Published online Dec 21, 2016. doi: 10.3748/wjg.v22.i47.10406
Peer-review started: August 28, 2016
First decision: September 28, 2016
Revised: October 15, 2016
Accepted: November 16, 2016
Article in press: November 16, 2016
Published online: December 21, 2016
Processing time: 114 Days and 9.2 Hours
To evaluate the value of pre-treatment 18F-FDG PET/CT in patients with HCC following liver radioembolization.
We identified 34 patients with HCC who underwent an FDG PET/CT scan prior to hepatic radioembolization at our institution between 2009 and 2013. Patients were seen in clinic one month after radioembolization and then at 2-3 mo intervals. We assessed the influence of FDG tumor uptake on outcomes including local liver control (LLC), distant liver control (DLC), time to distant metastases (DM), progression free survival (PFS) and overall survival (OS).
The majority of patients were males (n = 25, 74%), and had Child Pugh Class A (n = 31, 91%), with a median age of 68 years (46-84 years). FDG-avid disease was found in 19 (56%) patients with SUVmax ranging from 3 to 20. Female patients were more likely to have an FDG-avid HCC (P = 0.02). Median follow up of patients following radioembolization was 12 months (1.2-62.8 mo). FDG-avid disease was associated with a decreased 1 year LLC, DLC, DM and PFS (P < 0.05). Using multivariate analysis, FDG avidity predicted for LLC, DLC, and PFS (all P < 0.05).
In this retrospective study, pre-treatment HCC FDG-avidity was found to be associated with worse LLC, DLC, and PFS following radioembolization. Larger studies are needed to validate our initial findings to assess the role of F-18-FDG PET/CT scans as biomarker for patients with HCC following radioembolization.
Core tip: Positron emission tomography (PET)/computed tomography is not currently incorporated in the workup for hepatocellular carcinoma. We reviewed PET scans and analyzed outcomes for patient with hepatocellular carcinoma who had been treated with radioembolization and we showed that patients with FDG avid disease had worse control of the disease inside the liver.
- Citation: Abuodeh Y, Naghavi AO, Ahmed KA, Venkat PS, Kim Y, Kis B, Choi J, Biebel B, Sweeney J, Anaya DA, Kim R, Malafa M, Frakes JM, Hoffe SE, El-Haddad G. Prognostic value of pre-treatment F-18-FDG PET-CT in patients with hepatocellular carcinoma undergoing radioembolization. World J Gastroenterol 2016; 22(47): 10406-10414
- URL: https://www.wjgnet.com/1007-9327/full/v22/i47/10406.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i47.10406
Hepatocellular carcinoma (HCC) ranks fifth in cancer incidence and third in cancer related deaths worldwide[1]. The majority of patients diagnosed with HCC are not candidates for surgical resection or liver transplant and require different liver directed therapies for disease management[2,3]. One of these recognized treatments for HCC is hepatic radioembolization with Yttrium-90 (Y-90) microspheres, which demonstrated good results in controlling liver disease with a relatively safe toxicity profile[4].
Fluorodeoxyglucose positron emission tomography (FDG-PET) has proven to provide prognostic information in multiple solid tumors[5-8] but is not routinely used in the work up for HCC due to a low sensitivity (50%-70%)[9-11]. Multiple studies have shown a correlation between standardized uptake value (SUV) of HCC on FDG-PET scans and outcomes following different systemic and locoregional treatments[12-22]. In this study, we assessed the prognostic value of pre-treatment FDG-PET/CT scans in HCC patients undergoing liver radioembolization with Y-90 microspheres.
Institutional review board (IRB) approval was obtained to retrospectively review charts for patients with HCC treated with Y-90 glass microsphere radioembolization at our institution between August 2009 and October 2013. At our institution, upon diagnosis of HCC, patients are evaluated by a multidisciplinary hepatology team with referral to radiation oncology and interventional radiology if the decision was made to administer Y-90 glass microspheres for disease management. From a well-maintained database for all patients treated with radioembolization for HCC we were able to retrospectively identify patients who had PET/CT for various reasons prior to diagnosis and treatment.
All PET/CT images were reviewed at our institution by one of three nuclear radiologists with at least 10-year experience. FDG avidity was defined as maximal standardized uptake value (SUVmax) ≥ 3 of the liver lesion and/or higher than background activity in the surrounding normal liver tissue.
As part of this study, various reasons to obtain PET/CT were reviewed and collected. All other scans were also reviewed by our radiologist to characterize disease. Barcelona-Clinic Liver Cancer (BCLC) staging and classification system[23] incorporating Okuda[24] staging system was used to classify patients. Factors in staging system were also recorded separately to be included in the analysis including: performance status, Child-Pugh class, liver function, portal invasion, presence of extrahepatic disease, and tumor burden in the liver.
Pre-treatment evaluation included: clinical assessment, laboratory work up with a comprehensive metabolic panel to evaluate hepatic and renal function, alpha fetoprotein (AFP) level, and multiphasic CT scan or MRI scan of the liver. HCC diagnosis was made clinically and radiographically with confirmatory biopsy for further confirmation when felt necessary.
Prior to radioembolization, an angiographic evaluation of hepatic vasculature was performed followed by hepatic injection of Tc99m macroaggregated albumin (MAA) to determine the lung shunt fraction. The volume of liver to be treated was measured based on cross-sectional imaging. This was then used to calculate the radiation activity based on the formula for Y90 glass microspheres {A(Gbq) = [D(Gy) ×M(kg)]/50}, A is the activity, D the nominal target dose, and M the liver mass for the planned target volume (PTV) (i.e., segment, lobe, or whole liver) being treated)[25,26]. All treatments were performed in an outpatient setting. Four patients were treated with two radioembolization treatments to separate lobes.
All patients were seen one month after treatment in the interventional radiology and radiation oncology clinic with clinical examination, complete blood count, complete metabolic panel, AFP level and multiphasic cross-sectional imaging (CT scan or MRI liver protocol) to assess response to treatment and progression. Subsequent follow-ups were done at 2-3 mo intervals. Time-to-event outcomes were calculated from the time of radioembolization. The time to disease progression in the treated liver lobe/segment was defined as local liver control (LLC), and the time to progression in the liver outside the treated lesion was defined as distant liver control (DLC). Progression free survival (PFS) was calculated from the time of radioembolization to the time of intrahepatic, extrahepatic progression, death, or last follow-up. The rate of distant metastases (DM) was calculated after excluding patients with extrahepatic disease at time of radioembolization from analysis for DM. Overall survival (OS) was calculated from the time of radioembolization to the time of death.
The primary endpoint for this study was LLC. Secondary endpoints included DLC, DM, PFS, and OS. Outcomes were calculated from the date of radioembolization with patients censored at last follow-up or death.
Patient, tumor and treatment characteristics were compared between FDG-avid and non-FDG-avid HCC lesions via Pearson χ2 or Fisher’s Exact Test for categorical variables and Mann-U Whitney for continuous variables on univariate analysis (UVA) when appropriate.
Factors predictive of time-to-event outcome were estimated on UVA with Kaplan-Meier (comparison via log-rank test) and Cox proportional hazard analysis for categorical and continuous variables, respectively. Significant variables or close but not significant variables might interact and affect outcomes. Therefore, variables with marginally significant effect (P < 0.1) on univariate analysis, were accounted for in our Cox-regression multivariate analysis (MVA). LLC was based on distinct tumor volumes treated, whereas DLC, PFS, DM, and OS were based on the patients treated. Patients with extrahepatic disease on presentation were excluded from DM analysis. Statistical analysis was performed using Statistical Product and Service Solutions version 22.0 (SPSS®, Chicago, IL). All statistical analysis was reviewed by a biomedical statistician.
Thirty-four patients with HCC undergoing radioembolization procedures with pre-treatment FDG-PET/CT scans were identified. The reasons for obtaining PET/CT scan were as follows: initial work up of liver mass in 23 patients (67%), history of a prior non-HCC cancer in 6 patients (18%), to rule out metastatic disease in 3 patients (9%), or due to atypical non-diagnostic findings on prior imaging in 2 (6%) patients. Eighteen patients (53%) had a PET/CT scan performed at an outside institution but were reviewed by our nuclear radiologists.
In those 34 patients, radioembolization was delivered to a total of 38 liver lobes and segments. Median age of patients was 68 years (range 46-84 years), with the majority being male (74%, n = 25), and fourteen (41%) patients had no known previous cirrhotic liver on presentation. There were 20 patients (59%) with cirrhosis that was secondary to hepatitis C, hepatitis B, alcohol, and non-alcoholic steatohepatitis in 10 patients (50%), 2 patients (10%), 4 patients (20%) and 4 patients (20%), respectively. Extra-hepatic disease was present in 3 (9%) patients and the majority of patients (n = 32, 94%) were not candidates for liver resection or transplant. Two patients (6%) were referred for downstaging using radioembolization prior to surgical resection. Table 1 details patient, tumor, and treatment characteristics.
| n (%) | ||
| FDG avidity | Yes | 19 (56) |
| No | 15 (44) | |
| ECOG-PS1 | 0 | 17 (50) |
| 1 | 14 (41) | |
| ≥ 2 | 3 (9) | |
| Gender | Female | 9 (27) |
| Male | 25 (74) | |
| Grade | G1 | 18 (53) |
| G2 | 6 (18) | |
| G3 | 1 (3) | |
| Unknown | 9 (27) | |
| Presence of cirrhosis | 20 (59) | |
| Causes of cirrhosis (n = 20) | Viral hepatitis | 12 (60) |
| Hepatitis B | 2 (10) | |
| Hepatitis C | 10 (50) | |
| Alcohol | 4 (20) | |
| NASH2 | 4 (20) | |
| Prior treatment | 15 (44) | |
| Child pugh class | A | 31 (91) |
| B | 3 (9) | |
| BCLC stage3 | A | 4 (12) |
| B | 10 (29) | |
| C | 19 (56) | |
| D | 1 (3) | |
| Tumor burden4 | ≤ 50% | 29 (85) |
| > 50% | 5 (15) | |
| Portal vein thrombosis | 5 (15) | |
| Extrahepatic disease | 3 (9) | |
| Median (Range) | ||
| Age | 68 (46-84) | |
| PET SUVmax | 4 (2-20) | |
| Initial AFP | 14 (1-186000) | |
| Volume treated (mL) | 1146 (191-2340) | |
| Dose delivered (Gy) | 126.6 (110.5-478.6) | |
| Follow up (mo) | 12 (1-63) |
Percutaneous image-guided liver lesion biopsy was performed to confirm diagnosis in 31 patients. Histopathology grade was determined in 25 patients, 18 (53%) of which had a well-differentiated HCC, 6 moderately differentiated (18%) and one (3%) poorly differentiated HCC. Nine patients who did not undergo a biopsy or who had lesions of unknown grade were grouped together (n= 9; 26%). There were 31 patients (91%) with Child-Pugh Class (CP) A and 3 patients (9%) with CP B. Portal vein thrombosis was present in 5 patients (15%). Median AFP prior to treatment was 14 ng/mL (1-186000). Y-90 glass microspheres were injected intra-arterially to treat a median volume of 1146 cc (191-2340) of the liver with a median delivered dose of 126.6 Gy (110.5-478.6).
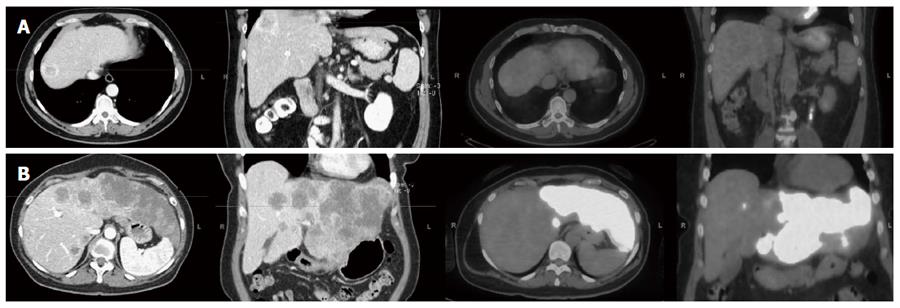
Nineteen patients (56%) were considered as having an FDG-avid HCC. Figure 1 shows two patients with different FDG avidity. Female gender was the only factor found to be associated with HCC FDG-avid disease (P = 0.047), Table 2.
| Non-avid patients | PET-avid patients | UVA | ||
| n (%) | n (%) | P value | ||
| ECOG-PS1 | 0 | 9 (60) | 8 (40) | 0.58 |
| 1 | 5 (33) | 9 (47) | ||
| ≥ 2 | 1 (7) | 2 (11) | ||
| Gender | Female | 1 (7) | 8 (42) | 0.0474 |
| Male | 14 (93) | 11 (58) | ||
| Pathology-differentiation | Well | 10 (67) | 8 (42) | 0.311 |
| Moderate | 1 (7) | 5 (26) | ||
| Poor | 0 (0) | 1 (5) | ||
| Unknown | 4 (27) | 5 (26) | ||
| BCLC stage2 | A | 3 (20) | 1 (5) | 0.123 |
| B | 6 (40) | 4 (21) | ||
| C+ | 6 (40) | 14 (74) | ||
| Tumor burden | ≤ 50% | 14 (93) | 15 (79) | 0.3554 |
| > 50% | 1 (7) | 4 (21) | ||
| Portal vein thrombosis | No | 14 (93) | 15 (79) | 0.24 |
| Yes | 5 (33) | 10 (53) | ||
| Extrahepatic disease | No | 14 (93) | 17 (90) | 1.004 |
| Yes | 1 (7) | 2 (11) | ||
| Prior treatment | No | 9 (60) | 10 (53) | 0.67 |
| Yes | 6 (40) | 9 (47) | ||
| Child-Pugh-class | A | 14 (93) | 17 (90) | 1.004 |
| B | 1 (7) | 2 (11) | ||
| Viral hepatitis | No | 8 (53) | 14 (74) | 0.22 |
| Yes | 7 (47) | 5 (26) | ||
| Cirrhosis | No | 14 (93) | 16 (84) | 0.41 |
| Yes | 10 (67) | 10 (53) | ||
| Median (Range) | Median (Range) | |||
| Age | 59 (46-84) | 71 (57-84) | 0.10 | |
| PET SUV3 | 2(2-2) | 6(3-20) | - | |
| Initial AFP | 12 (1-478) | 16 (2-186000) | 0.23 | |
| Volume treated (mL) | 1260 (400-2044) | 1091 (191-2340) | 0.39 | |
| Dose delivered (Gy) | 125.9 (111.1-478.6) | 128.5 (110.5-467.5) | 0.66 |
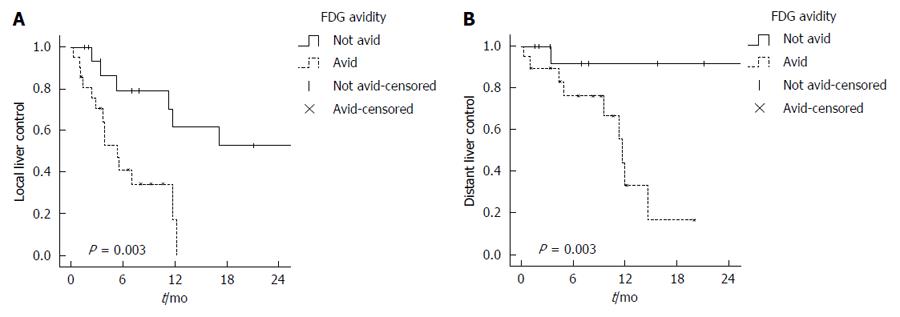
The median follow up for all patients was 12 mo (range 1-63 mo). The median LLC for all liver volumes treated (n = 38) was 11.3 mo. FDG-avid disease was associated with shorter LLC. In comparison to non FDG-avid disease, the 1-year rate of LLC was 17.2% vs 61.4% (P = 0.003) with a median LLC of 5 mo vs 17 mo, respectively (Figure 2A). On UVA, FDG-avid disease, tumor burden > 50% and extrahepatic disease were associated with worse LLC and there was a trend for worse LLC for female gender and poorly differentiated HCC. However, on MVA, only FDG-avidity [P = 0.002, HR = 6.3 (2-20)] and presence of extrahepatic disease [P < 0.001, HR = 38.9 (6.6-229.2)] were associated with worse LLC Table 3.
| Variables1 | LLC (n = 38) | DLC (n = 34) | DM (n = 31) | PFS (n = 34) | OS (n = 34) | ||||||||||
| UVA (P) | MVA (P) | HR (95%CI) | UVA (P) | MVA (P) | HR (95%CI) | UVA (P) | MVA (P) | HR (95%CI) | UVA (P) | MVA (P) | HR (95%CI) | UVA (P) | MVA (P) | HR (95%CI) | |
| FDG Avidity (avid vs non-avid) | 0.003 | 0.002 | 6.3 (2.0-20.4) | 0.003 | 0.015 | 57.7 (2.2-1496.9) | < 0.001 | NS | 0.015 | 0.008 | 4.04 (1.4-11.3) | 0.893 | - | ||
| ECOG (0 vs 1 vs≥ 2) | 0.120 | - | 0.038 | NS | 0.990 | - | 0.049 | NS | 0.002 | NS | |||||
| Gender (female vs male) | 0.100 | NS | 0.398 | - | 0.596 | - | 0.100 | NS | 0.122 | - | |||||
| Pathology (grade 1 vs 2 vs 3 vs unknown) | 0.059 | NS | 0.034 | NS | 0.048 | NS | 0.016 | NS | 0.688 | - | |||||
| Extrahepatic disease | < 0.001 | < 0.001 | 38.9 (6.6-229.2) | < 0.001 | NS | - | - | < 0.001 | < 0.001 | 47 (7.7-286) | < 0.001 | NS | |||
| Tumor Burden ( ≤ 50% vs > 50%) | < 0.001 | NS | 0.009 | NS | 0.990 | - | 0.012 | NS | 0.004 | NS | |||||
| Cirrhosis | 0.477 | - | 0.588 | - | 0.732 | - | 0.593 | - | 0.065 | NS | |||||
| Age (yr) | 0.405 | - | 0.405 | - | 0.238 | - | 0.232 | - | 0.100 | NS | |||||
The median disease control in the untreated liver or DLC was 26.3 mo. FDG-avid disease was associated with a faster progression inside the liver with 1 year DLC rate of 44.4%, as compared to 91.7% for non FDG-avid disease (P = 0.003) (Figure 2B). On both UVA and MVA, FDG-avidity was a predictor for DLC [P = 0.03, HR = 57.7 (2.2-1496.9)], Table 3. Significant variables on UVA include: performance status, poorly differentiated pathology, extrahepatic disease and tumor burden > 50%, but all were not statistically significant on MVA, Table 3.
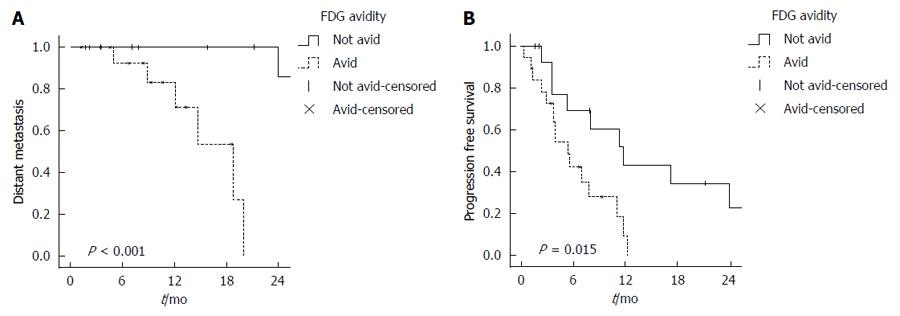
Initially, there were 3 patients with extrahepatic disease at the time of treatment. On UVA, the presence of this correlated with worse outcome in LLC, DLC, and OS (P < 0.05), but it was not included in statistical analysis for DM. Upon follow up, there were 4 patients who progressed in extrahepatic sites after radioembolization. After excluding patients with extrahepatic disease at the time of treatment from the analysis, the median for freedom from DM was not reached and freedom from DM at 1 and 2-year were 96% and 54%, respectively. On UVA, FDG-avid disease (Figure 3A) and poorly differentiated tumors were associated with worse DM, but none were significant on MVA (Table 3).
FDG-avid disease was associated with shorter PFS. The 1 year PFS was higher for non FDG-avid disease (43.3%) when compared to FDG-avid disease (9.4%) on univariate analysis (log-rank test, P = 0.015) (Figure 3B). On multivariate analysis, FDG-avid disease was a predictor for worse PFS [P = 0.008, HR = 4 (1.4-11.3)] (Table 3).
For all patients, the 1-year OS rate was 77%. There was no statistically significant difference in survival at one year between FDG-avid and non FDG-avid disease (77% vs 76.2%, P = 0.89). On UVA, other factors resulting in shorter OS were low performance status, presence of extrahepatic disease and tumor burden > 50% (all P < 0.05), with trending values for older age, presence of cirrhosis, and higher BCLC stage. The MVA analysis included component of staging system that were significant on UVA (Table 3).
In this retrospective analysis of 34 HCC patients undergoing 38 liver radioembolizations, we found that pre-treatment tumor FDG-avidity correlated with worse LLC, DLC, and PFS outcomes. However, these differences in LLC and DLC with FDG-avid disease did not translate into a difference in OS in our study.
FDG retention in malignant cells is dependent on intracellular glucose-6-phosphatase enzymatic activity[27]. HCCs contain varying levels of this enzyme[28,29], and therefore the reported sensitivity of FDG PET/CT scans in detecting hepatocellular carcinoma ranges between 50% and 70%[9-11]. The low sensitivity and variation in FDG uptake are the main reasons for not routinely including FDG PET/CT imaging in the initial HCC work up. Despite their high accuracy in diagnosing HCC, CT and MRI cannot distinguish well differentiated from poorly differentiated HCC[30]. Since a large number of HCCs do not get biopsied, FDG PET may play a non-invasive role in predicting tumor biology and behavior, as the variability of FDG uptake has been linked to HCC’s microvascular invasion and differentiation[31-33], and proliferative activity[34]. It was not possible to draw any conclusions regarding the relationship between FDG tumor uptake and histopathologic grade in our study since the patient population was skewed with only one patient having a poorly differentiated HCC.
Our analysis found female patients to be more likely to have FDG-avid disease. In a study by Salem et al[4], female patients with HCC treated with radioembolization were found to have shorter survival. The reason for this finding is still unknown, but might be related to the promotion of HCC in post menopausal patients due to the loss of estrogen’s protective effect[35,36]. However, unlike FDG avidity, gender was not associated with worse outcomes in our MVA. Given the small number of patients and retrospective nature of our study, drawing firm conclusions regarding the impact of gender on clinical outcomes following HCC radioembolization is limited.
The role of FDG-PET in the assessment of different treatment modalities for HCC has been described in several reports[16-22]. Pant et al[17] assessed pre-treatment FDG-avidity as a prognostic index in the management of HCC in 100 patients. In their study, FDG-avidity was defined as activity above the liver background, which is similar to what we used in our study. FDG-avid disease was associated with a higher radiological stage, increased risk of distant metastases, invasion into the portal vein, and higher tumor grade[17]. A study by Kornberg et al[20] consisting of 91 HCC patients undergoing transplantation showed tumor-to-background ratio of > 1 on pre-transplant scans was associated with worse recurrence free survival of 81% vs 21% (P = 0.02). Lee et al[21] showed in 59 patients, that ratio of tumor SUVmax to liver SUVmax with cut-off value of 1.15 was the most significant predictor for tumor recurrence at 1 year with rates of 97% vs 57% (P < 0.001). Another study from Korea by Kim et al[22] showed that FDG uptake-volume products was also an effective predictor for post-transplant recurrence.
Lee et al[19] assessed the value of FDG-PET scans in 29 patients treated with sorafenib. The max SUV was a statistically significant prognostic factor for OS and PFS. The study from Lee and colleagues used a SUVmax cut off value of 5. The group of patients with SUVmax lower than 5 had a significantly longer PFS and OS [19].
The role of FDG-PET scans for patients with hepatocellular carcinoma has also been assessed in patients following trans-arterial chemotherapy embolization (TACE)[16,18]. Cho et al[16] found after a mean follow-up of 8 months (range, 1-59 mo), among the 47 patients who underwent TACE, a higher ratio of tumor SUVmax to mean mediastinal SUV of ≥ 3.1 was associated with higher rates of recurrence and lower survival (94% vs 64%, P = 0.016). Kim et al[18] showed that SUVmax to mean liver SUV ratio of 1.83 was a predictor for disease progression and worse OS. In these 2 studies, different SUV ratios and cutoff values were used. The majority of PET scans evaluated in our study were performed in an outside facility, and due to the lack of standardized PET scan protocols, we opted to use a simpler way to classify FDG-avidity by comparing tumor uptake to liver background. Another study by Lee et al[37] evaluated the tumor-to-liver uptake ratio in a total of 214 patients treated with either external radiation or TACE and they found that a ratio more than 2 correlated on MVA with worse PFS and OS.
Our study is limited by its retrospective nature with a relatively small number of patients, short follow-up, and heterogeneous background of liver disease. It is difficult to find HCC patients that underwent an FDG-PET scan since this imaging modality is not routinely ordered due to previous reports of poor sensitivity[9-11]. More than half of FDG-PET/CT scans were performed at an outside facility and thus there was no standardized PET scan protocol used across studies. Therefore, we were not able to directly compare tumor SUV from different institutions. However, absolute SUV can vary significantly even within the same institution[38] and there are multiple reports on the equivalence of using qualitative SUV in relation to background in comparison to absolute SUV[39]. The selection of SUVmax of 3 might introduce bias in patient categorization, but based on previously mentioned reports, the selected value will ensure selecting patients with true active disease above normal liver background, which ranges between 1.5-2[40]. This provides a minimum of tumor to Liver SUV ratio of 1.5, which correlated with outcomes in several publications[41]. Regarding a potential selection bias, there were only 2 patients in our study with atypical features for HCC on diagnostic imaging, and the majority of PET scans were performed to exclude non-HCC etiologies prior to proper diagnostic imaging. Furthermore, since most of the HCC lesions were well differentiated or were not histopathologically graded after percutaneous biopsy, this lack of histopathology information and uneven distribution, limited our ability to correlate pathological grade with clinical endpoints. As expected, the majority of patients treated with this focal therapy were BCLC stage B and C, with smaller number in stage A and only one patient with stage D (Table 1). The stage incorporation and analysis was weakly associated with outcome, so the multivariate analysis included significant components of this staging system that had stronger associations with outcome on UVA (i.e., ECOG Performance status, extrahepatic disease, and tumor burden) (Table 3). Despite these limitations, our results were in line with other studies that found a similar prognostic benefit to pre-treatment FDG-PET/CT scans in patients getting treated for HCC[16-22].
In conclusion, previous studies have found a prognostic benefit to pre-treatment FDG-PET/CT scans following TACE, surgery, and sorafenib treatment of HCC. Similarly, in our study, we found high FDG-avidity to correlate with worse clinical outcomes of LLC, DLC, and PFS following radioembolization of HCC. As opposed to the initial reports on FDG-PET that showed limited utility in the diagnosis of HCC, our study supports the growing body of evidence for the prognostic utility of pre-treatment FDG-PET/CT scan in the management of HCC. Pre-treatment FDG-PET scan may compliment staging multiphasic cross sectional imaging studies, and help determine whether there is a need for more aggressive treatment approaches. Further investigation is needed to validate our initial findings as well as the optimal method to incorporate FDG-PET/CT scans in the initial work up of HCC.
Hepatocellular carcinoma (HCC) is the third leading cause of death from all cancers. The majority of patients with HCC present beyond eligibility criteria for surgical resection or transplant, and require local liver directed therapy. FDG PET/CT scan has a predictive and prognostic value in different types of cancers, but due to low sensitivity it is not incorporated in the workup for patients with HCC.
Different studies have shown a correlation between standardized uptake value of HCC on FDG-PET scans and outcomes following different systemic and locoregional treatments. In this study, the authors assessed the prognostic value of pre-treatment FDG-PET/CT scans in HCC patients undergoing liver radioembolization.
After radioembolization, FDG-avid HCC disease was associated with significant worse disease control in the liver. Hazard ratio for progression free survival was 4 (1.4-11.3) (P = 0.008) for FDG-avid disease as opposed to non-FDG-avid disease.
Further studies and investigations are needed to define the role of PET scans in HCC and to improve sensitivity for FDG or new tracers, so that this biomarker scan is incorporated in the prognostic workup.
The paper includes important information.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Nagamachi S, Vinh-Hung V S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25545] [Article Influence: 1824.6] [Reference Citation Analysis (7)] |
| 2. | Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. 1998;311-322. |
| 3. | Vauthey JN, Dixon E, Abdalla EK, Helton WS, Pawlik TM, Taouli B, Brouquet A, Adams RB. Pretreatment assessment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 150] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. |
| 5. | Sun G, Tian J, Gorospe EC, Johnson GB, Hunt CH, Lutzke LS, Leggett CL, Iyer PG, Wang KK. Utility of baseline positron emission tomography with computed tomography for predicting endoscopic resectability and survival outcomes in patients with early esophageal adenocarcinoma. J Gastroenterol Hepatol. 2013;28:975-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Chung HH, Cheon GJ, Kang KW, Kim JW, Park NH, Song YS. Preoperative PET/CT FDG standardized uptake value of pelvic lymph nodes as a significant prognostic factor in patients with uterine cervical cancer. Eur J Nucl Med Mol Imaging. 2014;41:674-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kidd EA, Siegel BA, Dehdashti F, Grigsby PW. Pelvic lymph node F-18 fluorodeoxyglucose uptake as a prognostic biomarker in newly diagnosed patients with locally advanced cervical cancer. Cancer. 2010;116:1469-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC, Avril N. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 753] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 9. | Teefey SA, Hildeboldt CC, Dehdashti F, Siegel BA, Peters MG, Heiken JP, Brown JJ, McFarland EG, Middleton WD, Balfe DM. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology. 2003;226:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 10. | Jeng LB, Changlai SP, Shen YY, Lin CC, Tsai CH, Kao CH. Limited value of 18F-2-deoxyglucose positron emission tomography to detect hepatocellular carcinoma in hepatitis B virus carriers. Hepatogastroenterology. 2003;50:2154-2156. [PubMed] |
| 11. | Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792-797. [PubMed] |
| 12. | Lee JW, Yun M, Cho A, Han KH, Kim DY, Lee SM, Lee JD. The predictive value of metabolic tumor volume on FDG PET/CT for transarterial chemoembolization and transarterial chemotherapy infusion in hepatocellular carcinoma patients without extrahepatic metastasis. Ann Nucl Med. 2015;29:400-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 13. | Parikh U, Marcus C, Sarangi R, Taghipour M, Subramaniam RM. FDG PET/CT in Pancreatic and Hepatobiliary Carcinomas: Value to Patient Management and Patient Outcomes. PET Clin. 2015;10:327-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Song HJ, Cheng JY, Hu SL, Zhang GY, Fu Y, Zhang YJ. Value of 18F-FDG PET/CT in detecting viable tumour and predicting prognosis of hepatocellular carcinoma after TACE. Clin Radiol. 2015;70:128-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Asman Y, Evenson AR, Even-Sapir E, Shibolet O. [18F]fludeoxyglucose positron emission tomography and computed tomography as a prognostic tool before liver transplantation, resection, and loco-ablative therapies for hepatocellular carcinoma. Liver Transpl. 2015;21:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 16. | Cho E, Jun CH, Kim BS, Son DJ, Choi WS, Choi SK. 18F-FDG PET CT as a prognostic factor in hepatocellular carcinoma. Turk J Gastroenterol. 2015;26:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Pant V, Sen IB, Soin AS. Role of 18F-FDG PET CT as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Kim MJ, Kim YS, Cho YH, Jang HY, Song JY, Lee SH, Jeong SW, Kim SG, Jang JY, Kim HS. Use of (18)F-FDG PET to predict tumor progression and survival in patients with intermediate hepatocellular carcinoma treated by transarterial chemoembolization. Korean J Intern Med. 2015;30:308-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Lee JH, Park JY, Kim DY, Ahn SH, Han KH, Seo HJ, Lee JD, Choi HJ. Prognostic value of 18F-FDG PET for hepatocellular carcinoma patients treated with sorafenib. Liver Int. 2011;31:1144-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Kornberg A, Küpper B, Tannapfel A, Büchler P, Krause B, Witt U, Gottschild D, Friess H. Patients with non-[18 F]fludeoxyglucose-avid advanced hepatocellular carcinoma on clinical staging may achieve long-term recurrence-free survival after liver transplantation. Liver Transpl. 2012;18:53-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, Lee MC, Lee DS. Prediction of tumor recurrence by 18F-FDG PET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Kim YI, Paeng JC, Cheon GJ, Suh KS, Lee DS, Chung JK, Kang KW. Prediction of Posttransplantation Recurrence of Hepatocellular Carcinoma Using Metabolic and Volumetric Indices of 18F-FDG PET/CT. J Nucl Med. 2016;57:1045-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 24. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] |
| 25. | Kennedy A, Nag S, Salem R, Murthy R, McEwan AJ, Nutting C, Benson A, Espat J, Bilbao JI, Sharma RA. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiation Oncol Biol Phy. 2007;68:13-23. |
| 26. | Nordion M. TheraSphere yttrium-90 microspheres package insert. Canada: MDS Nordion, Kanata 2004; . |
| 27. | Hellman RS, Krasnow AZ, Sudakoff GS. Positron Emission Tomography for Staging and Assessment of Tumor Response of Hepatic Malignancies. Seminars Intervent Radiol. 2006;23:21-32. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Torizuka T, Tamaki N, Inokuma T, Magata Y, Sasayama S, Yonekura Y, Tanaka A, Yamaoka Y, Yamamoto K, Konishi J. In vivo assessment of glucose metabolism in hepatocellular carcinoma with FDG-PET. J Nucl Med. 1995;36:1811-1817. [PubMed] |
| 29. | Messa C, Choi Y, Hoh CK, Jacobs EL, Glaspy JA, Rege S, Nitzsche E, Huang SC, Phelps ME, Hawkins RA. Quantification of glucose utilization in liver metastases: parametric imaging of FDG uptake with PET. J Comput Assist Tomogr. 1992;16:684-689. [PubMed] |
| 30. | Asayama Y, Yoshimitsu K, Nishihara Y, Irie H, Aishima S, Taketomi A, Honda H. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. AJR Am J Roentgenol. 2008;190:W28-W34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 31. | Kornberg A, Freesmeyer M, Bärthel E, Jandt K, Katenkamp K, Steenbeck J, Sappler A, Habrecht O, Gottschild D, Settmacher U. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 32. | Seo S, Hatano E, Higashi T, Hara T, Tada M, Tamaki N, Iwaisako K, Ikai I, Uemoto S. Fluorine-18 fluorodeoxyglucose positron emission tomography predicts tumor differentiation, P-glycoprotein expression, and outcome after resection in hepatocellular carcinoma. Clin Cancer Res. 2007;13:427-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 152] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 33. | Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, Fujii H, Shimahara Y. Preoperative positron emission tomography with fluorine-18-fluorodeoxyglucose is predictive of prognosis in patients with hepatocellular carcinoma after resection. World J Surg. 2006;30:1736-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Kitamura K, Hatano E, Higashi T, Narita M, Seo S, Nakamoto Y, Yamanaka K, Nagata H, Taura K, Yasuchika K. Proliferative activity in hepatocellular carcinoma is closely correlated with glucose metabolism but not angiogenesis. J Hepatol. 2011;55:846-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Shi L, Feng Y, Lin H, Ma R, Cai X. Role of estrogen in hepatocellular carcinoma: is inflammation the key? J Transl Med. 2014;12:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 36. | Hong EJ, Levasseur MP, Dufour CR, Perry MC, Giguère V. Loss of estrogen-related receptor α promotes hepatocarcinogenesis development via metabolic and inflammatory disturbances. Proc Natl Acad Sci USA. 2013;110:17975-17980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Lee JW, Oh JK, Chung YA, Na SJ, Hyun SH, Hong IK, Eo JS, Song BI, Kim TS, Kim do Y. Prognostic Significance of 18F-FDG Uptake in Hepatocellular Carcinoma Treated with Transarterial Chemoembolization or Concurrent Chemoradiotherapy: A Multicenter Retrospective Cohort Study. J Nucl Med. 2016;57:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 38. | Kumar V, Nath K, Berman CG, Kim J, Tanvetyanon T, Chiappori AA, Gatenby RA, Gillies RJ, Eikman EA. Variance of SUVs for FDG-PET/CT is greater in clinical practice than under ideal study settings. Clin Nucl Med. 2013;38:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 39. | Lowe VJ, Hoffman JM, DeLong DM, Patz EF, Coleman RE. Semiquantitative and visual analysis of FDG-PET images in pulmonary abnormalities. J Nucl Med. 1994;35:1771-1776. [PubMed] |
| 40. | Turcotte E, Leblanc M, Carpentier A, Bénard F. Optimization of whole-body positron emission tomography imaging by using delayed 2-deoxy-2-[F-18]fluoro-D: -glucose Injection following I.V. Insulin in diabetic patients. Mol Imaging Biol. 2006;8:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 41. | Sun DW, An L, Wei F, Mu L, Shi XJ, Wang CL, Zhao ZW, Li TF, Lv GY. Prognostic significance of parameters from pretreatment (18)F-FDG PET in hepatocellular carcinoma: a meta-analysis. Abdom Radiol (NY). 2016;41:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |