Published online Dec 21, 2016. doi: 10.3748/wjg.v22.i47.10388
Peer-review started: September 21, 2016
First decision: October 20, 2016
Revised: November 1, 2016
Accepted: November 16, 2016
Article in press: November 16, 2016
Published online: December 21, 2016
Processing time: 90 Days and 7.4 Hours
To examine the clinical features and risk factors for adverse outcomes in chronic hepatitis B (CHB) superimposed with hepatitis E virus (HEV).
This retrospective cohort study included 228 patients with acute HEV infection (showing clinical acute hepatitis symptomology and positivity for anti-HEV immunoglobulin M) with underlying CHB (confirmed by positivity for hepatitis B surface antigen and/or hepatitis B virus (HBV) DNA over 6 mo) who had been admitted to the Shanghai Public Health Clinical Center, which represents the regional tertiary hospital for infectious diseases in Shanghai city, China. Data for adverse outcomes were collected, and included severe liver diseases (defined as liver failure and/or acute liver decompensation) and liver-related mortality. Logistic regression modeling was performed to determine the risk factors for adverse outcomes.
The symptoms caused by superimposed acute hepatitis E (AHE) were much more severe in cirrhotic patients (n = 94) than in non-cirrhotic patients (n = 134), as evidenced by significantly higher liver complications (77.7% vs 28.4%, P < 0.001) and mortality rate (21.3% vs 7.5%, P = 0.002). Most of the cirrhotic patients (n = 85, 90.4%) had no prior decompensation. Among the non-cirrhotic patients, superimposed AHE caused progressively more severe diseases that corresponded with the CHB disease stages, from immune tolerant to immune reactivation phases. Few risk factors were identified in the cirrhotic patients, but risk factors for non-cirrhotic patients were found to be intermediate HBV DNA levels (OR: 5.1, P = 0.012), alcohol consumption (OR: 6.4, P = 0.020), and underlying diabetes (OR: 7.5, P = 0.003) and kidney diseases (OR: 12.7, P = 0.005). Only 28.7% of the cirrhotic patients and 9.0% of the non-cirrhotic patients had received anti-HBV therapy previously and, in all cases, the efficacy had been suboptimal.
CHB-related cirrhosis and intermediate HBV DNA level were associated with severe disease in superinfected patients, and successful antiviral treatment might counter this outcome.
Core tip: To determine whether status of chronic hepatitis B (CHB) affects clinical outcomes of hepatitis E virus (HEV) super-infections, we investigated immunological phases, hepatitis B virus (HBV) serum markers, HBV viral load and anti-viral treatments among 228 patients with HBV-HEV co-infection. Well-compensated patients were majorly affected by HEV super-infections, and hepatitis B e antigen-negative CHB patients had the worst clinical outcomes among non-cirrhotics. Lack of proper anti-HBV treatment may contribute to the worse outcomes. These data may help to facilitate development of vaccination programs that precisely target populations at risk of poor outcome from HBV-HEV co-infections.
- Citation: Chen C, Zhang SY, Zhang DD, Li XY, Zhang YL, Li WX, Yan JJ, Wang M, Xun JN, Lu C, Ling Y, Huang YX, Chen L. Clinical features of acute hepatitis E super-infections on chronic hepatitis B. World J Gastroenterol 2016; 22(47): 10388-10397
- URL: https://www.wjgnet.com/1007-9327/full/v22/i47/10388.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i47.10388
Viral hepatitis, including hepatitis A, B, C, D and E, causing acute or chronic liver diseases, represents severe threats to public health worldwide. In China, where the hepatitis B virus (HBV) is endemic, the rate of hepatitis B surface antigen (HBsAg) positivity among the general population remains around 7.2%, with 93 million people diagnosed as chronic HBV carriers despite the national HBV vaccination program. Chronic hepatitis B (CHB) and its related diseases lead to approximately 0.3 million deaths annually[1]. Infection with the hepatitis E virus (HEV) was previously thought limited to developing countries with poor sanitation, but it is gradually emerging as a prevalent disease in developed countries as well. HEV mainly causes sporadic symptomatic infections that are transmitted zoonotically and associated with several risk factors, including old age, male sex, socio-economic status (such as rural area dwelling) and occupational exposure[2-7]. On the other hand, the meat industry in China is in the stage of rapid development, rapidly replacing the traditional family farms, which can lead to increased viral spreading between domestic animals and contaminated meats will results in more human infections. Therefore, in areas where CHB is endemic, such as China, super-infections of HEV on the CHB background are not uncommon. In fact, it was reported that HBV-HEV co-infections represented 20%-40% of all symptomatic acute hepatitis E (AHE) infections[8-11].
It is well known that AHE superimposed on chronic liver diseases (CLDs), especially cirrhosis, frequently leads to severe disease, as exemplified by acute-on-chronic liver failure (ACLF)[8,12,13]. However, CLDs vary largely in their etiology, disease stages and treatments, and it is still unclear whether these factors affect the adverse clinical outcomes that have been observed with HEV super-infections. Research into such issues will provide valuable data for increasing the overall understanding of the devastating diseases caused by HEV super-infections and will facilitate the development of future vaccination programs to precisely targeting the risk populations at highest risk of having the worst outcome from HBV-HEV co-infections.
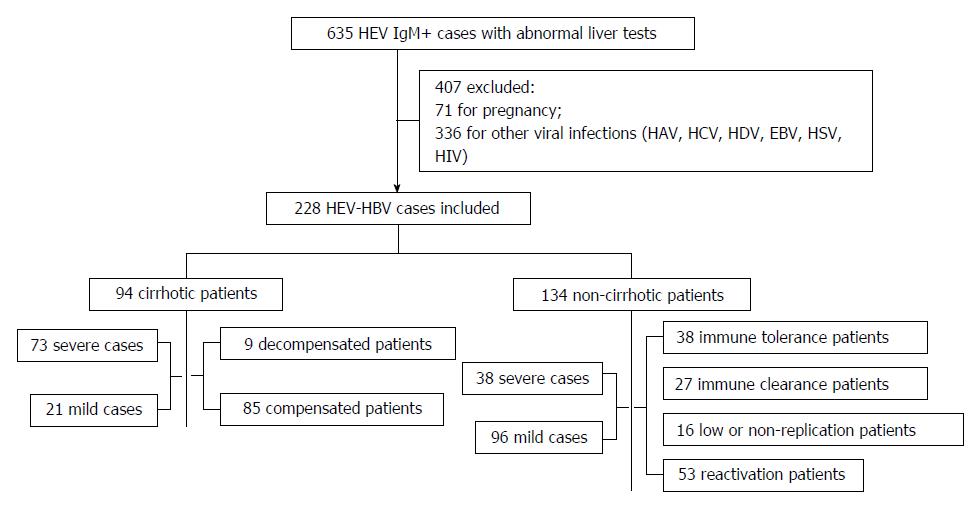
From September 2009 to September 2014, 635 acute HEV-infected patients (showing clinical acute hepatitis symptomology and anti-HEV immunoglobulin (Ig)M positivity) were admitted to the Shanghai Public Health Clinical Center, which represents the regional tertiary hospital for infectious diseases in Shanghai city, China. Four hundred and seven of those patients were excluded from this study due to pregnancy, other viral hepatitis etiology (e.g., hepatitis A, C or D virus) or infection with Epstein-Barr virus, herpes simplex virus, or human immunodeficiency virus; the remaining 228 HEV-HBV co-infection patients were included in this retrospective study (Figure 1).
Anti-HEV IgM level was determined in serum from patients by use of enzyme-linked immunosorbent assay (ELISA) (MP Biomedicals Asia Pacific, Singapore). Liver function was assessed by measuring standard serum markers using a fully-automated biochemical analyzer (7600 Series; Hitachi, Tokyo, Japan). HBV DNA levels were quantified by real-time PCR (ABI 7500; Applied Biosystems Inc., Foster City, CA, United States), with lower detection limit of 500 copies/mL. HBV serological markers were detected by ELISA (ARCHITECT i2000 SR; Abbott, Wiesbaden, Germany). Routine blood panel was detected with an automated hematology analyzer (XT-2000i; Sysmex, Kobe, Japan). Prothrombin time (PT) was measured by an automatic coagulometer (STA-R; Diagnostica Stago, Asnieres-sur-Seine, France). The PT-international normalized ratio (INR) was calculated.
CHB was confirmed by HBsAg and/or HBV DNA positivity over the previous 6 mo[14]. Cases of AHE were identified by two consecutive positive tests for anti-HEV IgM along with record of clinical acute hepatitis symptomology in the hospital’s clinical database. Liver failure and liver decompensation, or both, were considered as the severe liver diseases in these cases. Diagnosis of liver failure was based upon INR ≥ 1.5 or prothrombin activity ≤ 40% and rapidly progressing jaundice, with total bilirubin at 10 times the upper limit of normal, or a daily increase of ≥ 17.1 μmol/L[15]. Liver decompensation was defined by acute development of one or more major complications of the liver, including ascites, hepatic encephalopathy (HE), gastrointestinal hemorrhage or bacterial infections[16]. Patient mortality was defined as death related to liver disease within 3 mo from the disease onset. Laboratory data on the day of admission were collected for analysis.
Cirrhosis was diagnosed based upon liver biopsy results and/or radiological evidence (computed tomography, abdominal ultrasound or magnetic resonance imaging showing nodular liver surface or evidence of increased portal venous pressure, such as splenomegaly or the presence of intra-abdominal collateral vessels)[17]. The four stages of the natural course of CHB were used in this study: immune tolerant (IT), immune clearance (IC), low or non-replication (LR), and reactivation (RA)[18-20]. The IT stage was defined by the presence of HBeAg, high levels of HBV DNA, normal or minimally elevated serum alanine aminotransferase (ALT), normal or minimal liver histological activity, and scant fibrosis. The IC stage was characterized by fluctuating but progressively decreasing HBV DNA levels, elevated ALT and hepatic necro-inflammation. The LR stage was defined by negativity for HBeAg and positivity for anti-hepatitis B e antigen (anti-HBe), undetectable or low levels of HBV DNA, persistently normal ALT levels and inactive liver histology. The RA stage was characterized by HBeAg negativity with anti-HBe positivity, detectable serum HBV DNA levels, ALT elevation and moderate or severe necro-inflammation with variable fibrosis on liver biopsy[20].
Pre-anti-HBV therapy was defined as taking nucleos/tide analogues (NUCs) for more than 3 mo before the disease onset. Post-anti-HBV therapy was defined as taking NUCs immediately after the diagnosis of HBV-HEV co-infections. Pre-anti-HBV therapy effectiveness was defined as > 2 log decline in HBV DNA within 3 mo of treatment.
Demographic data, information regarding alcohol consumption, cigarette usage and drug history, and information of past medical history were retrieved from the hospital’s clinical database. Drug history focused on the prior exposure to potential hepatotoxic medication before the onset of current symptoms. For past medical history, the definition of other liver diseases was the presence of one or more of the following diseases: chronic hepatitis C, alcoholic liver disease, fatty liver, autoimmune liver diseases, schistosomiasis, primary hepatic carcinoma, hepatic cyst and hepatic hemangioma. In addition, the definition of other extrahepatic underlying diseases was the presence of one or more previous associated comorbidities affecting various relevant major organ systems, including diabetes, hypertension, chronic respiratory diseases (e.g., chronic obstructive pulmonary disease, bronchial asthma, bronchiectasis, tuberculosis, phthisis), chronic kidney diseases (e.g. kidney stone, renal cyst, chronic renal insufficiency, chronic glomerulonephritis) and extrahepatic tumors.
Statistical analyses were performed using SPSS 18.0 software. Descriptive statistics (median and range for continuous variables, frequency and percentage for categorical variables) were used to summarize the baseline demographics and disease characteristics for HBV-HEV co-infected patients. The χ2 test or Fisher’s exact test and Mann-Whitney U test or ANOVA test were used to compare categorical and continuous variables, respectively. Categorical variables included baseline characteristics (i.e., smoker, sex), liver complications (i.e., ascites, HE), adverse clinical outcomes (i.e., severe diseases, mortality) and medical history information (i.e., diabetes, chronic respiratory diseases). Continuous variables included age, laboratory parameters, jaundice and HBV DNA. Multivariate logistic analysis was used for examining the risk factors for severe liver diseases and patient mortality in non-cirrhotic and cirrhotic HBV-HEV co-infected patients, respectively. P values < 0.05 were considered statistically significant.
The data showed that HEV super-infections led to severe adverse outcomes, with 48.7% of the patients having liver failure and/or decompensation and the short-term mortality rate reaching 13.2% (Table 1). Since 94 (41.2%) patients had pre-existing CHB-related cirrhosis, which generated worse clinical outcomes by HEV super-infections, we further compared the clinical features and laboratory parameters between the cirrhotic and non-cirrhotic patients (Table 1). HEV super-infections were found to cause much more severe diseases with significantly more liver complication events in the cirrhotic patients (77.7%; Table 1) and their short-term mortality rate was nearly 20%. These findings were significantly different than those in the non-cirrhotic patients (vs 28.4%, P < 0.001 and vs 7.5%, P = 0.002, respectively). The cirrhotic and non-cirrhotic patients also differed significantly in findings from several biochemistry tests; specifically, levels of transaminase, serum albumin, platelets and leukocytes were all significantly lower, but INR was significantly higher in the cirrhotic patients (Table 1).
| Characteristic | Total patients | Cirrhotic patients | Non-cirrhotic patients | P value |
| (n = 228) | (n = 94) | (n = 134) | ||
| Baseline characteristics | ||||
| Age (yr) (Q1-Q3) | 49 (37-58) | 55 (44.8-62.3) | 44 (35.0-53.3) | < 0.001 |
| Male sex, n (%) | 167 (73.3) | 74 (78.7) | 93 (69.4) | 0.118 |
| Alcohol, n (%) | 74 (34.5) | 37 (39.4) | 37 (27.6) | 0.062 |
| Smoker, n (%) | 72 (31.6) | 30 (31.9) | 42 (31.3) | 0.927 |
| Clinical features, n (%) | ||||
| Bilirubin > 10-fold ULN | 76 (33.3) | 33 (35.1) | 43 (32.1) | 0.634 |
| Ascites | 83 (36.4) | 62 (66) | 21 (15.7) | < 0.001 |
| Infection | 73 (32) | 47 (50) | 26 (19.4) | < 0.001 |
| HE | 39 (17.1) | 24 (25.5) | 15 (11.2) | < 0.010 |
| GH | 11 (4.8) | 11 (11.7) | 0 | < 0.001 |
| Outcomes of disease, n (%) | ||||
| Severe disease | 111 (48.7) | 73 (77.7) | 38 (28.4) | < 0.001 |
| Mortality | 30 (13.2) | 20 (21.3) | 10 (7.5) | < 0.010 |
| Laboratory parameters | ||||
| ALT (IU/L) | 239.5 (62.5-773.5) | 115 (10.8-456.8) | 429.5 (89.8-966.8) | < 0.001 |
| AST (IU/L) | 144 (63-422.5) | 112 (54.5-364.5) | 185 (74.5-475.0) | 0.077 |
| Tbil (μmol/L) | 61.5 (19.0-257.6) | 68.1 (22.8-296.5) | 59.6 (16.9-246.8) | 0.245 |
| ALB (g/dL) | 37.8 (32.1-40) | 33.1 (28.7-36.7) | 38.1 (34.4-40.9) | < 0.001 |
| INR | 1.2 (1.0-1.6) | 1.4 (1.2-1.8) | 1.1 (1.0-1.3) | < 0.001 |
| LEU count (109/L) | 5.3 (4.1-7.1) | 4.6 (3.3-6.6) | 6.0 (4.6-7.2) | < 0.001 |
| Platelet count (109/L) | 115 (78-163) | 83 (53.8-114.3) | 147.5 (97.8-182.3) | < 0.001 |
| NEU count (109/L) | 1.4 (1.0-1.9) | 1.1 (0.8-1.5) | 1.7 (1.2-2.2) | 0.080 |
To determine whether clinical outcomes would be affected by the severity of underlying cirrhosis, patients with decompensated and well-compensated cirrhosis were compared. There were only 9 decompensated patients (9.6%), in contrast to the 85 well-compensated ones. Except for a higher proportion of HE in the decompensated group (66.7% vs well-compensated group: 21.2%, P = 0.008), there was no difference in short-term mortality rates (21.2% vs 22.2%, P = 0.973), and biochemistry tests between the two groups were also similar (Table 2).
| Characteristic | Decompensated | Compensated | P value |
| (n = 9) | (n = 85) | ||
| Baseline characteristics | |||
| Age, yr (Q1-Q3) | 58 (54.0-62.5) | 55 (41.5-62.5) | 0.183 |
| Male sex | 8 (88.9) | 66 (77.7) | 0.679 |
| Alcohol | 2 (22.2) | 35 (41.2) | 0.475 |
| Smoker | 3 (33.3) | 27 (31.8) | 0.982 |
| Clinical features | |||
| Bilirubin > 10-fold ULN | 2 (22.2) | 32 (37.7) | 0.480 |
| Ascites | 8 (88.9) | 54 (63.5) | 0.160 |
| Infection | 6 (66.7) | 41 (48.2) | 0.486 |
| HE | 6 (66.7) | 18 (21.2) | < 0.010 |
| GH | 1 (11.1) | 10 (11.8) | 0.982 |
| Outcomes of disease | |||
| Severe disease | 9 (100) | 64 (75.3) | 0.200 |
| Mortality | 2 (22.2) | 18 (21.2) | 0.973 |
| Laboratory parameters | |||
| ALT ( IU/L) | 51 (37.0-114.5) | 122 (41.5-545.0) | 0.094 |
| AST (IU/L) | 81 (49.0-188.5) | 118 (53.0-399.5) | 0.253 |
| Tbil (μmol/L) | 39.5 (22.5-165.8) | 85.7 (22.4-322.4) | 0.433 |
| ALB (g/dL) | 28.7 (26.6-34.9) | 33.2 (29.1-37.3) | 0.114 |
| INR | 1.5 (1.2-1.6) | 1.4 (1.2-1.8) | 0.995 |
| LEU count (109/L) | 3.9 (2.0-7.5) | 4.6 (3.5-6.3) | 0.516 |
| Platelet count (109/L) | 54 (44.0-84.5) | 85 (58.8-117.3) | 0.080 |
| NEU count (109/L) | 2.8 (1.2-6.4) | 3.0 (1.8-4.4) | 0.933 |
When examining the roles of HBV-related markers and anti-viral therapy, extremely low rates of anti-HBV treatment, both pre- (28.7%) and post- (42.6%), were unexpectedly identified. Consistent with this low level of anti-viral therapy, > 70% of the cirrhotic patients had positive HBV DNA tests and > 40% even had high levels of HBV DNA (> 5 × 105 IU/mL; Table 3). Although anti-HBV therapy was not significantly associated with clinical outcomes, higher percentages of pre- and post-anti-viral therapy were found respectively in patients with mild diseases (38.1% vs 26.0%, P = 0.281 in patients with severe diseases; Table 3) and surviving cases (52.8% vs 30.0%, P = 0.081 in fatal cases; Supplementary Table 1).
| Cirrhotic | Severe | Mild | P value | |
| (n = 94) | (n = 73) | (n = 21) | ||
| Baseline characteristics | ||||
| Age (yr) (Q1-Q3) | 55 (44.8-62.3) | 56 (45.5-62.5) | 52 (38-62) | 0.254 |
| Male sex | 74 (78.7) | 61 (83.6) | 13 (61.9) | 0.065 |
| Smoker | 30 (31.9) | 27 (37) | 3 (14.3) | < 0.050 |
| Alcohol | 37 (39.4) | 31 (42.5) | 6 (28.6) | 0.251 |
| Potential hepatoxic medications | 8 (8.5) | 8 (11) | 0 | 0.192 |
| Pre-existing comorbidities, n (%) | ||||
| Hypertension | 12 (12.8) | 8 (11) | 4 (19.1) | 0.456 |
| Diabetes | 10 (10.6) | 9 (12.3) | 1 (4.8) | 0.448 |
| Respiratory diseases | 4 (4.3) | 4 (5.5) | 0 | 0.572 |
| Kidney diseases | 10 (10.6) | 9 (12.3) | 1 (4.8) | 0.448 |
| Other liver diseases | 24 (25.5) | 21 (28.8) | 3 (14.3) | 0.180 |
| HBeAg positivity | 32 (34) | 22 (30.1) | 10 (47.6) | 0.136 |
| HBV DNA positivity | 69 (73.4) | 52 (71.2) | 17 (81) | 0.374 |
| HBV DNA < 500 IU/mL | 25 (26.6) | 21 (28.8) | 4 (19.1) | 0.374 |
| 500 ≤ HBV DNA < 5 × 105 IU/mL | 31 (33) | 22 (30.1) | 9 (42.9) | 0.275 |
| HBV DNA ≥ 5 × 105 IU/mL | 38 (40.4) | 30 (41.1) | 8 (38.1) | 0.805 |
| Pre-anti-HBV therapy | 27 (28.7) | 19 (26) | 8 (38.1) | 0.281 |
| Effective | 11 (11.7) | 9 (12.3) | 2 (9.5) | 0.982 |
| Post-anti-HBV therapy | 40 (42.6) | 34 (46.6) | 6 (28.6) | 0.141 |
Finally, male sex (OR: 3.1, P = 0.038) and alcohol usage (OR: 3.5, P = 0.060; Supplementary Table 2) were identified to be likely associated with severe diseases in cirrhotic patients, while pre-existing kidney disease was linked to short-term mortality among cirrhotic patients with severe liver diseases (OR: 13.7, P = 0.008; Supplementary Table 3).
The RA group, or the HBeAg-negative CHB patients, had the worst outcomes among different stages of CHB (Table 4). The proportions of severe diseases (liver failure and/or decompensation) and short-term mortality were 41.5% and 17.0%, respectively, which were highest among non-cirrhotic groups and even close to the figures observed in cirrhotic patients (41.5% vs 77.7% and 17% vs 21.3% respectively; Table 1 and Table 4). In contrast, the incidence of severe diseases (13.2%) and mortality (2.6%) was lowest in IT patients, while the proportions in the IC and LR group fell between those for the IT and RA groups (Table 4). Regarding findings from the biochemistry tests, the different CHB groups also differed significantly in their level of serum bilirubin (P = 0.000), INR (P = 0.015) and level of leukocytes (P = 0.035).
| Characteristic | IT | IC | LC | RA | P value |
| (n = 38) | (n = 27) | (n = 16) | (n = 53) | ||
| Baseline characteristics | |||||
| Age (yr ) (Q1-Q3) | 43 (30-52) | 42 (34-51) | 48 (39.3-58.8) | 47 (36.0-56.5) | 0.134 |
| Male sex | 17 (44.7) | 22 (81.5) | 13 (81.3) | 41 (77.4) | < 0.010 |
| Alcohol | 7 (18.4) | 5 (18.5) | 5 (31.3) | 20 (37.7) | 0.136 |
| Smoker | 8 (21.1) | 8 (29.6) | 4 (25) | 22 (41.5) | 0.191 |
| Clinical features | |||||
| Jaundice > 10 ULN | 6 (15.8) | 6 (22.2) | 6 (37.5) | 25 (47.2) | < 0.010 |
| Ascites | 4 (10.5) | 3 (11.1) | 0 | 14 (26.4) | < 0.050 |
| Infection | 4 (10.5) | 2 (7.4) | 4 (25) | 16 (30.2) | < 0.050 |
| HE | 1 (2.6) | 1 (3.7) | 0 | 13 (24.5) | < 0.010 |
| Outcomes of disease | |||||
| Severe disease | 5 (13.2) | 6 (22.2) | 5 (31.3) | 22 (41.5) | < 0.050 |
| Mortality | 1 (2.6) | 0 | 0 | 9 (17) | < 0.010 |
| Laboratory parameters | |||||
| ALT (IU/L) | 276.5 (77.5-520.5) | 454 (214-822) | 597.5 (156.75-1188.5) | 447 (70-1034.5) | 0.231 |
| AST (IU/L) | 162 (66.3-471.0) | 186 (108-527) | 266 (55.5-841.0) | 200 (70.5-421.0) | 0.854 |
| Tbil (μmol/L) | 22.1 (10.5-42.9) | 38.8 (15.0-162.3) | 160.3 (63.9-205.5) | 148.4 (29.8-339.0) | < 0.001 |
| ALB (g/dL) | 38.4 (35.0-40.8) | 39.4 (35.1-42.0) | 36.5 (32.9-39.4) | 36.5 (33.5-41.2) | 0.199 |
| INR | 1.1 (1.0-1.2) | 1.1 (1.0-1.2) | 1.1 (0.9-1.3) | 1.1 (1-2) | < 0.050 |
| LEU count (109/L) | 5 (4.3-6.5) | 6.2 (5.0-6.9) | 7.2 (5.4-9.0) | 6.1 (4.4-7.1) | < 0.050 |
| Platelet count (109/L) | 155 (102.3-205.3) | 149 (103-190) | 177.5 (124.3-200.0) | 126 (93-159) | 0.072 |
| NEU count (109/L) | 2.7 (2.3-3.7) | 3.4 (2.8-4.2) | 3.9 (2.8-5.5) | 3.7 (2.1-4.8) | 0.144 |
Next, we assessed the involvement of HBV markers, anti-HBV therapy and other host factors in the development of adverse clinical outcomes among the non-cirrhotic patients (Table 5). Proportions of HBeAg positivity (29.0% vs 56.3%, P = 0.004) and high HBV DNA levels (> 5 × 105 IU/mL; 29.0% vs 53.1%, P = 0.011) were much lower, while the proportion with intermediate HBV DNA levels (500-5 × 105 IU/mL; 47.4% vs 18.8%, P = 0.001) was much higher in the non-cirrhotic patients with severe liver diseases.
| Characteristic | Non-cirrhotic (n = 134) | Severe (n = 38) | Mild (n = 96) | P value |
| Baseline characteristics | ||||
| Age (yr) (Q1-Q3) | 44 (35-53.3) | 47.5 (36-56.3) | 44 (32.5-53) | 0.167 |
| Male sex | 93 (69.4) | 30 (80.0) | 63 (65.6) | 0.131 |
| Alcohol | 37 (27.6) | 18 (47.4) | 19 (19.8) | < 0.010 |
| Smoker | 42 (31.3) | 16 (42.1) | 26 (27.1) | 0.091 |
| Potential hepatoxic medications | 18 (13.4) | 9 (23.7) | 9 (9.4) | < 0.050 |
| Pre-existing comorbidities | ||||
| Hypertension | 13 (9.7) | 4 (10.5) | 9 (9.4) | 0.986 |
| Diabetes | 15 (11.2) | 9 (23.7) | 6 (6.3) | < 0.050 |
| Respiratory diseases | 9 (6.7) | 3 (7.9) | 6 (6.3) | 0.713 |
| Kidney diseases | 10 (7.5) | 8 (21.1) | 2 (2.1) | < 0.010 |
| Extrahepatic tumors | 2 (1.5) | 1 (2.6) | 1 (1.0) | 0.488 |
| Other liver diseases | 24 (17.9) | 6 (15.8) | 18 (18.8) | 0.687 |
| HBeAg positive | 65 (48.5) | 11 (29.0) | 54 (56.3) | < 0.010 |
| HBV DNA positivity | 98 (73.1) | 29 (76.3) | 69 (71.9) | 0.601 |
| HBV DNA < 500 IU/mL | 36 (26.9) | 9 (23.7) | 27 (28.1) | 0.556 |
| 500 ≤ HBV DNA < 5 × 105 IU/mL | 36 (26.9) | 18 (47.4) | 18 (18.8) | < 0.010 |
| HBV DNA ≥ 5 × 105 IU/mL | 62 (46.3) | 11 (29.0) | 51 (53.1) | < 0.050 |
| Pre-anti-HBV therapy | 12 (9) | 2 (5.3) | 10 (10.4) | 0.508 |
| Effective | 3 (2.2) | 1 (2.6) | 2 (2.1) | 0.991 |
| Post-anti-HBV therapy | 30 (22.4) | 10 (26.3) | 20 (20.8) | 0.493 |
We further found that both the pre- and post-anti-HBV treatment rates were extremely low in the non-cirrhotic CHB patients, only 9.0% and 22.4% respectively. The low number of treated patients precluded any significant result from the statistical analysis; however, a higher percentage of previous anti-HBV therapy was still noted for the patients with mild disease (10.4% vs 5.3%, P = 0.508) (Table 5).
In addition, alcohol consumption (OR: 6.4, P = 0.020), pre-existing diabetes (OR: 7.5, P = 0.003) and kidney diseases (OR: 12.7, P = 0.005) were identified as independent predictors for severe diseases by the multivariate logistic regression analysis with adjustment for confounding factors (Table 6).
| Risk factors | Odds ratio for severe liver diseases | |||
| Odds ratio (95%CI)1 | P value1 | Odds ratio (95%CI)2 | P value2 | |
| Non-cirrhotic patients (n = 134) | ||||
| Male sex | 2 (0.8-4.8) | 0.135 | ||
| Age | 1 (0.9-1.1) | 0.135 | ||
| Alcohol | 3.7 (1.6-8.2) | < 0.01 | 6.4 (1.3-31.4) | < 0.05 |
| Smoker | 2.8 (1.2-6.7) | < 0.05 | 0.4 (0.1-2.4) | 0.341 |
| Potential hepatoxic medications | 3 (1.1-8.3) | < 0.05 | 2 (0.56-7.2) | 0.296 |
| Diabetes | 4.7 (1.5-14.2) | < 0.01 | 7.5 (2-28.5) | < 0.01 |
| Hypertension | 1.1 (0.3-3.9) | 0.839 | ||
| Respiratory diseases | 1.3 (0.3-5.4) | 0.732 | ||
| Kidney diseases | 12.5 (2.5-62.3) | < 0.01 | 12.7 (2.1-76) | < 0.01 |
| Extrahepatic tumors | 2.6 (0.2-42.1) | 0.509 | ||
| Other liver disease | 0.8 (0.3-2.2) | 0.687 | ||
| HBeAg positive | 0.3 (0.1-0.7) | < 0.01 | 0.4 (0.1-1.3) | 0.118 |
| HBV DNA status | ||||
| HBV DNA < 500 IU/mL | Reference | < 0.01 | Reference | < 0.05 |
| 500 ≤ HBV DNA < 5 × 105 IU/mL | 3 (1.1-8.1) | < 0.05 | 5.1 (1.4-18.2) | < 0.05 |
| HBV DNA ≥ 5 × 105 IU/mL | 0.7 (0.2-1.8) | 0.392 | 2 (0.5-8.6) | 0.347 |
| Pre-anti-HBV therapy | 0.5 (0.1-2.3) | 0.356 | ||
| Effective | 1.3 (0.1-14.4) | 0.847 | ||
| Post-anti-HBV therapy | 1.4 (0.6-3.3) | 0.493 | ||
Despite the national HBV vaccination campaign in China, HBV remains endemic, with 7% of the total Chinese population identified as afflicted with CHB[21,22]. Meanwhile, recent epidemiological study in China has indicated a past HEV infection rate of 20%-40% (anti-HEV IgG positivity) and an additional 1% of new infections annually[23-25]. Due to such high prevalence of HBV and HEV, co-infections by both viruses are not rare in China and nearly 20%-40% of all symptomatic HEV infections were determined to occur on CHB backgrounds, as reported previously and observed in the present study[7-10]. On one hand, the underlying CHB could predispose the co-infected patients to more severe symptoms than HEV mono-infections[10,11]. On the other hand, HEV infection may also aggravate the clinical outcome of HBV infection, especially under conditions of liver cirrhosis[26]. Notably, HEV super-infections were reported as the second most prevalent precipitating factor in triggering ACLF in CHB patients[27,28], revealing a mutual influence among the viral co-infections. However, CHB patients vary significantly in their stages of infection, viral activity, treatment strategies and liver functional reserves[14], all of which can greatly affect clinical outcomes during HEV super-infections. To characterize the roles of these HBV-related factors, we analyzed a large dataset of 228 HBV-HEV co-infected patients retrospectively.
Underlying HBV-related cirrhosis is a crucial determining factor for disease severity[11]. Most cirrhotic patients with superimposed HEV-infections in our study presented with liver failure, decompensation or both, as compared to the non-cirrhotics. Interestingly, comparison of patients with prior compensated cirrhosis to those with decompensated cirrhosis showed no difference in short-term mortality rates for the two groups. This finding was surprising but in agreement with the recent studies on ACLF; whereas, the short-term mortality in patients with prior decompensation were not worse than that in compensated patients[16,28]. Additionally, a small proportion of previously-decompensated patients was identified in the current study, indicating that HEV superinfection was a major threat to well-compensated cirrhotic patients.
Cirrhosis is considered prerequisite for ALCF in Western countries; however, in Asia, it is well recognized that ACLF can develop in non-cirrhotic patients, such as the CHB patients[13,29]. To date, little is known regarding the incidence of and risk factors for ACLF in non-cirrhotics. In the present study, we found a continuous increase in incidence of liver failure and decompensation that followed the progression of CHB in co-infected patients. Although the observed outcomes in our study were liver failure and decompensation, distinctive from the definition of ACLF, these data still strongly suggested that no such distinct predisposing boundary existed for ACLF between the cirrhotic patients and non-cirrhotic patients. For example, the proportions of severe diseases and mortality in non-cirrhotic RA patients were not far from those in the cirrhotics. Therefore, these data indicate the involvement of underlying liver functional reserves in the subsequent development of more severe diseases in non-cirrhotics, suggesting that patients with HBeAg-negative CHB are the most vulnerable CHB population for symptomatic HEV infections and should be targeted for possible prophylactic vaccination[30]. Consistent with this theory, HBeAg negativity and intermediate HBV DNA levels were identified as independent predictors for adverse outcomes in co-infected non-cirrhotics.
Successful anti-HBV treatment can significantly reduce the adverse clinical outcomes in CHB patients, even in patients with decompensated cirrhosis[31-33]. Therefore, it is tempting to ask whether clearance of HBV would also affect the disease severity and mortality in HBV-HEV co-infections. No conclusions regarding this issue could be made from the data of previous studies, probably due to the mixed analysis of cirrhotic and non-cirrhotic patients and the relatively small sample sizes[10,11]. In the present study, we were able to see a trend towards higher pre-treatment rates in both cirrhotic and non-cirrhotic subgroups when comparing patients with mild versus severe liver diseases. We also found a remarkably low proportion of previous anti-viral treatment in our HBV-HEV co-infected cohort. Moreover, regarding anti-HBV treatment after the onset of HEV super-infections, which may help to control potential HBV-reactivation, we found that the overall proportions of patients receiving anti-viral treatment were also low. Yet, we found a trend towards a higher percentage of post-anti-HBV treatment in the cirrhotic patients who survived. It is tempting to speculate that such low rates of both pre- and post-antiviral therapy were likely to contribute to the adverse outcomes in co-infected patients. Although the small number of samples precluded our ability to detect any statistically significant differences, we still strongly believe that appropriate and effective treatment will help to substantially reduce the incidence of symptomatic HEV super-infections and to prevent its related adverse outcomes.
Furthermore, we assessed the link between some pre-existing comorbidities and the adverse clinical outcomes in co-infected patients. We identified alcohol intake, previous diabetes and chronic kidney diseases as independent predictors, in addition to HBV markers. Previous studies have also identified alcohol use as a risk factor for HEV-infection and disease severity[7,34]. It is well known that kidney function may become detrimentally impacted by liver failure[35,36]. Accordingly, underlying kidney diseases might predispose HBV-HEV-infected patients to subsequent kidney failure in the setting of severe liver injury, which in turn may lead to multi-organ failure and will almost certainly increase the mortality rate. In addition, our data was in agreement with that in a previous report which demonstrated diabetes as an independent risk factor for severe viral hepatitis[37]. Therefore, treating clinicians should pay close attention to the possibility of superimposed HEV infection in CHB patients with underlying diabetes and kidney diseases, as it might lead to worse outcomes, especially if left undiagnosed.
Several limitations inherent to our retrospective study design must be considered when interpreting our findings. Since the analyzed data were retrieved from the clinical database of our hospital, we may have missed some crucial data that would have been otherwise recognized in a prospective analysis of the patients. Some diagnoses may have been inaccurate, and we had no control over the follow-up duration (by which we may have gained insight into longer-term outcomes). However, as highlighted in this article, our cohort of HBV-HEV co-infected patients was much larger than in previous studies and might compensate for the above-mentioned limitations. The major findings reported here were strongly supported by the data, which ultimately contribute to a better understanding of HEV-HBV co-infections.
We thank Dr. Jianjun Sun (Shanghai Public Health Clinical Center), Prof. Hongying Shi and Dr. Wenyue Liu (Wenzhou Medical University) for their assistance in statistical analysis, and all the staff in charge of the clinical database for their assistance in data acquisition.
Superimposed acute hepatitis E (AHE) on chronic liver diseases (CLDs) often led to severe diseases, such as acute-on-chronic liver failure. However, CLDs vary largely in their etiology, disease stages and treatments, and it remains unclear whether these factors affect the adverse clinical outcomes of HEV super-infections. In areas such as China where chronic hepatitis B (CHB) is endemic, super-infections by hepatitis E virus (HEV) on the CHB background are not uncommon, but the clinical features and the risk factors for adverse outcomes in CHB superimposed with HEV are not clear.
This research investigated CHB-related cirrhosis, immunological phases, hepatitis B virus (HBV) serum markers, HBV viral load and anti-viral treatments among a large cohort of HBV-HEV co-infected patients to explore the clinical features and risk factors for adverse outcomes in patients with CHB superimposed with HEV. This study provides valuable data for understanding the devastating diseases caused by HEV-HBV super-infections.
Underlying CHB-related cirrhosis was found to pose a great risk for adverse outcomes in patients with superimposed AHE. Hepatitis B e antigen-negative CHB and intermediate HBV DNA levels were shown to be associated with severe diseases in non-cirrhotic super-infected patients. The rather low rate of anti-HBV therapy suggested that successful antiviral treatment might reduce the risks of superimposed AHE.
This study provides valuable data for understanding the devastating diseases caused by HEV-HBV super-infections and may facilitate the HEV vaccination program to precisely target populations most at risk of poor outcomes.
Acute hepatitis E was defined as clinical acute hepatitis symptomology and anti-HEV immunoglobulin M positivity. Chronic hepatitis B was confirmed by positivity for hepatitis B surface antigen and/or HBV DNA within the previous 6 mo. Super-infection was defined as a second infection superimposed on an earlier one, especially by a different microbial agent of exogenous or endogenous origin.
This paper contributes to a better understanding of clinical features among the HEV-HBV co-infections.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B,B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Asfeha GG, Bock T, Bivigou-Mboumba B S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 942] [Article Influence: 85.6] [Reference Citation Analysis (4)] |
| 2. | Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. Hepatitis E. Lancet. 2012;379:2477-2488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 744] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 3. | Pauli G, Aepfelbacher M, Bauerfeind U, Blümel J, Burger R, Gärtner B, Gröner A, Gürtler L, Heiden M, Hildebrandt M. Hepatitis E Virus. Transfus Med Hemother. 2015;42:247-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Arends JE, Ghisetti V, Irving W, Dalton HR, Izopet J, Hoepelman AI, Salmon D. Hepatitis E: An emerging infection in high income countries. J Clin Virol. 2014;59:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Aggarwal R. Hepatitis e: epidemiology and natural history. J Clin Exp Hepatol. 2013;3:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Pavio N, Meng XJ, Doceul V. Zoonotic origin of hepatitis E. Curr Opin Virol. 2015;10:34-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (3)] |
| 7. | Kmush BL, Nelson KE, Labrique AB. Risk factors for hepatitis E virus infection and disease. Expert Rev Anti Infect Ther. 2015;13:41-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Shalimar D, Vadiraja PK, Nayak B, Thakur B, Das P, Datta Gupta S, Panda SK, Acharya SK. Acute on chronic liver failure because of acute hepatic insults: Etiologies, course, extrahepatic organ failure and predictors of mortality. J Gastroenterol Hepatol. 2016;31:856-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Zhang X, Ke W, Xie J, Zhao Z, Xie D, Gao Z. Comparison of effects of hepatitis E or A viral superinfection in patients with chronic hepatitis B. Hepatol Int. 2010;4:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Chow CW, Tsang SW, Tsang OT, Leung VK, Fung KS, Luk WK, Chau TN. Comparison of acute hepatitis E infection outcome in patients with and without chronic hepatitis B infection: a 10 year retrospective study in three regional hospitals in Hong Kong. J Clin Virol. 2014;60:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Cheng SH, Mai L, Zhu FQ, Pan XF, Sun HX, Cao H, Shu X, Ke WM, Li G, Xu QH. Influence of chronic HBV infection on superimposed acute hepatitis E. World J Gastroenterol. 2013;19:5904-5909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (2)] |
| 12. | Ramachandran J, Eapen CE, Kang G, Abraham P, Hubert DD, Kurian G, Hephzibah J, Mukhopadhya A, Chandy GM. Hepatitis E superinfection produces severe decompensation in patients with chronic liver disease. J Gastroenterol Hepatol. 2004;19:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Kumar A, Saraswat VA. Hepatitis E and Acute-on-Chronic Liver Failure. J Clin Exp Hepatol. 2013;3:225-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | McMahon BJ. Chronic hepatitis B virus infection. Med Clin North Am. 2014;98:39-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Organization Committee of 13th Asia-Pacific Congress of Clinical Microbiology and Infection. 13th Asia-Pacific Congress of Clinical Microbiology and Infection Consensus Guidelines for diagnosis and treatment of liver failure. Hepatobiliary Pancreat Dis Int. 2013;12:346-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, Durand F, Gustot T, Saliba F, Domenicali M. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426-137, 1426-137. [PubMed] |
| 17. | Suk KT, Baik SK, Yoon JH, Cheong JY, Paik YH, Lee CH, Kim YS, Lee JW, Kim DJ, Cho SW. Revision and update on clinical practice guideline for liver cirrhosis. Korean J Hepatol. 2012;18:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 519] [Cited by in RCA: 557] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 19. | Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29 Suppl 1:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 20. | Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 909] [Cited by in RCA: 966] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 21. | Liu J, Zhang S, Wang Q, Shen H, Zhang M, Zhang Y, Yan D, Liu M. Seroepidemiology of hepatitis B virus infection in 2 million men aged 21-49 years in rural China: a population-based, cross-sectional study. Lancet Infect Dis. 2016;16:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (1)] |
| 22. | Zhang Q, Qi W, Wang X, Zhang Y, Xu Y, Qin S, Zhao P, Guo H, Jiao J, Zhou C. Epidemiology of Hepatitis B and Hepatitis C Infections and Benefits of Programs for Hepatitis Prevention in Northeastern China: A Cross-Sectional Study. Clin Infect Dis. 2016;62:305-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Zhu FC, Huang SJ, Wu T, Zhang XF, Wang ZZ, Ai X, Yan Q, Yang CL, Cai JP, Jiang HM. Epidemiology of zoonotic hepatitis E: a community-based surveillance study in a rural population in China. PLoS One. 2014;9:e87154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 24. | Guo QS, Yan Q, Xiong JH, Ge SX, Shih JW, Ng MH, Zhang J, Xia NS. Prevalence of hepatitis E virus in Chinese blood donors. J Clin Microbiol. 2010;48:317-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 82] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Huang SJ, Liu XH, Zhang J, Ng MH. Protective immunity against HEV. Curr Opin Virol. 2014;5:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Hoan NX, Tong HV, Hecht N, Sy BT, Marcinek P, Meyer CG, Song le H, Toan NL, Kurreck J, Kremsner PG. Hepatitis E Virus Superinfection and Clinical Progression in Hepatitis B Patients. EBioMedicine. 2015;2:2080-2086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Shi Y, Yang Y, Hu Y, Wu W, Yang Q, Zheng M, Zhang S, Xu Z, Wu Y, Yan H. Acute-on-chronic liver failure precipitated by hepatic injury is distinct from that precipitated by extrahepatic insults. Hepatology. 2015;62:232-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 242] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 28. | Li H, Chen LY, Zhang NN, Li ST, Zeng B, Pavesi M, Amorós À, Mookerjee RP, Xia Q, Xue F, Ma X, Hua J, Sheng L, Qiu DK, Xie Q, Foster GR, Dusheiko G, Moreau R, Gines P, Arroyo V, Jalan R. Characteristics, Diagnosis and Prognosis of Acute-on-Chronic Liver Failure in Cirrhosis Associated to Hepatitis B. Sci Rep. 2016;6:25487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 29. | Arroyo V, Jalan R. Acute-on-Chronic Liver Failure: Definition, Diagnosis, and Clinical Characteristics. Semin Liver Dis. 2016;36:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 30. | Wang X, Li M, Li S, Wu T, Zhang J, Xia N, Zhao Q. Prophylaxis against hepatitis E: at risk populations and human vaccines. Expert Rev Vaccines. 2016;15:815-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 31. | Chen CH, Lin CL, Hu TH, Hung CH, Tseng PL, Wang JH, Chang JY, Lu SN, Chien RN, Lee CM. Entecavir vs. lamivudine in chronic hepatitis B patients with severe acute exacerbation and hepatic decompensation. J Hepatol. 2014;60:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 32. | Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, Kim TY, Sohn JH, Tak WY, Han KH. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61:1809-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 33. | Hung CH, Hu TH, Lu SN, Lee CM, Chen CH, Kee KM, Wang JH, Tsai MC, Kuo YH, Chang KC. Tenofovir versus entecavir in treatment of chronic hepatitis B virus with severe acute exacerbation. Antimicrob Agents Chemother. 2015;59:3168-3173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Dalton HR, Bendall RP, Rashid M, Ellis V, Ali R, Ramnarace R, Stableforth W, Headdon W, Abbott R, McLaughlin C. Host risk factors and autochthonous hepatitis E infection. Eur J Gastroenterol Hepatol. 2011;23:1200-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Lv Y, Fan D. Hepatopulmonary Syndrome. Dig Dis Sci. 2015;60:1914-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Gonwa TA, Wadei HM. Kidney disease in the setting of liver failure: core curriculum 2013. Am J Kidney Dis. 2013;62:1198-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Singh KK, Panda SK, Shalimar SK. Patients with Diabetes Mellitus are Prone to Develop Severe Hepatitis and Liver Failure due to Hepatitis Virus Infection. J Clin Exp Hepatol. 2013;3:275-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |