Published online Dec 21, 2016. doi: 10.3748/wjg.v22.i47.10440
Peer-review started: August 1, 2016
First decision: August 22, 2016
Revised: October 18, 2016
Accepted: November 13, 2016
Article in press: November 13, 2016
Published online: December 21, 2016
Processing time: 145 Days and 13.2 Hours
To investigate the relationship between plasma ghrelin level, Helicobacter pylori (H. pylori) infection status and the severity of atrophy in hemodialysis patients.
One hundred eights patients who received hemodialysis and 13 non-hemodialysis H. pylori-negative controls underwent gastroduodenoscopy to evaluate the severity of gastric atrophy. Serum levels of pepsinogen (PG) were measured as serum markers of gastric atrophy. H. pylori infection was evaluated by anti-H. pylori IgG antibody, rapid urease test and culture test. We classified H. pylori infection status as non-infection, present infection and past infection. In addition, plasma acyl-ghrelin and desacyl-ghrelin levels were measured by enzyme-linked immunosorbent assay.
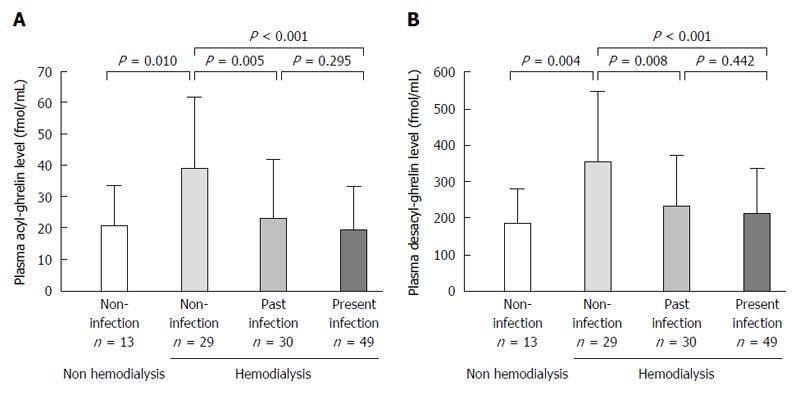
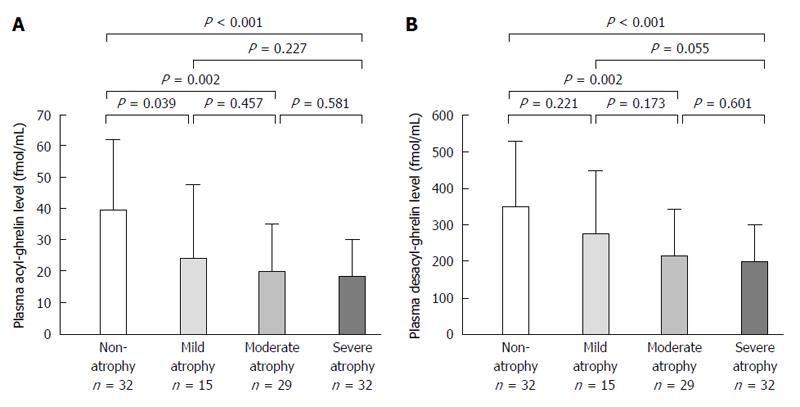
Infection rate of H. pylori was 45.4% (49/108). Acyl-ghrelin level in the non-infection group (39.4 ± 23.0 fmol/mL) was significantly higher than in the past (23.4 ± 19.9 fmol/mL, P = 0.005) and present infection groups (19.5 ± 14.0 fmol/mL, P < 0.001). Furthermore, desacyl-ghrelin level in the non-infection group (353.2 ± 190.2 fmol/mL) was significantly higher than those in the past (234.9 ± 137.5 fmol/mL, P = 0.008) and present infection groups (211.8 ± 124.2 fmol/mL, P < 0.001). Acyl-ghrelin was positively correlated with the PG I level and PG I/II ratio (|R| = 0.484, P < 0.001 and |R| = 0.403, P < 0.001, respectively). Both ghrelins were significantly decreased in accordance with the progress of endoscopic atrophy (both P < 0.001) and acyl-ghrelin was significantly lower in patients with mild, moderate and severe atrophy (24.5 ± 23.1 fmol/mL, 20.2 ± 14.9 fmol/mL and 18.3 ± 11.8 fmol/mL) than in those with non-atrophy (39.4 ± 22.2 fmol/mL, P = 0.039, P = 0.002 and P < 0.001, respectively).
In hemodialysis patients, plasma ghrelin level was associated with the endoscopic and serological severity of atrophy related to H. pylori infection.
Core tip: Ghrelin enhances the orexigenic effect, protein anabolism, anti-inflammatory actions and cardiovascular protection in hemodialysis patients. Helicobacter pylori (H. pylori) infection and gastric mucosal atrophy affect ghrelin level in subjects with normal renal function. However, it is unclear whether ghrelin in hemodialysis patients relates to H. pylori status or gastric mucosal atrophy. This study demonstrated that plasma ghrelin level was associated with endoscopic and serological severity of atrophy related with H. pylori infection in hemodalysis patients. Therefore, eradication therapy of H. pylori before the progression of atrophy might improve ghrelin level associated with nutrition status, such as protein-energy wasting.
- Citation: Ichikawa H, Sugimoto M, Sakao Y, Sahara S, Ohashi N, Kato A, Sugimoto K, Furuta T, Andoh A, Sakao T, Yasuda H. Relationship between ghrelin, Helicobacter pylori and gastric mucosal atrophy in hemodialysis patients. World J Gastroenterol 2016; 22(47): 10440-10449
- URL: https://www.wjgnet.com/1007-9327/full/v22/i47/10440.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i47.10440
The number of end-stage kidney disease patients receiving hemodialysis is more than 1.1 million worldwide, and the size of this population is expanding at a rate of 7% per year with the progress in medical and hemodialysis machine technique[1]. Hemodialysis patients often suffer from gastrointestinal conditions such as bleeding, peptic ulcer, gastric cancer and other severe abdominal symptoms during their long treatment period. Helicobacter pylori (H. pylori) infection causes atrophic gastritis, peptic ulcer and gastric cancer[2-5], and is thought to be one of the major risk factors for gastrointestinal morbidity in hemodialysis patients. However, a recent report noted that the prevalence of H. pylori infection in hemodialysis patients was significantly lower than in subjects with normal renal function[6]. It is therefore necessary to investigate whether the actual condition of gastroduodenal diseases in hemodialysis patients relates to H. pylori infection.
Hemodialysis patients have many risk factors that affect mortality, such as traditional cardiovascular risk factors, chronic inflammation and nutritional disturbance[7]. Among these risk factors, the state of metabolic and nutritional derangement, called protein-energy wasting (PEW), has a major impact on mortality in these patients[8,9]. PEW is defined as a state of decreased body stores of protein and energy fuels (body protein and fat masses) and is diagnosed if three features are present: (1) abnormal nutrition markers (i.e., low serum levels of albumin, transthyretin or cholesterol); (2) reduced body mass (i.e., low or reduced body or fat mass or weight loss with reduced intake of protein and energy); and (3) reduced muscle mass (i.e., muscle wasting or sarcopenia, and reduced mid-arm muscle circumference)[10]. Recently, it was suggested that ghrelin, an orexigenic peptide released primarily from endocrine cells in the stomach, is important in the pathogenesis of PEW in hemodialysis patients[7,11,12]. Ghrelin has multiple favorable functions including enhancement of orexigenic effect, protein anabolism, anti-inflammatory actions, and cardiovascular protection[11,13,14]. In general, plasma ghrelin level increases after fasting and decreases after eating and plasma ghrelin level was found to associate with nutritional disorder in patients with advanced-stage cancer and anorexia nervosa[14]. A low ghrelin level increases the risk of cardiovascular mortality and morbidity in hemodialysis patients[15], and in recent clinical trials, administration of ghrelin improved appetite and food intake in dialysis patients[16,17]. The usefulness of monitoring plasma ghrelin at fixed intervals as a biomarker for mortality in hemodialysis patients has been proven[7]. Therefore, we think that it is important to know what factors could influence the ghrelin level in dialysis patients.
H. pylori-positive patients have low levels of gastric mucosal and/or plasma ghrelin and a small number of ghrelin-positive cells in the gastric mucosa[18]. Although, in subjects with normal renal function, the relationship between plasma ghrelin level and the severity of gastric mucosal atrophy is observed[18], the association between ghrelin level and H. pylori infection and between ghrelin level and severity of gastric mucosal atrophy in hemodialysis patients is unknown.
Clarifying the factors associated with PEW in hemodialysis patients is important in improving prognosis. We therefore investigated the relationship among plasma ghrelin level, H. pylori status and endoscopic and serological gastric mucosal atrophy in hemodialysis patients.
Approval for the study protocol was given in advance by the Institutional Review Board of Hamamatsu University School of Medicine (Number: 23-185) and written informed consent was obtained from patients enrolled in this study. The registration number of this clinical research is UMIN 000023336.
One hundred eight patients receiving maintenance hemodialysis at 8 Dialysis center in Shizuoka, Japan (Sanaru Sun-Clinic, Sano Clinic, Yamashita Clinic, Tadokoro Clinic, Hiryu Clinic, Sanarudai Asahi-Clinic, Satsuki no Mori Clinic and Hamana clinic), who had received regular hemodialysis for 4 h three times per week and 13 non-hemodialysis H. pylori-negative control subjects (eGFR > 50) in Hamamatsu University Hospital were enrolled in this study. After written informed consent was obtained, all hemodialysis patients and control subjects underwent gastroduodenoscopy and blood samples were taken for measurement of plasma ghrelin level on a non-dialysis day. Of 108 hemodialysis patients, 78 patients who received regular hemodialysis at Hamana clinic (Hamamatsu, Japan) was used in previous report which investigated associations with plasma grhrelin level and gastric mucosal atoprophy as single center study[19]. In this study, because previous our study could not show significant association with grhrelin level and present H. pylori infection (present infection and present non-infection including of non-infection and past infection), we focused in associations with plasma grhrelin level and infection status of H. pylori (non-infection, past infection and present infection) and we did as multicenter trial.
Exclusion criteria were a history of H. pylori eradication, history of gastrectomy, significant clinical illness (e.g., cancer) and lack of informed consent.
Blood samples were taken from all patients while fasting on the morning of a non-dialysis day. Samples were collected in an aprotinin/EDTA-containing tube and immediately centrifuged at 1500 ×g for 15 min at 4 °C. The resultant plasma was agitated with the addition of 1 mol/L hydrochloric acid equal to one-tenth of the plasma volume and stored at -80 °C until assay. We used a two-site sandwich enzyme-linked immunosorbent assay specific for acyl ghrelin and des-acyl ghrelin (Active Ghrelin ELISA Kit and Des-acyl Ghrelin ELISA Kit, SCETI, Tokyo, Japan).
The diagnosis of H. pylori infection was achieved with an anti-H. pylori IgG serological test (E plate Eiken H. pylori antibody®; Eiken Chemical Co. Ltd., Tochigi, Japan), a rapid urease test (RUT) (Helicocheck®; Otsuka Co., Tokyo, Japan) and culture test (E-MR82; Eiken Chemical Co, Ltd., Tochigi, Japan). When positive results were observed with at least one of the detection systems, we diagnosed as positive for H. pylori. We classified H. pylori infection status into three groups: present infection (positive results were observed with at least one of the detection systems), past infection (negative with all three detection systems and positive for endoscopic gastric mucosal atrophy) and non-infection (negative with all three detection systems and no endoscopic gastric mucosal atrophy) (Table 1). Past infection group had not been received previously H. pylori eradication therapy with proton pump inhibitor and two kinds of antimicrobial agents. Hemodialysis patients in this group were considered to be naturally eradicated H. pylori infection without receiving eradication therapy.
| (1) Anti-H. pylori IgG | Endoscopic gastric mucosal atrophy | |
| (2) RUT | ||
| (3) Culture test | ||
| Present infection | Positive at least one detection systems | |
| Past infection | Negative with all of three detection systems | Positive for atrophy |
| Non-infection | Negative with all of three detection systems | No atrophy |
Endoscopic diagnosis of gastric mucosal atrophy was shown significant correlations with morphological and histological diagnosis[20,21]. In addition, because 30% of hemodialysis patients enrolled in this study were taking anti-coagulant agents, we diagnosed gastric mucosal atrophic by endoscopic findings, not pathological evaluation using gastric biopsy specimens, to prevent hemorrhage events. The endoscopic gastric mucosal atrophic pattern was evaluated according to the Kimura-Takemoto classification and expressed in six grades: Close (C)-I, C-II, C-III, Open (O)-I, O-II and O-III[22]. We categorized the severity of gastric mucosal atrophy into four groups as follows: non-atrophy; mild, C-I and C-II; moderate, C-III and O-I; severe, O-II and O-III.
Serum levels of PG I and PG II were measured using a commercially available kit (Pepsinogen CLEIA®; Fuji Rebio. Ltd, Tokyo, Japan) by chemiluminescence enzyme immunoassay (EIA), and PG I/PG II ratio was calculated as a serological marker of gastric mucosal atrophy[23].
Normally numerical values were expressed as mean ± standard deviation (SD). Statistical differences in plasma ghrelin levels and serum pepsinogen levels among subgroups were assessed using one-way ANOVA (analysis of variance) with Scheffe’s multiple comparison test. Continuous variables were compared using t-test or Mann-Whitney U-test and categorical variables were compared using the χ2 test. All P-values were two-sided. P < 0.05 was considered statistically significant. Calculations were carried out using StatView 5.0 statistical software (SAS Institute, Cary, NC, United States).
The mean age of hemodialysis patients was 68.8 ± 11.2 years and the mean duration of hemodialysis was 8.7 ± 8.6 years (Table 2). The causes of end-stage renal disease in patients receiving hemodialysis were chronic glomerulonephritis (52/108, 48.1%), diabetic nephropathy (29/108, 26.9%), nephrosclerosis (7/108, 6.5%), and others (20/108, 18.5%).
| Age (yr) | 68.8 ± 11.2 |
| Male/female (n/n) | 68/40 |
| Duration of hemodialysis (yr) | 8.7 ± 8.6 |
| Body weight (kg) | 51.4 ± 11.4 |
| Body mass index (kg/m2) | 20.2 ± 3.5 |
| Albumin (g/dL) | 3.4 ± 0.4 |
| Total cholesterol (mg/dL) | 159.5 ± 36.4 |
| Cholinesterase (U/L) | 230.3 ± 87.2 |
| Infection of H. pylori: non/past/present | 29/30/49 |
| Acyl-ghrelin (fmol/mL) | 25.9 ± 19.8 |
| Desacyl-ghrelin (fmol/mL) | 256.2 ± 158.4 |
| Pepsinogen I (ng/mL) | 349.9 ± 294.5 |
| Pepsinogen II (ng/mL) | 43.5 ± 32.3 |
| Pepsinogen I/II ratio | 8.3 ± 4.7 |
Among the 108 hemodialysis patients, 49 (45.4%) had current H. pylori infection (present infection group) (Table 2) while 30 (27.8%) were in the past infection group, irrespective of eradication history (Table 2). Seventy-six patients (70.4%) had endoscopic gastric mucosal atrophy (46 patients in the present infection group and 30 patients in the past infection group). Although plasma ghrelin levels affected by body weights, sex and BMI, there were no significant differences in these parameters among three different groups related with H. pylori infection (non-infection, past infection and present infection groups) and among different groups of severity of gastric mucosal atrophy (Tables 3 and 4). Total cholesterol and cholinesterase in the H. pylori present infection group or the severe gastric mucosal atrophy group were lower than those in the H. pylori non-infection group or the non-atrophy group, but not significant (Tables 3 and 4).
| H. pylori status | Non-infection | Past infection | Present infection | P value |
| Number | 29 | 30 | 49 | |
| Male/female (n/n) | 19/10 | 22/8 | 27/22 | 0.275 |
| Age (yr) | 67.9 ± 10.7 | 71.7 ± 11.3 | 67.6 ± 11.3 | 0.119 |
| Body weight (kg) | 51.2 ± 12.1 | 49.0 ± 8.3 | 51.9 ± 12.4 | 0.468 |
| BMI (kg/m2) | 19.9 ± 3.4 | 19.3 ± 2.3 | 21.0 ± 4.0 | 0.160 |
| Albumin (g/dL) | 3.5 ± 0.3 | 3.5 ± 0.4 | 3.3 ± 0.4 | 0.156 |
| Total cholesterol (mg/dL) | 167.5 ± 36.1 | 149.5 ± 29.0 | 161.5 ± 44.2 | 0.175 |
| Cholinesterase (U/L) | 247.0 ± 109.3 | 228.5 ± 64.3 | 207.5 ± 75.3 | 0.310 |
| Acyl-ghrelin (fmol/mL) | 39.4 ± 23.0 | 23.4 ± 19.9a | 19.5 ± 14.0a | < 0.001 |
| Desacyl-ghrelin (fmol/mL) | 353.2 ± 190.2 | 234.9 ± 137.5a | 211.8 ± 124.2a | 0.001 |
| Pepsinogen I (ng/mL) | 551.8 ± 319.2 | 286.5 ± 268.2a | 269.3 ± 238.0a | < 0.001 |
| Pepsinogen II (ng/mL) | 46.4 ± 24.5 | 28.5 ± 17.4a | 51.0 ± 39.9 | 0.002 |
| Pepsinogen I/II | 12.2 ± 4.5 | 9.3 ± 4.1a | 5.3 ± 2.9a | < 0.001 |
| Mucosal atrophy | Non-atrophy | Mild atrophy | Moderate atrophy | Severe atrophy | P value |
| Number | 32 | 15 | 29 | 32 | |
| Male/female (n/n) | 19/13 | 10/5 | 19/10 | 20/12 | 0.969 |
| Age (yr) | 67.5 ± 11.8 | 64.5 ± 12.3 | 71.9 ± 10.9 | 69.3 ± 10.0 | 0.293 |
| Body weight (kg) | 50.7 ± 12.0 | 53.6 ± 10.5 | 50.6 ± 11.4 | 51.5 ± 11.4 | 0.708 |
| BMI (kg/m2) | 19.9 ± 3.4 | 20.3 ± 2.8 | 20.1 ± 3.6 | 20.6 ± 3.9 | 0.896 |
| Albumin (g/dL) | 3.5 ± 0.3 | 3.5 ± 0.3 | 3.3 ± 0.4 | 3.4 ± 0.5 | 0.351 |
| Total cholesterol (mg/dL) | 166.4 ± 34.8 | 159.9 ± 34.9 | 153.6 ± 37.5 | 151.4 ± 40.4 | 0.510 |
| Cholinesterase (U/L) | 246.0 ± 104.3 | 250.5 ± 77.7 | 201.9 ± 59.3 | 218.6 ± 77.6 | 0.283 |
| Acyl-ghrelin (fmol/mL) | 39.4 ± 22.2 | 24.5 ± 23.1a | 20.2 ± 14.9a | 18.3 ± 11.8a | < 0.001 |
| Desacyl-ghrelin (fmol/mL) | 345.7 ± 183.7 | 276.1 ± 169.4 | 212.6 ± 129.4a | 196.8 ± 104.4a | < 0.001 |
| Pepsinogen I (ng/mL) | 525.9 ± 316.6 | 461.9 ± 330.3 | 284.2 ± 207.3a | 181.0 ± 198.8a | < 0.001 |
| Pepsinogen II (ng/mL) | 45.9 ± 23.8 | 49.3 ± 32.0 | 45.8 ± 40.8 | 36.3 ± 31.6 | 0.120 |
| Pepsinogen I/II | 11.6 ± 4.7 | 10.0 ± 4.6 | 6.9 ± 3.4a | 5.3 ± 3.3a | < 0.001 |
Mean plasma acyl- and desacyl-ghrelin levels of the 108 patients were 25.9 ± 19.8 fmol/mL and 256.2 ± 158.4 fmol/mL, respectively (Table 2). Acyl-ghrelin level in the non-infection group (39.4 ± 23.0 fmol/mL) was significantly higher than in the past (23.4 ± 19.9 fmol/mL, P = 0.005) and present infection groups (19.5 ± 14.0 fmol/mL, P < 0.001) (Figure 1A and Table 3). Furthermore, desacyl-ghrelin level in the non-infection group (353.2 ± 190.2 fmol/mL) was significantly higher than those in the past (234.9 ± 137.5 fmol/mL, P = 0.008) and present infection groups (211.8 ± 124.2 fmol/mL, P < 0.001) (Figure 1B and Table 3). However, there were no significant differences in acyl- or desacyl-ghrelin levels between the present and past infection groups (Figure 1A and B).
The acyl- or desacyl-ghrelin levels in the non-infection group of hemodialysis patients were significantly higher than those in H. pylori infection-negative non-hemodialysis control subjects (P = 0.010 and 0.004, respectively) (Figure 1A and B).
Acyl-ghrelin and desacyl-ghrelin levels were significantly decreased in accordance with the progress of endoscopic gastric mucosal atrophy (both P < 0.001) (Table 4). Acyl-ghrelin levels were significantly lower in patients with mild (C-I and C-II), moderate (C-III and O-I) and severe atrophy (O-II and O-III) (24.5 ± 23.1 fmol/mL, 20.2 ± 14.9 fmol/mL and 18.3 ± 11.8 fmol/mL) than in those with non-atrophy (39.4 ± 22.2 fmol/mL, P = 0.039, P = 0.002 and P < 0.001, respectively) but not in those with mild atrophy (Figure 2A and Table 4). Furthermore, desacyl-ghrelin levels were also significantly lower in patients with moderate and severe atrophy (212.6 ± 129.4 fmol/mL and 196.8 ± 104.4 fmol/mL) than in those with non-atrophy (345.7 ± 183.7 fmol/mL, P = 0.002 and P < 0.001, respectively) (Figure 2B and Table 4).
Serum PG I, PG II levels and PG I/II ratio were 349.9 ± 294.5 ng/mL, 43.5 ± 32.3 ng/mL and 8.3 ± 4.7, respectively (Table 2). These values were higher than those generally reported in subjects with normal renal function[24-27]. Serum PG I level and PG I/II ratio were significantly decreased in accordance with the progress of endoscopic atrophy (both P < 0.001) (Table 4).
The acyl-ghrelin level was positively correlated with serum PG I level and PG I/II ratio (|R| = 0.484, P < 0.001 and |R| = 0.403, P < 0.001, respectively). In addition, desacyl-ghrelin level was also positively correlated with serum PG I level and PG I/II ratio (|R| = 0.538, P < 0.001 and |R| = 0.426, P < 0.001, respectively).
Serum PG I level in the non-infection group (551.8 ± 319.2 ng/mL) was significantly higher than those in the past (286.5 ± 268.2 ng/mL, P = 0.001) and present infection groups (269.3 ± 238.0 ng/mL, P < 0.001) (Table 3). Furthermore, PGI/II ratio in the non-infection group (12.2 ± 4.5) was significantly higher in the past (9.3 ± 4.1, P = 0.014) and present infection groups (5.3 ± 2.9, P < 0.001) (Table 3).
In this study, we showed that plasma acyl and des-acyl ghrelin levels were significantly associated with the endoscopic and serological severity of gastric mucosal atrophy in patients who were hemodialyzed, and that both ghrelin levels graduately decreased according to the advanced endoscopic and serological severity of atrophy. In general, although H. pylori infection down-regulated plasma and gastric ghrelin levels in subjects with normal renal function[28-30], these results also demonstrate the possibility that not only long-standing enhanced gastric mucosal inflammation induced by H. pylori infection but also gastric mucosal atrophy influenced plasma and gastric mucosal acyl- and desacyl-ghrelin levels[18]. In fact, in subjects with normal renal function, plasma ghrelin level was lower in H. pylori-positive patients with severe mucosal atrophy than in those without[18]. Here, our observation might suggest that the interaction with plasma ghrelin levels, gastric mucosal atrophy and H. pylori infection in patients who were hemodialyzed was similar to that in subjects with normal renal function. From the observation that improvement of PEW in hemodialysis patients was important for improving prognosis, measures for H. pylori infection in hemodialysis patients will be required not only for prevention of gastric cancer but also for improvement of nutritional disturbance and prognosis.
Although ghrelin is released from tissues including the small and large intestines, lung, kidney, the nucleus of the hypothalamus and A cells of the pancreatic islet, ghrelin-producing endocrine cells had been found mainly in the oxyntic mucosa of the stomach[11]. Ghrelin plays central and peripheral biological roles in food intake, gastric motility and acid secretion, and major factors that influenced plasma ghrelin levels were sex, body weight, hormonal parameter and H. pylori infection in the gastric mucosa[28-30]. Of these, H. pylori infection inhibited ghrelin production from gastric endocrine cells, leading to lower plasma and gastric mucosal ghrelin levels[18,31,32]. Moreover, expression of gastric ghrelin mRNA significantly increased after either peptic ulcer healing or H. pylori eradication therapy[33]. Recently, Osawa et al[18] reported that lower PG I levels and PG I/II ratio which denoted advanced gastric mucosal atrophy were significantly associated with lower plasma ghrelin levels in current H. pylori-positive patients with normal renal function. In this study, we divided hemodialysis patients into non-, past, and present H. pylori-infection groups. Previously, we did the follow-up survey of H. pylori infection in hemodialysis patients[6]. When the natural history of H. pylori infection was investigated using more than 300 patients for a 4 year-follow up survey, although nobody received eradication therapy, the prevalence of H. pylori infection was reported to be 51.6% at first year, 42.9% two years later, and 38.3% four years later, indicating that the infection rate gradually decreased during dialysis treatment. In other words, 26.7% of dialysis patients naturally cured H. pylori infection over four years. Therefore, H. pylori infection might be actually eradicated after beginning hemodialysis treatment. We used “past H. pylori-infection” group as patients group who had been naturally cured H. pylori infection without receiving eradication therapy. The acyl-ghrelin level in the non-infection group was significantly higher than that in the present infection group, as reported in previous studies in subjects with normal renal function[18,29]. In addition, we saw no significant differences in acyl- and desacyl-ghrelin level between the present and past infection groups and both ghrelin levels in the past infection group were significantly lower than those in the non-infection group, whereas there was no current H. pylori infection in the past infection group. In this study, most hemodialysis patients in the past infection group had advanced gastric mucosal atrophy (i.e., severe atrophy, 36.7% and moderate atrophy, 33.3%, respectively). Importantly, this observation suggested that the determination of plasma ghrerin level was influenced more by the severity of gastric mucosal atrophy than current H. pyori infection in hemodialysis patients. In fact, plasma ghrelin levels and gastric ghrelin mRNA levels depended on the number of ghrelin immunoreactive cells in the gastric mucosa[18,28,34]. Chronic persistent damage to the gastric mucosa by H. pylori infection might affect ghrelin production, leading to changes in food intake and body weight. Because ghrelin level was associated with mortality related to cardiovascular disease and PEW in hemodialysis patients, alternative management, such as H. pylori eradication therapy, before the progression of gastric mucosal atrophy might be necessary to prevent the decrease of ghrelin level in hemodialysis patients[12,15].
PG I and II used to be considered promising biomarkers for predicting the status of gastric mucosa, such as the severity of gastric mucosal atrophy and the risk of gastric cancer development[35,36]. In Japan, recently, the combination of serum PG level and H. pylori-IgG level had been used mainly at health check-ups as useful markers for gastric cancer screening, namely, the ABC method[37]. However, because the kidney was the key organ for eliminating serum PGs[38], serum PG levels were elevated in chronic kidney disease (CKD) patients including those who received hemodialysis compared with control subjects with normal renal function[27,39,40]. Araki et al[40] reported that serum PG I and PG II levels and PG I/II ratio measured by radioimmunoassay (RIA), which strong correlation was proved between assays of EIA and RIA[41-43], in H. pylori-negative subjects with normal renal function were 50.7 ± 28.7 ng/mL, 10.2 ± 6.1 ng/mL and 5.5 ± 2.4, respectively, while in this study, those parameters were 551.8 ± 319.2 ng/mL, 46.4 ± 24.5 ng/mL and 12.2 ± 4.5 in the non-infection group and 268.3 ± 238.0 ng/mL, 51.0 ± 39.9 ng/mL and 5.3 ± 2.9 in the present infection group, respectively. Therefore, it was considered difficult to use serum PG as a promising biomarker of gastric atrophy and gastric cancer in hemodialysis patients using the same standard for subjects with normal renal function.
In this study, the serum PG I level and PG I/II ratio in the non-infection group were significantly higher than those in the past and present infection groups. In addition, the serological severity of gastric mucosal atrophy evaluated by PG levels had a significant positive correlation with endoscopic evaluations. Therefore, serum PG may be a biomarker for gastric mucosal atrophy and represent the new standard for hemodialysis patients. In addition, because there was a significant positive correlation between PG and ghrelin levels even in hemodialysis patients, the serum PG level and PG I/II ratio might be useful determinations not only for gastric mucosal atrophy related to gastric cancer development but also nutrition status. However, in this study, because the serum PG levels of each subject showed a wide distribution and the sample number was small, we could not determine the standard PG value to evaluate the severity of gastric mucosal atrophy in hemodialysis patients. Thus, further large-scale studies are needed to verify whether the serum PG level can become the parameter of serological severity of gastric mucosal atrophy and nutrition status in hemodialysis patients.
Our study had several limitations, as follows. First, our study had a relatively small sample size. Second, we were unable to evaluate the control subjects with normal renal function. We therefore compared the data of control subjects with normal renal function in the previous reports. Third, we could not evaluate whether H. pylori eradication therapy affected plasma ghrelin levels because we did not have data on plasma ghrelin levels before and after eradication therapy in the same subject.
In conclusion, We investigated the relationships among plasma ghrelin level, H. pylori status and endoscopic and serological gastric mucosal atrophy in hemodialysis patients. Acyl and des-acyl ghrelin levels decreased significantly with the progress of gastric mucosal atrophy caused by H. pylori infection for long-term periods. Importantly. the status of gastric mucosal atrophy was the major determinant of plasma ghrelin level. Because ghrelin influences the pathogenesis of PEW, inflammation and cardiovascular risk in CKD patients, leading to a poor outcome in hemodialysis patients, it is necessary to clarify the factors which affect the ghrelin level and to consider countermeasures to improve the ghrelin level. To increase ghrelin secretion in hemodialysis patients, improvement of gastric mucosal status may be the most important approach. Eradication therapy before the progression of gastric mucosal atrophy might be necessary to prevent the decrease of ghrelin level in hemodialysis patients.
The authors would like to thank the gastroenterologists (Iwaizumi M, Tani S, Takano R, Tamura S, Kuriyama S, Kawasaki S, Terai T and Hamaya Y) and nephrologists (Tsuji N, Ishigaki S, Kitajima K, Iwakura T, Isobe S, Ono M, Fujikura T, Tsuji T and Fujigaki Y) at Hamamatsu University School of Medicine for their assistance with sample collection. We also thank Nephrologists enrolled patients in this study (Hisanori Azekura, Sanaru Sun-Clinic; Koji Sano, Sano Clinic; Fuyuki Yamashita, Yamashita Clinic; Shigeru Tadokoro, Tadokoro Clinic; Ken Fukuta, Hiryu Clinic; Asahi Shimomura, Sanarudai Asahi-Clinic; Kunihiro Hayakawa, Satsuki no Mori Clinic). Of 108 hemodialysis patients, 78 patients who received regular hemodialysis at Hamana Clinic (Hamamatsu, Japan) were used in previous report[19].
Ghrelin enhances the orexigenic effect, protein anabolism, anti-inflammatory actions and cardiovascular protection in hemodialysis patients. Recently, it was suggested that ghrelin is important in the pathogenesis of protein-energy wasting (PEW), which is the state of metabolic and nutritional derangement, in hemodialysis patients. Helicobacter pylori (H. pylori) infection and gastric mucosal atrophy affect plasma and gastric mucosal ghrelin level in subjects with normal renal function. However, it is unclear whether ghrelin level in hemodialysis patients relates to H. pylori status or gastric mucosal atrophy.
PEW has a major impact on mortality and morbidity in hemodialysis patients, especially in cardiovascular events. Administration of ghrelin improves appetite and food intake in hemodialysis patients, finally improves nutrition status in hemodialysis patients. Therefore, clarifying the factors associated with plasma and gastric mucosal ghrelin levels affected to PEW in hemodialysis patients is important in improving their prognosis.
Acyl and des-acyl ghrelin levels in hemodialysis patients decreased significantly with the progress of gastric mucosal atrophy caused by H. pylori infection for long-term periods. Importantly, the status of gastric mucosal atrophy was the major determinant of plasma ghrelin level.
These data suggested that eradication therapy of H. pylori before the progression of gastric mucosal atrophy might be required to prevent the decrease of ghrelin level in hemodialysis patients. In addition, eradication therapy before the progression of atrophy would be expected to improve mortality in hemodialysis patients by improving nutrition status, such as PEW, as well as to prevent increased risk fo gastric carcinogenesis. However, because this study is preliminary, The authors think that it will be better to clarify the relationship between plasma and gastric mucosal ghrelin levels and PEW markers by enrolling many hemodialysis patients in multicenter study and the long-term observation study.
Ghrelin is mainly released from the oxyntic mucosa of the stomach and has multiple favorable functions. PEW is defined as a state of decreased body stores of protein and energy fuels and is diagnosed if three features are present: abnormal nutrition markers; reduced body mass; and reduced muscle mass. Pepsinogen (PG) I and II, the two main precursors of pepsin, are both produced by chief cells and mucous neck cells of the stomach. PG II is also produced by pyloric gland cells. PG I and II used to be considered biomarkers for predicting the status of gastric mucosa.
This clinical study considers the investigation of the plasma ghrelin level in hemodialysis patients and it association with gastric mucosal atrophy and H. pylori status. The main finding of this study was that the status of gastric mucosal atrophy was the major determinant of plasma gherlin level. The authors investigated 108 patients who received hemodialysis. The plasma gherlin level was evaluated using ELISA. The results are presented and discussed sufficiently well; the 2 figures and 3 tables give good overview about the results and are presented correctly.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chmiela M, Iwakura H, Ozkok E, Vorobjova T, Yamaoka Y S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Lysaght MJ. Maintenance dialysis population dynamics: current trends and long-term implications. J Am Soc Nephrol. 2002;13 Suppl 1:S37-S40. [PubMed] |
| 2. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 3. | Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric ulcer recurrence: a review. Gastroenterology. 1996;110:1244-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 354] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 4. | Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, Lai KC, Hu WH, Yuen ST, Leung SY. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA. 2004;291:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1015] [Cited by in RCA: 1046] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 5. | Take S, Mizuno M, Ishiki K, Nagahara Y, Yoshida T, Yokota K, Oguma K, Okada H, Shiratori Y. The effect of eradicating helicobacter pylori on the development of gastric cancer in patients with peptic ulcer disease. Am J Gastroenterol. 2005;100:1037-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Sugimoto M, Sakai K, Kita M, Imanishi J, Yamaoka Y. Prevalence of Helicobacter pylori infection in long-term hemodialysis patients. Kidney Int. 2009;75:96-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Mak RH, Cheung WW. Is ghrelin a biomarker for mortality in end-stage renal disease? Kidney Int. 2011;79:697-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Ikizler TA, Cano NJ, Franch H, Fouque D, Himmelfarb J, Kalantar-Zadeh K, Kuhlmann MK, Stenvinkel P, TerWee P, Teta D. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013;84:1096-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (1)] |
| 9. | Kovesdy CP, George SM, Anderson JE, Kalantar-Zadeh K. Outcome predictability of biomarkers of protein-energy wasting and inflammation in moderate and advanced chronic kidney disease. Am J Clin Nutr. 2009;90:407-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 10. | Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1218] [Cited by in RCA: 1338] [Article Influence: 74.3] [Reference Citation Analysis (0)] |
| 11. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5961] [Cited by in RCA: 5889] [Article Influence: 226.5] [Reference Citation Analysis (0)] |
| 12. | Carrero JJ, Nakashima A, Qureshi AR, Lindholm B, Heimbürger O, Bárány P, Stenvinkel P. Protein-energy wasting modifies the association of ghrelin with inflammation, leptin, and mortality in hemodialysis patients. Kidney Int. 2011;79:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Pradhan G, Samson SL, Sun Y. Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care. 2013;16:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 213] [Article Influence: 17.8] [Reference Citation Analysis (1)] |
| 14. | Akamizu T, Kangawa K. Ghrelin for cachexia. J Cachexia Sarcopenia Muscle. 2010;1:169-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Chou CC, Bai CH, Tsai SC, Wu MS. Low serum acylated ghrelin levels are associated with the development of cardiovascular disease in hemodialysis patients. Intern Med. 2010;49:2057-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Wynne K, Giannitsopoulou K, Small CJ, Patterson M, Frost G, Ghatei MA, Brown EA, Bloom SR, Choi P. Subcutaneous ghrelin enhances acute food intake in malnourished patients who receive maintenance peritoneal dialysis: a randomized, placebo-controlled trial. J Am Soc Nephrol. 2005;16:2111-2118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 141] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Ashby DR, Ford HE, Wynne KJ, Wren AM, Murphy KG, Busbridge M, Brown EA, Taube DH, Ghatei MA, Tam FW. Sustained appetite improvement in malnourished dialysis patients by daily ghrelin treatment. Kidney Int. 2009;76:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Osawa H, Nakazato M, Date Y, Kita H, Ohnishi H, Ueno H, Shiiya T, Satoh K, Ishino Y, Sugano K. Impaired production of gastric ghrelin in chronic gastritis associated with Helicobacter pylori. J Clin Endocrinol Metab. 2005;90:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Sakao Y, Sugimoto M, Ichikawa H, Sahara S, Tsuji T, Ohashi N, Kato A, Fujigaki Y, Sugimoto K, Furuta T. Severity of Gastric Mucosal Atrophy Is the Major Determinant of Plasma Ghrelin Level in Hemodialysis Patients. Am J Nephrol. 2016;44:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Calabrese C, Di Febo G, Brandi G, Morselli-Labate AM, Areni A, Scialpi C, Biasco G, Miglioli M. Correlation between endoscopic features of gastric antrum, histology and Helicobacter pylori infection in adults. Ital J Gastroenterol Hepatol. 1999;31:359-365. [PubMed] |
| 21. | Redéen S, Petersson F, Jönsson KA, Borch K. Relationship of gastroscopic features to histological findings in gastritis and Helicobacter pylori infection in a general population sample. Endoscopy. 2003;35:946-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Kimura K, Takemoto T. An Endoscopic Recognition of the Atrophic Border and its Significance in Chronic Gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (3)] |
| 23. | Miki K, Ichinose M, Ishikawa KB, Yahagi N, Matsushima M, Kakei N, Tsukada S, Kido M, Ishihama S, Shimizu Y. Clinical application of serum pepsinogen I and II levels for mass screening to detect gastric cancer. Jpn J Cancer Res. 1993;84:1086-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Yoshida T, Kato J, Inoue I, Yoshimura N, Deguchi H, Mukoubayashi C, Oka M, Watanabe M, Enomoto S, Niwa T. Cancer development based on chronic active gastritis and resulting gastric atrophy as assessed by serum levels of pepsinogen and Helicobacter pylori antibody titer. Int J Cancer. 2014;134:1445-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 25. | Zhang X, Xue L, Xing L, Wang J, Cui J, Mi J, Xing X, Wang J, Du Z, Misumi J. Low serum pepsinogen I and pepsinogen I/II ratio and Helicobacter pylori infection are associated with increased risk of gastric cancer: 14-year follow up result in a rural Chinese community. Int J Cancer. 2012;130:1614-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Watabe H, Mitsushima T, Yamaji Y, Okamoto M, Wada R, Kokubo T, Doi H, Yoshida H, Kawabe T, Omata M. Predicting the development of gastric cancer from combining Helicobacter pylori antibodies and serum pepsinogen status: a prospective endoscopic cohort study. Gut. 2005;54:764-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 27. | Tamura H, Tokushima H, Murakawa M, Matsumura O, Itoyama S, Mitarai T, Isoda K. Influences of Helicobacter pylori on serum pepsinogen concentrations in dialysis patients. Nephrol Dial Transplant. 1999;14:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Suzuki H, Masaoka T, Hosoda H, Ota T, Minegishi Y, Nomura S, Kangawa K, Ishii H. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut. 2004;53:187-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Isomoto H, Ueno H, Saenko VA, Mondal MS, Nishi Y, Kawano N, Ohnita K, Mizuta Y, Ohtsuru A, Yamashita S. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol. 2005;100:1711-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Chuang CH, Sheu BS, Yang HB, Lee SC, Kao AW, Cheng HC, Chang WL, Yao WJ. Gender difference of circulating ghrelin and leptin concentrations in chronic Helicobacter pylori infection. Helicobacter. 2009;14:54-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Osawa H. Ghrelin and Helicobacter pylori infection. World J Gastroenterol. 2008;14:6327-6333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 32. | Salles N, Ménard A, Georges A, Salzmann M, de Ledinghen V, de Mascarel A, Emeriau JP, Lamouliatte H, Mégraud F. Effects of Helicobacter pylori infection on gut appetite peptide (leptin, ghrelin) expression in elderly inpatients. J Gerontol A Biol Sci Med Sci. 2006;61:1144-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Jang EJ, Park SW, Park JS, Park SJ, Hahm KB, Paik SY, Sin MK, Lee ES, Oh SW, Park CY. The influence of the eradication of Helicobacter pylori on gastric ghrelin, appetite, and body mass index in patients with peptic ulcer disease. J Gastroenterol Hepatol. 2008;23 Suppl 2:S278-S285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Osawa H, Kita H, Ohnishi H, Nakazato M, Date Y, Bowlus CL, Ishino Y, Watanabe E, Shiiya T, Ueno H. Changes in plasma ghrelin levels, gastric ghrelin production, and body weight after Helicobacter pylori cure. J Gastroenterol. 2006;41:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Yanaoka K, Oka M, Mukoubayashi C, Yoshimura N, Enomoto S, Iguchi M, Magari H, Utsunomiya H, Tamai H, Arii K. Cancer high-risk subjects identified by serum pepsinogen tests: outcomes after 10-year follow-up in asymptomatic middle-aged males. Cancer Epidemiol Biomarkers Prev. 2008;17:838-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 36. | Watanabe M, Kato J, Inoue I, Yoshimura N, Yoshida T, Mukoubayashi C, Deguchi H, Enomoto S, Ueda K, Maekita T. Development of gastric cancer in nonatrophic stomach with highly active inflammation identified by serum levels of pepsinogen and Helicobacter pylori antibody together with endoscopic rugal hyperplastic gastritis. Int J Cancer. 2012;131:2632-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 37. | Masuyama H, Yoshitake N, Sasai T, Nakamura T, Masuyama A, Zuiki T, Kurashina K, Mieda M, Sunada K, Yamamoto H. Relationship between the degree of endoscopic atrophy of the gastric mucosa and carcinogenic risk. Digestion. 2015;91:30-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 38. | ten Kate RW, Pals G, Pronk JC, Bank RA, Eriksson AW, Donker AJ, Meuwissen SG. Renal handling of pepsinogens A and C in man. Clin Sci (Lond). 1988;75:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Aydemir S, Borazan A, Acikgoz S, Ustundag Y, Yilmaz A. The effects of continuous ambulatory peritoneal dialysis and hemodialysis on serum pepsinogen concentrations in patients with chronic renal failure. Tohoku J Exp Med. 2005;205:263-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Araki H, Miyazaki R, Matsuda T, Gejyo F, Koni I. Significance of serum pepsinogens and their relationship to Helicobacter pylori infection and histological gastritis in dialysis patients. Nephrol Dial Transplant. 1999;14:2669-2675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Huang SC, Miki K, Hirano K, Hayashi Y, Furihata C, Shimizu A, Ichinose M, Oka H, Matsushima T, Takahashi K. Enzyme-linked immunosorbent assay of serum pepsinogen I. Clin Chim Acta. 1987;162:85-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Pals G, Räsänen V, Meuwissen SG, Frants RR, Kostense PJ, Eriksson AW. Enzyme-linked immunosorbent assay and radioimmunoassay of serum pepsinogen A. Scand J Clin Lab Invest. 1987;47:29-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Kim N, Jung HC. The role of serum pepsinogen in the detection of gastric cancer. Gut Liver. 2010;4:307-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |