comparisons between two groups were performed using an unpaired Student’s t-test. Differences were considered to be significant at P < 0.05.
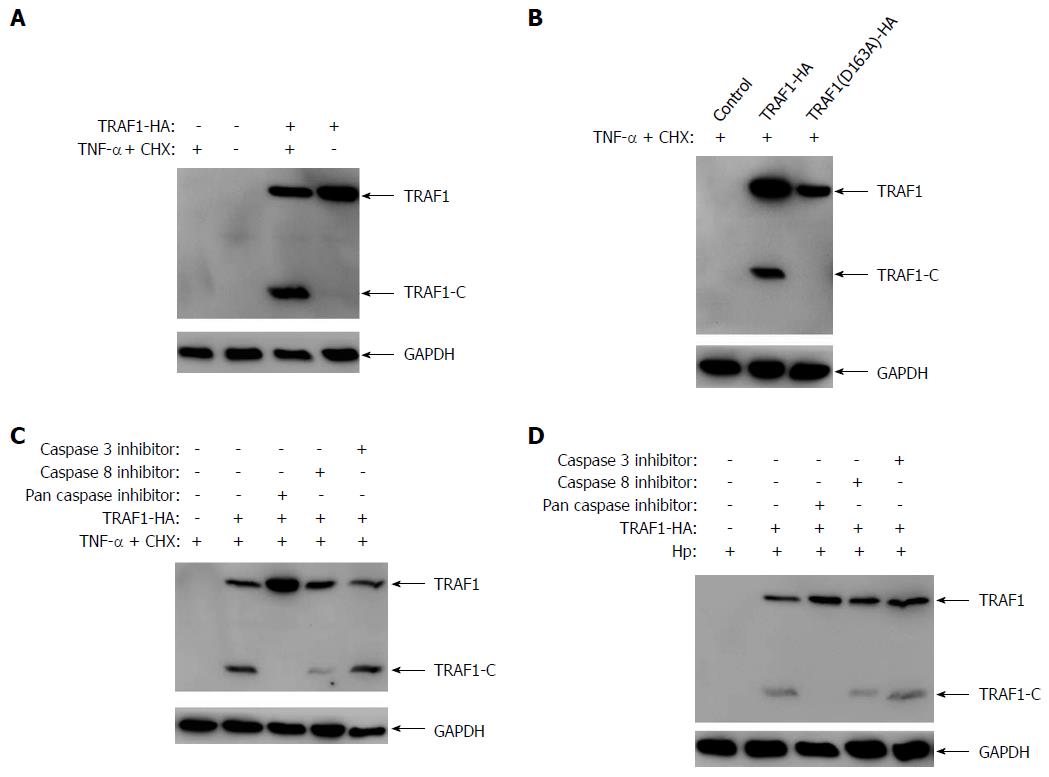
To confirm that TRAF1 could be cleaved under the condition of H. pylori infection, AGS cells transfected with HA-tagged TRAF1 were treated with the apoptosis inducer (0.3 μg/mL CHX and 80 ng/mL TNF-α) for 6 h, and then analyzed for the cleavage of TRAF1. Our results showed that TRAF1 could be significantly cleaved in AGS cells treated with the apoptosis inducer (Figure 1A). To confirm that the cleavage site of TRAF1 is located at aspartic acid 163 in the 160LVED163 motif, AGS cells were transfected with HA-tagged TRAF1 mutation (D163A) and treated with the apoptosis inducer. The result showed that the TRAF1 mutation (D163A) could not be cleaved by the apoptosis inducer, which suggested the cleavage site of TRAF1 is located at aspartic acid 163 (Figure 1B). To further confirm the cleavage of TRAF1 mediated by caspase-8, TRAF1 cleavage was examined in vitro using specific caspase inhibitors in the presence of the apoptosis inducer treatment or H. pylori infection. As shown in Figure 1C and 1D, TRAF1 cleavage was blocked completely by the addition of Z-VAD-FMK, a broad specificity inhibitor of caspases, and was mainly blocked by Z-IETD-FMK, a caspase-8-specific inhibitor. However, Z-YVAD-FMK, a caspase-3-specific inhibitor, had no apparent effect on TRAF1 cleavage. The results show caspase-8 is the major caspase involved in the cleavage of TRAF1 in cells infected with H. pylori.
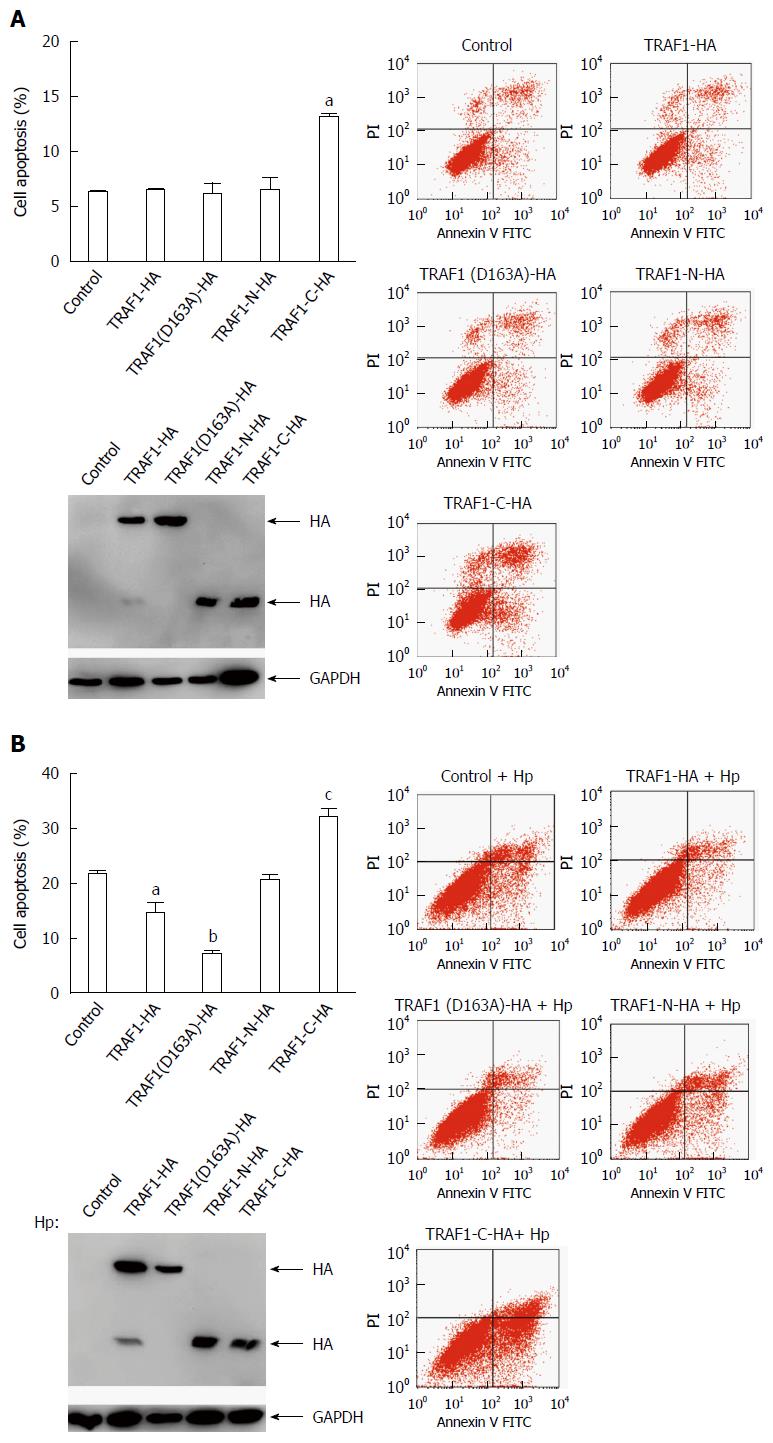
To confirm the pro-apoptotic role of the COOH-terminal TRAF1 fragment, AGS cells were transfected with HA-tagged TRAF1, TRAF1 (D163A), the NH2-terminal TRAF1 (TRAF1-N), the COOH-terminal TRAF1 (TRAF1-C), or empty vector respectively and cell apoptosis was analyzed by flow cytometry. The results showed transfection with TRAF1, TRAF1 (D163A), or TRAF1-N had no significant effect on cell apoptosis compared with empty vector control, whereas transfection with TRAF1-C significantly promoted cell apoptosis (Figure 2A). In addition, to confirm the pro-apoptotic role of the COOH-terminal TRAF1 fragment in AGS cells infected with H. pylori, AGS cells were also transfected with the above five plasmids respectively and infected with H. pylori for 24 h and analyzed for cell apoptosis (Figure 2B). The results showed transfection with TRAF1-N has no significant effect on cell apoptosis. Transfection with TRAF1 or TRAF1 (D163A) significantly inhibited H. pylori-induced apoptosis, whereas transfection with TRAF1-C significantly promoted H. pylori-induced apoptosis, thereby indicating the pro-apoptotic role of the COOH-terminal TRAF1 fragment in H. pylori infected cells.
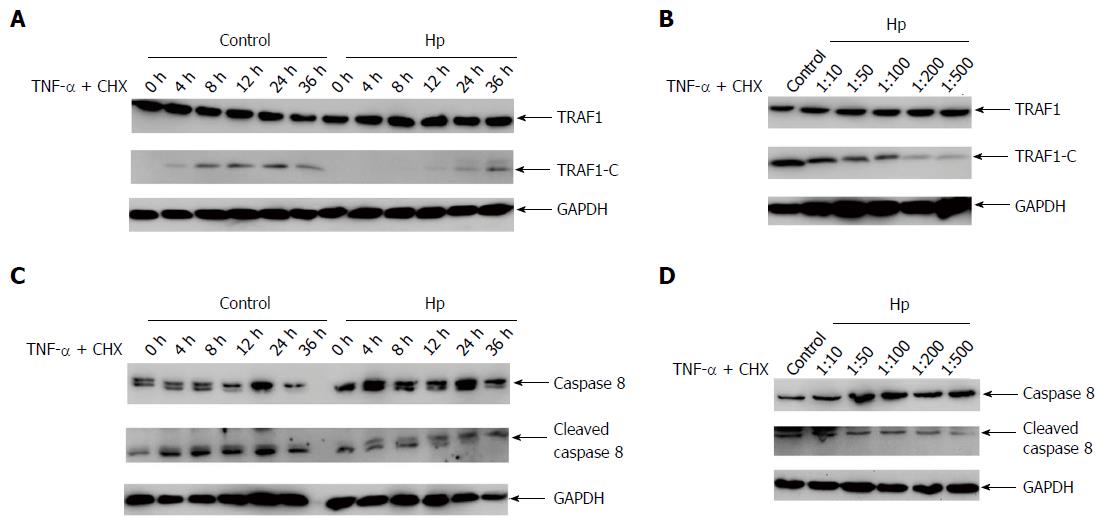
To investigate the effect of H. pylori infection on TRAF1 cleavage in cells, the expression of TRAF1 and its cleavage were examined in AGS cells when co-cultured with H. pylori or sterile saline alone in the presence of the apoptosis inducer for the specific infection times and cell/bacteria ratios. The cleavage of TRAF1 in AGS cells decreased significantly 4 h after H. pylori infection compared with the uninfected cells (Figure 3A). The effect of cell/bacteria ratios (1:10-1:500) on TRAF1 cleavage was then tested. A significantly decreased cleavage was observed at a ratio of 1:50, and the cleavage of TRAF1 gradually decreased as the bacteria amounts increased (Figure 3B). As caspase-8 is the major caspase involved in the cleavage of TRAF1, we next analyzed the activation of caspase-8. As shown in Figure 3C and D, the cleaved caspase-8 was significantly decreased when co-cultured with H. pylori in the presence of the apoptosis inducer for the specific infection times as well as the indicated cell/bacteria ratios, suggesting that the activity of caspase-8 was inhibited. These results indicate that H. pylori infection significantly inhibits the cleavage of TRAF1.
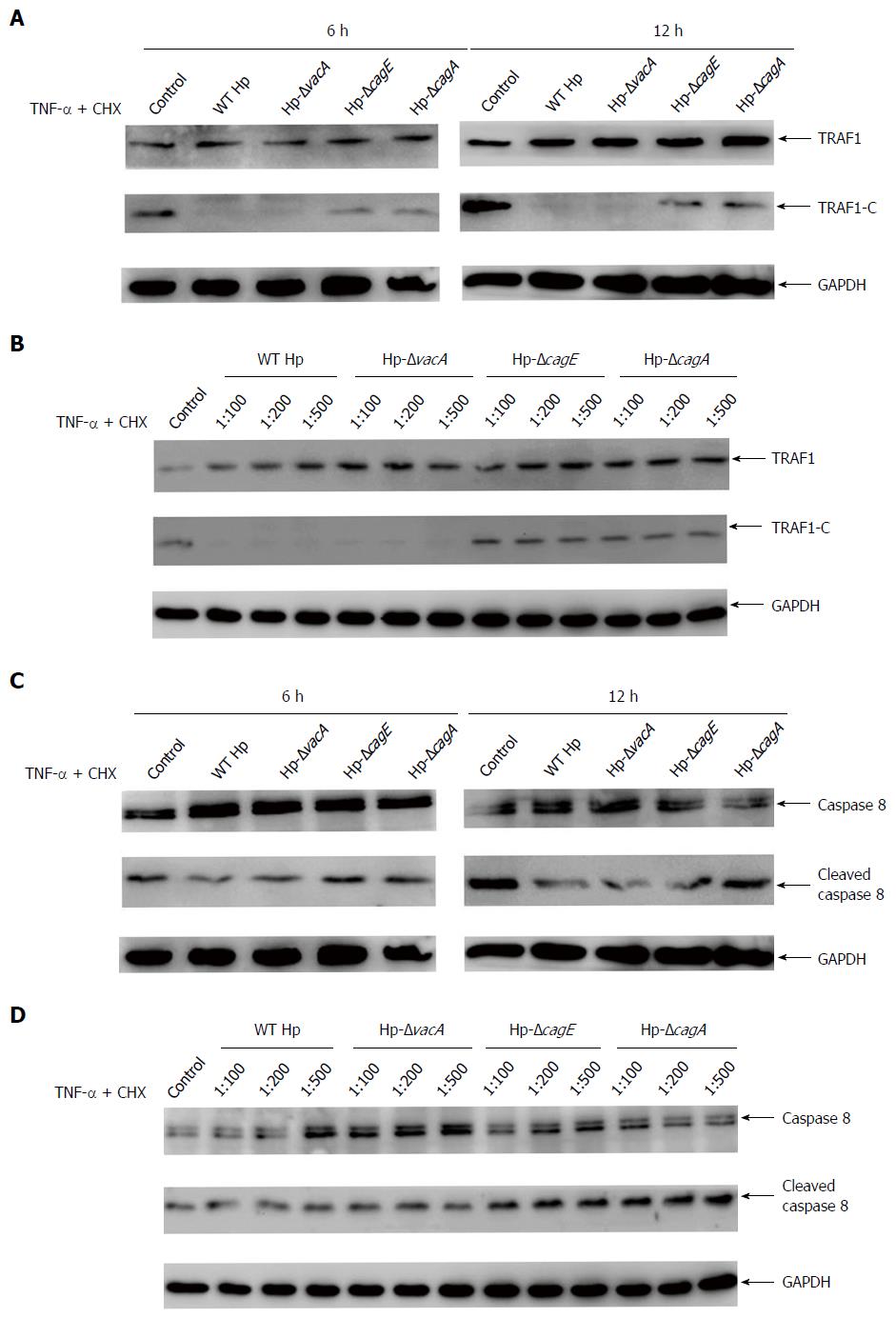
As CagA is a major virulence factor in H. pylori-related gastric pathogenesis, we next investigated whether CagA could affect TRAF1 cleavage. The isogenic cagA-, vacA- and cagE-null mutants were used. H. pylori lacking CagE cannot form an effective type IV secretion system and inhibits the translocation of CagA into cells, whereas knocking-out of cagA and vacA leads to no expression of the VacA and CagA virulence factors, respectively.
AGS cells were infected with the wild-type H. pylori or the isogenic H. pylori mutants in the presence of the apoptosis inducer, and analyzed for the TRAF1 cleavage. The results showed the TRAF1 cleavage significantly increased in 6 h and 12 h after infection with cagE- and cagA-null mutants compared with wild-type H. pylori, while the vacA-null mutant had no significant changes compared with wild-type H. pylori (Figure 4A). A significantly increased cleavage was also observed in cagE- and cagA-null mutants at the indicated cell/bacteria ratios (1:100-1:500) (Figure 4B). This suggested that the effect of cagE- and cagA-null mutants on the inhibition of TRAF1 cleavage was significantly attenuated compared with wild-type H. pylori in the presence of the apoptosis inducer. We also analyzed the uncleaved and cleaved caspase-8 forms at the indicated infection time and cell/bacteria ratios. As shown in Figure 4C and D, the activity of caspase-8 significantly increased in the cagE- and cagA-null mutants compared with wild-type H. pylori. Thus, these results suggested that the virulence factor CagA plays an important role in the H. pylori-mediated inhibition of TRAF1 cleavage.
In the present study, we show the previously unidentified role of TRAF1 cleavage regulated by H. pylori infection. TRAF1 was found to be cleaved via caspase-8 in AGS cells treated with an apoptosis inducer, and the COOH-terminal TRAF1 fragment was found to promote H. pylori-induced cell apoptosis. However, H. pylori infection was found to inhibit the cleavage of TRAF1 as well as inhibit the activation of caspase-8 in the presence of the apoptosis inducer. We also found that the H. pylori virulence factor CagA is mainly involved in the H. pylori-mediated inhibition of TRAF1 cleavage.
TRAF1 is a member of the TRAF family and is dysregulated in various diseases, such as atheroma, lymphoma, and solid tumors[16-19]. Some studies suggested that TRAF1 plays an antiapoptotic role[8,9] and could suppress TNFR-induced cell apoptosis[20,21]. Immunohistochemistry of gastric mucosal specimens from H. pylori-positive patients with chronic gastritis (CG), intestinal metaplasia (IM), dysplasia, and gastric carcinoma (GC) showed that positive TRAF1 expression was detected in 34.8%, 53.3%, 72.7%, and 88.9% specimens of CG, IM, dysplasia, and GC, respectively, suggesting that TRAF1 may be involved in H. pylori-induced gastric carcinogenesis[11]. Our previous study showed that the up-regulation of TRAF1 could be induced by H. pylori in both gastric epithelial cells and mice. The up-regulation of TRAF1 inhibited cell apoptosis and increased the viability of gastric epithelial cells infected with H. pylori. We also determined that H. pylori infection significantly enhanced the formation of the antiapoptotic complex containing TRAF1, TRAF2, cIAP1, and cIAP2, which might play a major role in suppressing the activation of caspase-8 and in inhibiting cell apoptosis[12].
Though the full-length TRAF1 protein exerts an anti-apoptotic role during H. pylori infection, TRAF1 could be converted into a pro-apoptotic protein after cleavage[13-15]. So, it is important to explore the regulation of TRAF1 cleavage by H. pylori. In our study, we found the cleavage of TRAF1 as well as the activation of caspase-8 induced by the apoptosis inducer could be significantly inhibited by H. pylori infection. As the COOH-terminal TRAF1 fragment has a pro-apoptotic role, the inhibition of TRAF1 cleavage may further protect H. pylori-infected cells from apoptosis. Our results showed that the cleavage of TRAF1 decreased significantly after 4 h following H. pylori infection and the cleavage of TRAF1 gradually decreased as the bacteria amounts increased, which suggests the inhibition of TRAF1 cleavage is related to the infection time and the bacterial amounts.
Another important finding is that the inhibition of TRAF1 cleavage was mainly dependent on the H. pylori virulence factor CagA. Our results suggested that the effects of cagE- and cagA-null mutants on the inhibition of TRAF1 cleavage were significantly attenuated compared with wild-type H. pylori or the vacA-null mutant in the presence of the apoptosis inducer. However, the mechanisms are not completely understood. It is possible that CagA-positive H. pylori up-regulates the expression of TRAF1, TRAF2, cIAP1, and cIAP2, which can increase formation of the antiapoptotic complex to suppress caspase-8 activation and then inhibit TRAF1 cleavage. It is also possible that H. pylori inhibits caspase-8 activation via other signaling pathways mediated by activation of NF-κB, thereby inhibiting TRAF1 cleavage. NF-κB is a major transcription regulator involved in inflammation, cell proliferation, and apoptosis, which are considered to be anti-apoptotic and to have an oncogenic role[22]. H. pylori infection could activate NF-κB through host-bacterial interactions via multiple signaling pathways[23,24]. Among these pathways, CagA is the major bacterial component to induce activation of NF-κB through interaction with multiple signaling regulators, such as receptor tyrosine kinase c-Met, guanine exchange factor α-Pix, and Ras, thereby leading to activation of phosphatidylinositol-3-kinase (PI3K)/Akt kinase, transforming growth factor beta-activated kinase 1 (TAK1), and MAPK/ERK signaling pathways[25-28].
In summary, our study suggested that during its evolutionary adaptation to the host gastric environment and to counteract detrimental conditions, H. pylori infection induced the up-regulation of TRAF1 as well as inhibited its cleavage. Therefore, the dysregulation and increase of TRAF1 may be advantageous for H. pylori by allowing this pathogen long-term habitation in the gastric mucosa without triggering cell apoptosis. However, this change may increase the risk of gastric carcinoma.
We would like to express our great appreciation to Weiwei Liu for his statistical review of our data.
Our previous study showed that tumor necrosis factor receptor-associated factor 1 (TRAF1) is up-regulated by Helicobacter pylori (H. pylori) infection in both gastric epithelial cells and mice. The up-regulation of TRAF1 has been shown to inhibit cell apoptosis and to increase the viability of infected cells, suggesting that TRAF1 may be an important molecule during the pathogenesis of H. pylori-related gastric cancer. Interestingly, several studies have shown that TRAF1 can be converted into a pro-apoptotic protein after cleavage by caspase-8 in the presence of Fas ligand or TNF-α-induced apoptosis. However, whether the cleavage of TRAF1 could be influenced by H. pylori infection and the related mechanism are not known.
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer-related deaths worldwide. Though numerous studies have identified an association between H. pylori infection and the development of gastric carcinoma, the mechanisms underlying the carcinogenic potential of H. pylori are still not completely understood.
The authors report our first results from a study of H. pylori-mediated TRAF1 cleavage. This study demonstrates for the first time that the cleavage of TRAF1 and the activation of caspase-8 were significantly inhibited by H. pylori infection in the presence of an apoptosis inducer. In addition, the virulence factor CagA was found to be mainly involved in the inhibition of TRAF1 cleavage.
This results suggest that, during its evolutionary adaptation to the host environment and to counteract detrimental conditions, H. pylori infection induced the up-regulation of TRAF1 as well as inhibited its cleavage. The up-regulation of TRAF1 inhibited cell apoptosis and increased the viability of infected cells, suggesting that TRAF1 may be a potential target for prevention and diagnosis during the pathogenesis of gastric cancer related to H. pylori infection.
Cytotoxin-associated gene A (CagA), an important virulence factor and the only bacterial oncoprotein, is delivered into gastric epithelial cells via type IV secretion of H. pylori. Upon delivery, CagA perturbs multiple host signaling pathways by interacting with the host signaling molecules, resulting in cytopathic effects and subsequent cell transformation.
The following are comments and questions to authors. I hope to take consideration for further improvement: (1) the detailed characteristics of AGS cell and the reason why this cell line was selected needs to be explained; (2) the authors revealed TRAF1 was cleaved by an apoptosis inducer and it was attenuated by a caspase-8 inhibitor. Do the authors have data that caspase-8 itself cleaved TRAF1? (3) for easy understanding, a correlation diagram in H. pylori and TRAF1 cleavage should be presented.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Pellicano R, Suzuki H, Vaziri F, Yamaoka Y S- Editor: Qi Y L- Editor: Filipodia E- Editor: Wang CH
| 1. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21373] [Article Influence: 2137.3] [Reference Citation Analysis (3)] |
| 2. | Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;1:1311-1315. [PubMed] |
| 3. | Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med. 2002;347:1175-1186. [PubMed] |
| 4. | Bessède E, Dubus P, Mégraud F, Varon C. Helicobacter pylori infection and stem cells at the origin of gastric cancer. Oncogene. 2015;34:2547-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Fischer W. Assembly and molecular mode of action of the Helicobacter pylori Cag type IV secretion apparatus. FEBS J. 2011;278:1203-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Tohidpour A. CagA-mediated pathogenesis of Helicobacter pylori. Microb Pathog. 2016;93:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Lee SY, Choi Y. TRAF1 and its biological functions. Adv Exp Med Biol. 2007;597:25-31. [PubMed] |
| 8. | Guo F, Sun A, Wang W, He J, Hou J, Zhou P, Chen Z. TRAF1 is involved in the classical NF-kappaB activation and CD30-induced alternative activity in Hodgkin’s lymphoma cells. Mol Immunol. 2009;46:2441-2448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Xie P, Hostager BS, Munroe ME, Moore CR, Bishop GA. Cooperation between TNF receptor-associated factors 1 and 2 in CD40 signaling. J Immunol. 2006;176:5388-5400. [PubMed] |
| 10. | Sughra K, Birbach A, de Martin R, Schmid JA. Interaction of the TNFR-receptor associated factor TRAF1 with I-kappa B kinase-2 and TRAF2 indicates a regulatory function for NF-kappa B signaling. PLoS One. 2010;5:e12683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Wang F, Luo LD, Pan JH, Huang LH, Lv HW, Guo Q, Xu CX, Shen SR. Comparative genomic study of gastric epithelial cells co-cultured with Helicobacter pylori. World J Gastroenterol. 2012;18:7212-7224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Wan XK, Yuan SL, Tao HX, Diao LP, Wang YC, Cao C, Liu CJ. The Upregulation of TRAF1 Induced by Helicobacter pylori Plays an Antiapoptotic Effect on the Infected Cells. Helicobacter. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Jang HD, Chung YM, Baik JH, Choi YG, Park IS, Jung YK, Lee SY. Caspase-cleaved TRAF1 negatively regulates the antiapoptotic signals of TRAF2 during TNF-induced cell death. Biochem Biophys Res Commun. 2001;281:499-505. [PubMed] |
| 14. | Leo E, Deveraux QL, Buchholtz C, Welsh K, Matsuzawa S, Stennicke HR, Salvesen GS, Reed JC. TRAF1 is a substrate of caspases activated during tumor necrosis factor receptor-alpha-induced apoptosis. J Biol Chem. 2001;276:8087-8093. [PubMed] |
| 15. | Irmler M, Steiner V, Ruegg C, Wajant H, Tschopp J. Caspase-induced inactivation of the anti-apoptotic TRAF1 during Fas ligand-mediated apoptosis. FEBS Lett. 2000;468:129-133. [PubMed] |
| 16. | Schwenzer R, Siemienski K, Liptay S, Schubert G, Peters N, Scheurich P, Schmid RM, Wajant H. The human tumor necrosis factor (TNF) receptor-associated factor 1 gene (TRAF1) is up-regulated by cytokines of the TNF ligand family and modulates TNF-induced activation of NF-kappaB and c-Jun N-terminal kinase. J Biol Chem. 1999;274:19368-19374. [PubMed] |
| 17. | Zirlik A, Bavendiek U, Libby P, MacFarlane L, Gerdes N, Jagielska J, Ernst S, Aikawa M, Nakano H, Tsitsikov E. TRAF-1, -2, -3, -5, and -6 are induced in atherosclerotic plaques and differentially mediate proinflammatory functions of CD40L in endothelial cells. Arterioscler Thromb Vasc Biol. 2007;27:1101-1107. [PubMed] |
| 18. | Zapata JM, Krajewska M, Krajewski S, Kitada S, Welsh K, Monks A, McCloskey N, Gordon J, Kipps TJ, Gascoyne RD. TNFR-associated factor family protein expression in normal tissues and lymphoid malignancies. J Immunol. 2000;165:5084-5096. [PubMed] |
| 19. | Rajandram R, Bennett NC, Wang Z, Perry-Keene J, Vesey DA, Johnson DW, Gobe GC. Patient samples of renal cell carcinoma show reduced expression of TRAF1 compared with normal kidney and functional studies in vitro indicate TRAF1 promotes apoptosis: potential for targeted therapy. Pathology. 2012;44:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680-1683. [PubMed] |
| 21. | Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299-5305. [PubMed] |
| 22. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [PubMed] |
| 23. | Doger FK, Meteoglu I, Ozkara E, Erkul ZK, Okyay P, Yükselen V. Expression of NF-kappaB in Helicobacter pylori infection. Dig Dis Sci. 2006;51:2306-2309. [PubMed] |
| 24. | Backert S, Naumann M. What a disorder: proinflammatory signaling pathways induced by Helicobacter pylori. Trends Microbiol. 2010;18:479-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 25. | Brandt S, Kwok T, Hartig R, König W, Backert S. NF-kappaB activation and potentiation of proinflammatory responses by the Helicobacter pylori CagA protein. Proc Natl Acad Sci USA. 2005;102:9300-9305. [PubMed] |
| 26. | Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, Nagai S, Koyasu S, Gilman RH, Kersulyte D. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 27. | Lim JW, Kim KH, Kim H. alphaPix interacts with Helicobacter pylori CagA to induce IL-8 expression in gastric epithelial cells. Scand J Gastroenterol. 2009;44:1166-1172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Lamb A, Yang XD, Tsang YH, Li JD, Higashi H, Hatakeyama M, Peek RM, Blanke SR, Chen LF. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |