Published online Dec 21, 2016. doi: 10.3748/wjg.v22.i47.10353
Peer-review started: July 25, 2016
First decision: September 7, 2016
Revised: September 21, 2016
Accepted: October 27, 2016
Article in press: October 27, 2016
Published online: December 21, 2016
Processing time: 150 Days and 23.3 Hours
To investigate the effects of active vitamin D3 on autophagy and interleukin (IL)-1β expression in Salmonella-infected intestinal epithelial cells (IECs).
Caco-2 cells, NOD2 siRNA-, Atg16L1 siRNA- or vitamin D receptor (VDR) siRNA-transfected Caco-2 cells were pretreated with 1,25-dihydroxyvitamin D3 (1,25D3), and then infected by wild-type S. typhimurium strain SL1344. The conversion of LC3-I to LC3-II was detected by Western blot analysis and LC3+ autophagosome was analyzed by immunofluorescence. Caco-2 cells or VDR siRNA-transfected cells were pretreated with 1,25D3, and then infected by SL1344. Membrane protein and total RNA were analyzed by Western blot and RT-PCR for VDR and Atg16L1 protein and mRNA expression, respectively. Atg16L1 siRNA-transfected Caco-2 cells were pretreated by 1,25D3 and then infected with SL1344. Total RNA was analyzed by RT-PCR for IL-1β mRNA expression.
The active form of vitamin D, 1,25D3, showed enhanced VDR-mediated Atg16L1 mRNA expression, membranous Atg16L1 protein expression leading to enhanced autophagic LC3II protein expression and LC3 punctae in Salmonella-infected Caco-2 cells which was counteracted by Atg16L1 and VDR siRNA, but Atg16L1 mediated suppression of IL-1β expression. Thus, active vitamin D may enhance autophagy but suppress inflammatory IL-1β expression in Salmonella-infected IECs.
Active vitamin D might enhance autophagic clearance of Salmonella infection, while modulation of inflammatory responses prevents the host from detrimental effects of overwhelming inflammation.
Core tip: In this study, the enhanced autophagy expression and down-regulation of inflammatory responses in Salmonella-infected intestinal epithelial cells by active vitamin D provide a rationale of its alternative therapy for invasive bacterial infection and other inflammatory disorders.
- Citation: Huang FC. Vitamin D differentially regulates Salmonella-induced intestine epithelial autophagy and interleukin-1β expression. World J Gastroenterol 2016; 22(47): 10353-10363
- URL: https://www.wjgnet.com/1007-9327/full/v22/i47/10353.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i47.10353
The incidence of food-borne human infections caused by Salmonella enteritidis and by multi-drug-resistant strains of S. typhimurium has increased substantially[1], with similar trends being reported from Europe[2] and Taiwan[3]. Intestinal epithelial cells (IECs) serve as not only a barrier to bacteria colonizing the gut but also rather as an integral and essential component of the innate mucosal immune system of the host.
Although clear evidence of the beneficial effects of vitamin D on a variety of systemic diseases exists, there has been renewed interest in this vitamin, which has a broad range of activities on microbial infections. Recent research has begun to unravel important roles of vitamin D in the regulation of innate immunity[4]. Vitamin D may play a role in protecting against infection during pregnancy and bacterial vaginosis. Four studies included in a systemic review[5] demonstrated the therapeutic effect of vitamin D supplementation for colitis. Thus, supplementation with vitamin D3 could provide a novel strategy to reduce antibiotic use and indirectly prevent the emerging epidemic of bacterial resistance.
The role of autophagy has been expanded in recent years to include diverse immunological effector and regulatory functions. Increasing evidence indicates the potential of autophagy in controlling infections by directing intracellular or ingested pathogens to lysosomes, leading to their destruction[6]. Several studies have linked autophagy to host defense against several intracellular bacterial pathogens that use different strategies to establish infection, such as Listeria monocytogenes, Shigella flexneri[7] and S. enterica serovar typhimurium[8]. Therefore, dissecting the molecular mechanisms by which Salmonella utilizes autophagy has the potential to lead to the identification of novel drug candidates to prevent and treat Salmonella infection and related intracellular infections.
Autophagy plays an essential role in the clearance of Salmonella by alveolar macrophages. Previous studies have linked nucleotide-binding oligomerization domain-containing protein 2 (NOD2) function to autophagy[9,10]. NOD2 is critical for the autophagic response to invasive bacteria, which recruits autophagy-related protein 16-like 1 (ATG16L1) to the plasma membrane at bacterial entry sites. The observation that NOD2 is a vitamin D target gene[11,12] and vitamin D receptor (VDR) transcriptionally regulates ATG16L1 as a VDR target gene[13,14] also links vitamin D signaling to autophagy. Atg16L1 is required for autophagy in IECs and protection of mice from Salmonella infection[15]. 1,25-dihydroxyvitamin D3 (1,25D3), the active form of vitamin D, up-regulates NOD2 mRNA expressions in Salmonella-infected IECs[16], and the interaction of NOD2 and Arg16L1 plays a critical role in Salmonella-induced autophagy in IECs[17]. These results strongly suggest that stimulation of NOD2 and Atg16L1 expression in human epithelial cells by 1,25D3 would recruit Atg16L1 to boost autophagy, which would then contribute to innate immune responses of vitamin D to bacterial infection.
Therefore, this study was designed to investigate the effects of active vitamin D3 on Salmonella-induced autophagy via enhanced NOD2 and Atg16L1 mRNA expression and membranous recruitment of proteins in IECs.
The wild-type Salmonella enterica serovar typhimurium (S. typhimurium) strain used in this work was SL1344. Salmonella inoculum was prepared as described previously[16,18]. Bacteria were grown overnight in static cultures with minimal aeration in LB medium. The bacteria were collected by centrifugation at 14000 g for 5 min, washed with sterile PBS, and resuspended in tissue culture medium without antibiotics.
Caco-2 cells were purchased from the American Type Culture Collection (Manassas, VA, United States) and were cultured as described previously[16,18,19]. Briefly, Caco-2 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in a 5% CO2 atmosphere at 37 °C. Passage 10-30 was used for all experiments. The cells were seeded in tissue culture plates and grown to 60%-75% confluence. About 1 h before addition of bacteria, the cells were washed and placed in antibiotic-free medium.
Cytosolic, membranous and nuclear extracts from untreated and treated cultured cells were prepared by the method previously described[16,19,20]. Protein concentrations in cell fractions were determined using a Bio-Rad assay kit.
Equal amounts of total protein from colon tissue or cultured cells were separated by SDS-PAGE and then transferred to nitrocellulose membranes by semi-dry blotting as previously described[16,19,20]. After blocking the membranes with 5% non-fat dry milk, they were probed with antibodies to either ATG16L1 (Cell Signaling, Beverly, MA, United States), NOD2 (Cayman Chemical, Ann Arbor, MI, United States), LC3B (Cell Signaling) or total GAPDH (Santa Cruz Biotechnology, Dallas, TX, United States). After washes, the membranes were incubated with appropriate horseradish peroxidase-associated secondary antibodies before signals were visualized with the enhanced chemiluminescence detection system (Amersham Bioscience, Piscataway, NJ, United States).
Total RNA was prepared from control or infected cells with the Trizol reagent (Invitrogen Corporation, Carlsbad, CA, United States), following the manufacturer’s directions. The RNA was reverse transcribed using the GeneAmp kit (Roche Diagnostics, Nutley, NJ, United States) as described in detail earlier[16].
Real-time reverse transcription PCR analyses were performed in a fluorescence temperature cycler (LightCycler; Roche Diagnostics) as described previously[16,19,20] to determine the NOD2 and Atg16L1 and interleukin-1β (IL-1β) mRNA expression levels using the comparative threshold cycle (ΔΔCt) method of relative quantitation.
The immunofluorescence study of LC3 was performed as previously described[16,19,20]. Briefly, cultured cells after treatment and infection were washed, fixed and permeabilized and then incubated with rabbit anti-LC3B (Cell Signaling Technology). Secondary antibody was goat anti-rabbit IgG conjugated with Alexa Fluor 594 fluorochrome (Invitrogen Molecular Probes, Eugene, OR, United States). After extensive washing, the nucleic acid stain 4,6-diamidino-2-phenyindole dihydrochloride (DAPI) was added to visualize the nucleus. Then, the coverslips were examined under immunoflourescence conditions by using a Zeiss Axio Observer Z1 (Carl Zeiss, Jena, Germany). The percentage of cells with endogenous LC3 punctate was determined by counting the number of positively stained cells from 100 randomly chosen cells from three separate experiments.
RNA interference (RNAi) experiments in cultured cells were performed as described previously[16,19,20]. Cells were transfected according to the manufacturer’s protocol, which was modified in our Lab. After 48 to 72 h incubation in a 37 °C incubator, the cells were infected by bacteria, and then the cells were lysed and RNA or proteins extracted over ice for further experiments.
Representative cell populations from each condition were examined under light microscopy. No significantly morphologic change was observed under any condition. Cell viability in untreated or treated cells was found to be > 90% as analyzed by trypan blue exclusion (data not shown).
All above experiments were carried out at least twice with similar results. Statistical analysis was performed using the paired Student’s t-test and ANOVAs (StatView; SAS Institute, Cary, NC, United States). P values < 0.05 were considered significant.
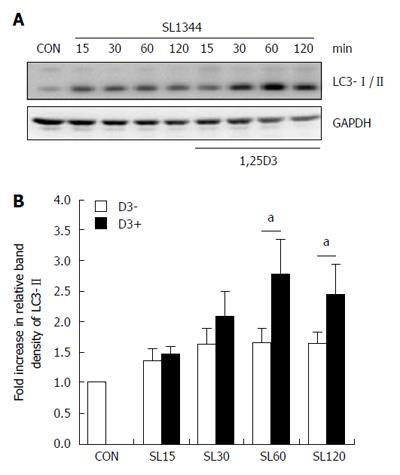
In order to explore if active vitamin D plays a role in Salmonella-induced autophagy in IECs, Caco-2 cells were pretreated with 1,25D3, and then infected by wild-type S. typhimurium strain SL1344. The conversion of LC3-I to LC3-II was detected by Western blot analysis and LC3+ autophagosome was analyzed by immunofluorescence. As shown in Figure 1, Salmonella-induced autophagy in Caco-2 cells was accompanied by an increase in the conversion of LC3-I to LC3-II (Figure 1A and B) and increased LC3 punctae-containing cells in immunofluorescent analysis (Figure 2C and D), while 1,25D3 enhanced Salmonella-induced LC3-II protein and LC3+ autophagosome expressions in Caco-2 cells. This suggested that 1,25D3 up-regulated the autophagic process in Salmonella-infected Caco-2 cells.
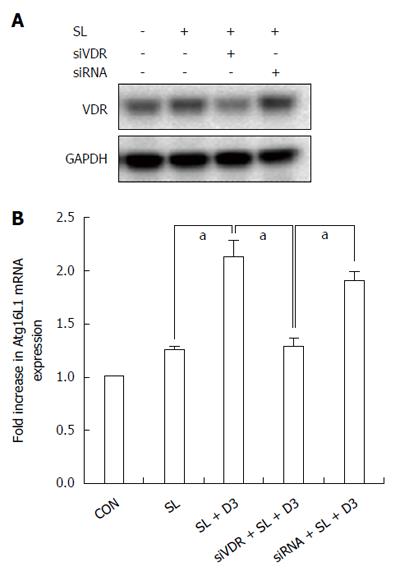
To examine if 1,25D3 up-regulates Salmonella-induced Atg16L1 mRNA expression in IECs, Caco-2 cells were untreated or pretreated with 1,25D3 and then infected with wild-type Salmonella strain SL1344 for 1 h. Total RNA was analyzed by real-time quantitative PCR for Atg16L1 mRNA expression. As shown in Figure 3B, 1,25D3 enhanced Atg16L1 mRNA expression in Salmonella-infected Caco-2 cells compared to Salmonella infection alone.
To investigate if VDR was involved in the 1,25D3-mediated up-regulation of Atg16L1 mRNA in Salmonella-infected cells, VDR siRNA-transfected cells were untreated or treated with 1,25D3 for 6 h and infected by S. typhimurium wild-type strain SL1344. Knockdown of VDR was confirmed by Western blot (Figure 3A). Following knockdown of VDR, the 1,25D3-enhanced Atg16L1 mRNA expression in Salmonella-infected cells was diminished in VDR-silenced cells (Figure 3B), but not in control siRNA-silenced cells. Therefore, specific suppression by siRNA targeting VDR attenuated the enhanced effect of 1,25D3 on Atg16L1 mRNA expression in Salmonella-infected Caco-2 cells.
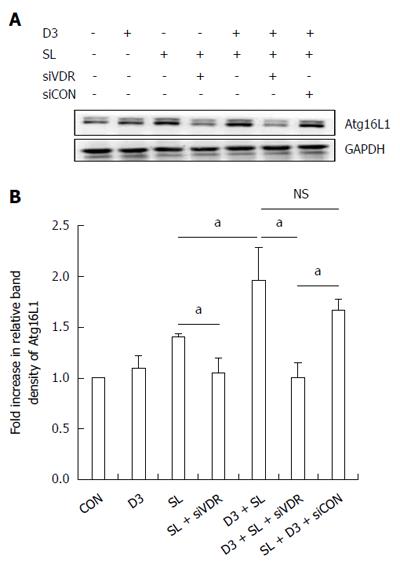
A previous study demonstrated that 1,25D3 enhanced NOD2 mRNA and membranous protein expression in IECs[16]. Likewise, we proceeded to investigate the effect of 1,25D3 on Atg16L1 protein expression in IECs. Western blot of Atg16L1 proteins expression was analyzed in membranous extract of Salmonella-infected Caco-2 cells in the presence or absence of 1,25D3. As shown in Figure 4, 1,25D3 enhanced the membranous Atg16L1 protein expression in Salmonella-infected Caco-2 cells compared to Salmonella infection only. Following knockdown of VDR, the 1,25D3-enhanced Atg16L1 membranous protein expression in Salmonella-infected cells was diminished in VDR-silenced cells (Figure 4), but not in control siRNA-silenced cells. Therefore, specific suppression by siRNA targeting VDR attenuated the enhanced effect of 1,25D3 on Atg16L1 membranous protein expression in Salmonella-infected Caco-2 cells.
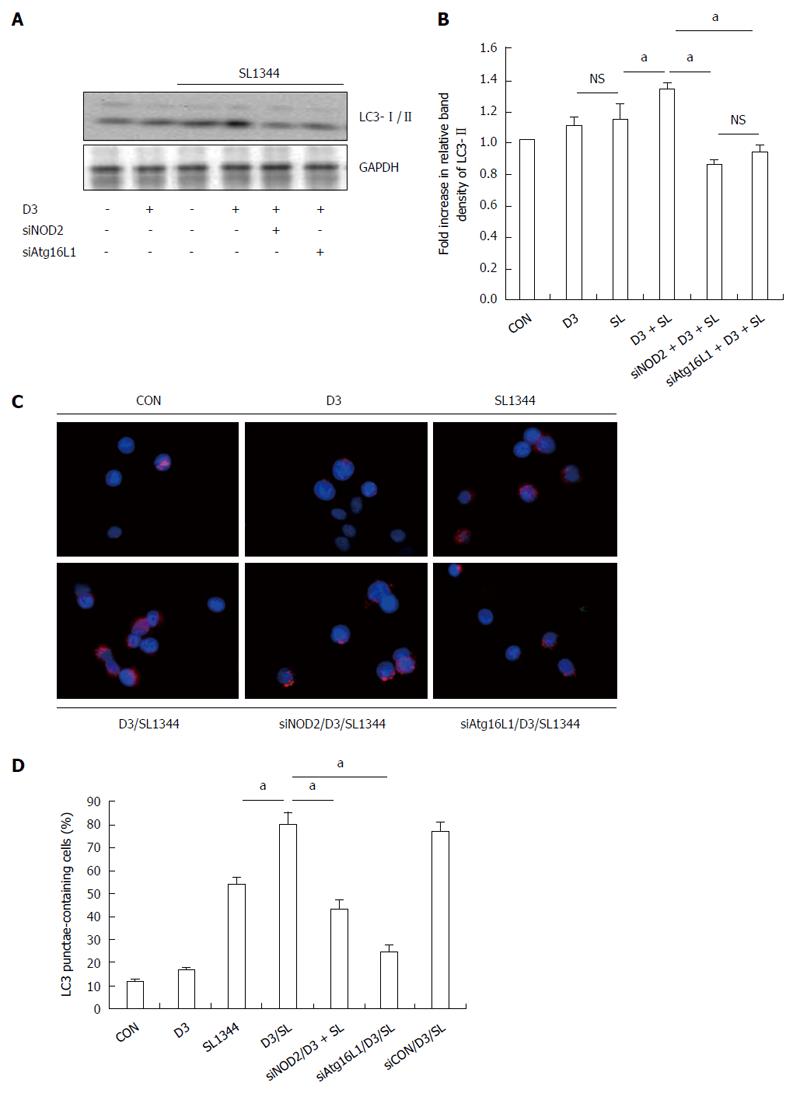
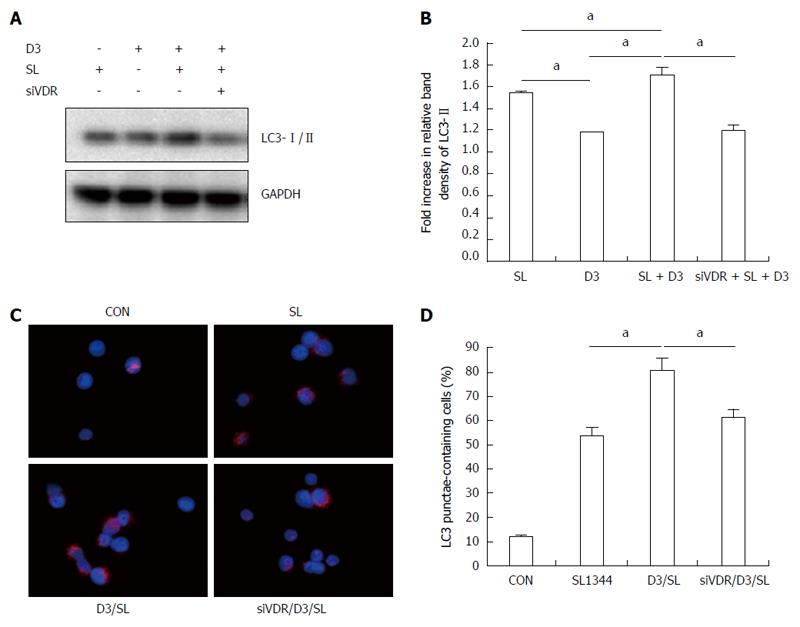
Based on the above observation that 1,25D3 enhanced NOD2 and Atg16L1 mRNA expressions in Salmonella-infected Caco-2 cells and the interaction of NOD2 and Arg16L1 in Salmonella-induced autophagy in IECs[17], we investigated the involvement of NOD2 or Atg16L1 in 1,25D3-enhanced autophagy in Salmonella-infected IECs. NOD2 siRNA- or Atg16L1 siRNA-transfected Caco-2 cells were untreated or pretreated with 1,25D3 and then infected with wild-type Salmonella strain SL1344 for 1 h. Knockdown of NOD2 or Atg16L1 with specific siRNA in Caco-2 cells was confirmed by Western blot in a previous report[21]. The conversion of LC3-I to LC3-II was detected by Western blot analysis (Figure 2A and B). The Caco-2 cells were fixed, permeabilized, immunostained with antibody to endogenous LC3 and visualized by fluorescence microscopy. Immunofluorescence study (Figure 2C) showed numerous LC3 punctae (red) in Salmonella-infected cells. Pre-treatment of the cells with 1,25D3 significantly increased the LC3II protein as above, while NOD2 siRNA- or Atg16L1-transfected Caco-2 cells significantly diminished 1,25D3-enhanced conversion of LC3 (Figure 2A and B) to a level below that detected in the SL1344 infection only group. The 1,25D3-enhanced percentage of cells showing accumulation of LC3 punctae was significantly diminished by NOD2 or Atg16L1 siRNA (Figure 2C and D). Our results suggest that NOD2 and Atg16L1 become involved in the effect of 1,25D3 on autophagy expression in Salmonella-infected Caco-2 cells.
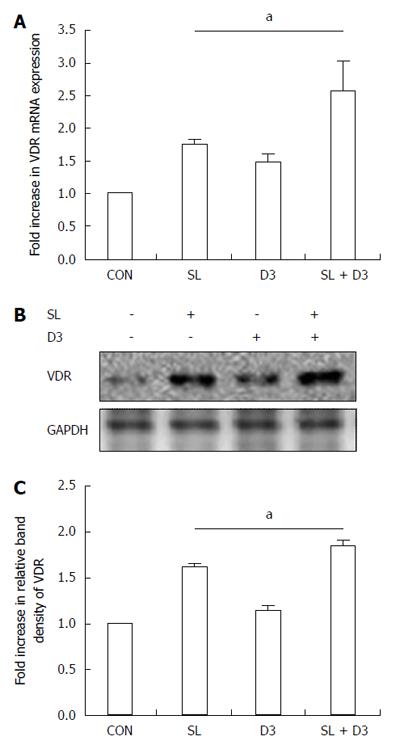
In order to determine if 1,25D3 up-regulates VDR mRNA and protein expressions in Salmonella-infected Caco-2 cells, cultured cells were left untreated or stimulated with 1,25D3 for 6 h, followed by wild-type S. typhimurium strain SL1344 infection. Immunoblots were performed on whole cell lysates with antibody to detect VDR protein and real-time quantitative PCR to detect mRNA expression. As shown in Figure 5, VDR mRNA and protein were up-regulated in Salmonella-infected cells and even higher in 1,25D3-treated cells.
To investigate the role of VDR in 1,25D3-enhanced autophagy expression in Salmonella-infected Caco-2 cells, VDR siRNA-transfected Caco-2 cells were untreated or pretreated by 1,25D3 and then infected with wild-type Salmonella strain SL1344 for 1 h. The conversion of LC3-I to LC3-II was detected by Western blot analysis and LC3+ autophagosome was analyzed by immunofluorescence. 1,25D3 enhanced Salmonella-induced LC3-II protein and LC3+ autophagosome expressions in Caco-2 cells, while VDR siRNA-transfected Caco-2 cells significantly diminished the enhancement of 1,25D3 on the conversion of LC3 (Figure 6A and B) or the percentage of LC3 punctae (Figure 6C and D). Our results suggest that VDR is involved in the effect of 1,25D3 on autophagy expression in Salmonella-infected Caco-2 cells.
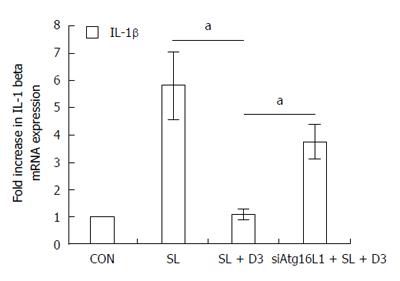
In order to determine the effect of 1,25D3 on IL-1β mRNA expression in Salmonella-infected Caco-2 cells and the involvement of Atg16L1, Atg16L1 siRNA-transfected Caco-2 cells were untreated or pretreated by 1,25D3 and then infected with wild-type Salmonella strain SL1344 for 1 h. Knockdown of Atg16L1 with specific siRNA in Caco-2 cells was confirmed previously. Total RNA was analyzed by real-time quantitative PCR for IL-1β mRNA expression. As shown in Figure 7, Salmonella-induced IL-1β mRNA expression in Caco-2 cells was down-regulated by 1,25D3. Following knockdown of Atg16L1, the 1,25D3-mediated suppression of IL-1β mRNA expression in Salmonella-infected cells was diminished in Atg16L1-silenced cells, but not in control siRNA-silenced cells (data not shown). Therefore, specific suppression by siRNA targeting Atg16L1 attenuated the suppressive effect of 1,25D3 on Salmonella-induced IL-1β mRNA expression in Caco-2 cells.
Vitamin D is an important mediator of intestinal epithelial defenses against infectious agents. Vitamin D deficiency predisposes to more severe intestinal injury in an infectious model of colitis[22]. However, the mechanism is unknown. Based on previous reports that 1,25D3 signaling induces NOD2 expression in human IECs[16] and activated NOD2 recruits ATG16L1 that control autophagy[23] to kill Salmonella[17], we investigated the effect of vitamin D on autophagy expression in IECs after Salmonella infection and the involvement of NOD2 and Atg16L1. We observed that 1,25D3 increased the Atg16L1 mRNA (Figure 3) and membranous protein expression (Figure 4) in Salmonella-infected Caco-2 cells and the enhancement of 1,25D3 on autophagy expression in Salmonella-infected Caco-2 cells (Figure 1) is NOD2- and Atg16L1-dependent (Figure 2). It suggests vitamin D enhances autophagy in Salmonella-infected IECs, depending on enhanced NOD2 and Atg16L1 expression. Atg16L1 is required for autophagy in IECs and protection of mice from Salmonella infection[15]. Additionally, the association of autophagy-related Atg16L1 gene polymorphism with sepsis severity in patients with sepsis and ventilator-associated pneumonia[24] provides the rationale to investigate the role of Atg16L1 gene polymorphism in patients with septic complication in Salmonella colitis and potential therapy of active vitamin D on invasive microorganisms.
ATG16L1 is a VDR target gene and VDR regulates autophagic activity through ATG16L1[13]. Intestinal epithelial VDR deletion leads to defective autophagy in a colitis model[14]. Lack of intestinal epithelial VDR down-regulates expression of Atg16L1 and the resulting microbial dysbiosis may sensitize the colonic mucosa to chemical-induced colitis. Low levels of intestinal epithelial VDR correlated with reduced ATG16L1 and dysbiotic microbial ecology dominated by intestinal Bacteroides known to contribute to the pathogenesis of inflammatory bowel disease (IBD). An increased risk of IBD following enteric infections with Salmonella was reported[25]. The abnormalities in the handling of intracellular bacteria through autophagy might play a role in Crohn’s disease pathogenesis[17,26-29]. This indicates the fundamental relationship between VDR, autophagy, and gut microbial assemblage that is essential for maintaining intestinal homeostasis, but also in contributing to the pathophysiology of IBD. 1,25D3 up-regulates the VDR through stabilization of the receptor in rat intestinal epithelial cells (IEC-6)[30] or the activation of gene expression in cells other than IECs[31]. Pretreatment with 1,25D3 can increase the VDR protein expression in Caco-2 cells[32]. Colonic epithelial VDR levels are markedly reduced in patients with inflammatory bowel diseases or in experimental colitis models, whereas vitamin D analog therapy that ameliorates colitis up-regulates epithelial VDR[33].
Thus, active vitamin D may enhance VDR expression to maintain intestinal homeostasis, induce autophagic defense against Salmonella infection and prevent IBD attack. We demonstrated that 1,25D3 enhances VDR mRNA and protein expression (Figure 5), which is involved in 1,25D3-enhanced Atg16L1 mRNA (Figure 3) and protein expression (Figure 4) in Salmonella-infected Caco-2 cells, subsequently resulting in enhanced Atg16L1-mediated autophagy (Figure 2). Collectively, our study suggests that 1,25D3 may have applicability for infectious diseases and autoimmune diseases through its actions on ATG16L1 and VDR.
Mice lacking VDR are much more susceptible to dextran sodium sulfate (DSS)-induced mucosal injury, leading to extensive ulceration and early death[34]. Chemical-induced colitis in the VDR knockout mice was accompanied by high colonic expression of TNF-α, IL-1α and IL-1β[35]. VDR knockout mice were hyper-responsive to exogenously injected lipopolysaccharide, and cultures of the peritoneal exudates of moribund DSS-treated VDR knockout mice were positive for bacterial growth. This suggests that VDR may suppress overwhelming inflammation and maintain intestinal epithelial integrity to prevent bacterial invasion during colitis. Treatment of IL-10 knockout mice with 1,25D3 resulted in the suppression of IBD symptoms[36]. 1,25D3 in the diet or delivered rectally decreased the severity and extent of inflammation in wild-type mice[35]. Furthermore, human studies documented some benefits of supplemental vitamin D in maintaining remission of Crohn’s disease[37]. However, the mechanism is not clear. Vitamin D hormone not only can induce VDR expression[38] but also can suppress TNF-α production[39]. Here, we observed that the enhancement of 1,25D3 on Atg16L1 expression depends on VDR (Figures 3 and 4) while 1,25D3 suppressed Atg16L1-mediated IL-1β expression (Figure 7). Thus, gut epithelial VDR signaling enhanced by active vitamin D could be a useful therapeutic target in the management of IBD and appears to play an essential role in controlling mucosal inflammation.
Sorbara et al[40] showed that Atg16L1 suppresses inflammatory cytokines induced by NOD2 signaling in an autophagy-independent manner. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production[41]. Atg16L1 polymorphisms exhibit an excessive production of IL-1β in human peripheral mononuclear cells[42]. Thus, in theory, vitamin D therapy can shift the balance to favor inhibition of inflammation. Here, we showed that 1,25D3 suppresses Salmonella-induced IL-1β mRNA expression depending on Atg16L1 (Figure 7). IL-1β is one of the key mediators of intestinal inflammation in IBD, with a role in amplifying mucosal inflammation[43], consistent with the finding that IL-1β is up-regulated in IBD patients[44] and animal models[45]. The IL-1β-induced increase in intestinal epithelial tight junction permeability has been postulated to be an important pathogenic mechanism contributing to intestinal inflammation. This could be a mechanism by which vitamin D therapy ameliorates IBD.
Almost all of the current data on how vitamin D can influence innate immune function has stemmed from studies of human cells. A limited number of animal models have been utilized with varying results, and future studies will need to improve this lack significantly. Finally, intestinal VDR expression was lower in patients with active Crohn’s disease despite having adequate serum levels of vitamin D[46], suggesting that epithelial responses to vitamin D may be diminished during active IBD and this may not be altered by increasing serum vitamin D alone. Thus, more clinical trials are needed to determine how vitamin D affects infection in vivo, and whether or what dose of active vitamin D administered orally or rectally can act as a therapy to ameliorate Salmonella colitis or IBD.
Conclusively, the study provides a link between vitamin D3-induced autophagy and anti-inflammation, both of which target the intracellular pathogen for eradication and prevention of overwhelming inflammation. The ability of the active form of vitamin D, 1,25D3 to induce autophagy has provided a new approach to treat invasive microbial infections in humans. Vitamin D therapy can shift the balance to favor inhibition of inflammation and enhancement of IEC autophagy against Salmonella infection, leading to the identification of novel drug candidates to treat Salmonella infection and prevent IBD. Perhaps, in the future, we might be able to treat or prevent certain infectious diseases with safe and inexpensive substances that induce expression of endogenous autophagy.
We thank the Stem Cell Research Core Laboratory (grant CLRPG8B0052) for technical support.
Salmonella spp. remain a major public health problem for the entire world. Intestinal epithelial cells (IECs) play an essential role in mucosal innate immunity of the host and autophagy has been highlighted as an innate immune defense against intracellular pathogens. Accumulated studies have suggested a critical role for vitamin D in mucosal innate immunity. NOD2 and Atg16L1, both vitamin D target genes, are required for autophagy in IECs. Therefore, we investigated the effects of active vitamin D3 on autophagy expression in Salmonella-infected IECs.
Previous study has demonstrated that 1,25-dihydroxyvitamin D3 (1,25D3) up-regulates NOD2 mRNA expressions in Salmonella-infected IECs and that the interaction of NOD2 and Arg16L1 plays a critical role in Salmonella-induced autophagy in IECs. However, the effect of vitamin D on Atg16L1-mediated autophagy and inflammation in Salmonella-infected IECs is not clear.
This is the first study to demonstrate the different effects of vitamin D on autophagy and IL-1β expression in Salmonella-infected IECs via Atg16L1 and VDR.
This study not only provides the mechanistic pathway for the effect of active vitamin D on Salmonella colitis but a rationale for its alternative therapy for invasive bacterial infection and other inflammatory disorders.
Autophagy is a unique process of membrane trafficking in which the membrane compartment (autophagosomes) engulfs both organelles and cytosolic macromolecules and delivers them to the lysosome for degradation. It is a well-characterized process that protects host cells from nutrient deprivation and other cellular stresses.
The authors demonstrated that active vitamin D enhances autophagy but suppresses inflammatory IL-1β expression in Salmonella-infected Caco-2. These results are interesting and novel. Previous studies have established the relationship between vitamin D, vitamin D receptor (VDR), NOD2 and Atg16L1. However, the effect of vitamin D on autophagy and inflammation in Salmonella-infected IECs via Atg16L1 and VDR has not been elucidated.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Rigby RG S- Editor: Yu J L- Editor: Filipodia E- Editor: Wang CH
| 1. | Glynn MK, Bopp C, Dewitt W, Dabney P, Mokhtar M, Angulo FJ. Emergence of multidrug-resistant Salmonella enterica serotype typhimurium DT104 infections in the United States. N Engl J Med. 1998;338:1333-1338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 362] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 2. | Parry CM. Antimicrobial drug resistance in Salmonella enterica. Curr Opin Infect Dis. 2003;16:467-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Lauderdale TL, Aarestrup FM, Chen PC, Lai JF, Wang HY, Shiau YR, Huang IW, Hung CL. Multidrug resistance among different serotypes of clinical Salmonella isolates in Taiwan. Diagn Microbiol Infect Dis. 2006;55:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | White JH. Vitamin D signaling, infectious diseases, and regulation of innate immunity. Infect Immun. 2008;76:3837-3843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Nicholson I, Dalzell AM, El-Matary W. Vitamin D as a therapy for colitis: a systematic review. J Crohns Colitis. 2012;6:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Sanjuan MA, Milasta S, Green DR. Toll-like receptor signaling in the lysosomal pathways. Immunol Rev. 2009;227:203-220. [PubMed] |
| 7. | Ogawa M, Yoshimori T, Suzuki T, Sagara H, Mizushima N, Sasakawa C. Escape of intracellular Shigella from autophagy. Science. 2005;307:727-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 632] [Cited by in RCA: 660] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 8. | Birmingham CL, Brumell JH. Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy. 2010;2:156-158. [PubMed] |
| 9. | Cooney R, Baker J, Brain O, Danis B, Pichulik T, Allan P, Ferguson DJ, Campbell BJ, Jewell D, Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 821] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 10. | Travassos LH, Carneiro LA, Ramjeet M, Hussey S, Kim YG, Magalhães JG, Yuan L, Soares F, Chea E, Le Bourhis L. Nod1 and Nod2 direct autophagy by recruiting ATG16L1 to the plasma membrane at the site of bacterial entry. Nat Immunol. 2010;11:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1014] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 11. | Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285:2227-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 281] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 12. | Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909-2912. [PubMed] |
| 13. | Sun J. VDR/vitamin D receptor regulates autophagic activity through ATG16L1. Autophagy. 2016;12:1057-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 14. | Wu S, Zhang YG, Lu R, Xia Y, Zhou D, Petrof EO, Claud EC, Chen D, Chang EB, Carmeliet G. Intestinal epithelial vitamin D receptor deletion leads to defective autophagy in colitis. Gut. 2015;64:1082-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 15. | Conway KL, Kuballa P, Song JH, Patel KK, Castoreno AB, Yilmaz OH, Jijon HB, Zhang M, Aldrich LN, Villablanca EJ. Atg16l1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology. 2013;145:1347-1357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 16. | Huang FC. The differential effects of 1,25-dihydroxyvitamin D3 on Salmonella-induced interleukin-8 and human beta-defensin-2 in intestinal epithelial cells. Clin Exp Immunol. 2016;185:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Homer CR, Richmond AL, Rebert NA, Achkar JP, McDonald C. ATG16L1 and NOD2 interact in an autophagy-dependent antibacterial pathway implicated in Crohn’s disease pathogenesis. Gastroenterology. 2010;139:1630-141, 1630-141. [PubMed] |
| 18. | Huang FC. De Novo sphingolipid synthesis is essential for Salmonella-induced autophagy and human beta-defensin 2 expression in intestinal epithelial cells. Gut Pathog. 2016;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Huang FC. The critical role of membrane cholesterol in salmonella-induced autophagy in intestinal epithelial cells. Int J Mol Sci. 2014;15:12558-12572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Huang FC. Differential regulation of interleukin-8 and human beta-defensin 2 in Pseudomonas aeruginosa-infected intestinal epithelial cells. BMC Microbiol. 2014;14:275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Huang FC. Regulation of Salmonella flagellin-induced interleukin-8 in intestinal epithelial cells by muramyl dipeptide. Cell Immunol. 2012;278:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Assa A, Vong L, Pinnell LJ, Avitzur N, Johnson-Henry KC, Sherman PM. Vitamin D deficiency promotes epithelial barrier dysfunction and intestinal inflammation. J Infect Dis. 2014;210:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 23. | Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T, Natsume T, Ohsumi Y, Yoshimori T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J Cell Sci. 2003;116:1679-1688. [PubMed] |
| 24. | Savva A, Plantinga TS, Kotanidou A, Farcas M, Baziaka F, Raftogiannis M, Orfanos SE, Dimopoulos G, Netea MG, Giamarellos-Bourboulis EJ. Association of autophagy-related 16-like 1 (ATG16L1) gene polymorphism with sepsis severity in patients with sepsis and ventilator-associated pneumonia. Eur J Clin Microbiol Infect Dis. 2014;33:1609-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Jess T, Simonsen J, Nielsen NM, Jørgensen KT, Bager P, Ethelberg S, Frisch M. Enteric Salmonella or Campylobacter infections and the risk of inflammatory bowel disease. Gut. 2011;60:318-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 26. | Lapaquette P, Darfeuille-Michaud A. Abnormalities in the handling of intracellular bacteria in Crohn’s disease. J Clin Gastroenterol. 2010;44 Suppl 1:S26-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Kuballa P, Huett A, Rioux JD, Daly MJ, Xavier RJ. Impaired autophagy of an intracellular pathogen induced by a Crohn’s disease associated ATG16L1 variant. PLoS One. 2008;3:e3391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 28. | Messer JS, Murphy SF, Logsdon MF, Lodolce JP, Grimm WA, Bartulis SJ, Vogel TP, Burn M, Boone DL. The Crohn’s disease: associated ATG16L1 variant and Salmonella invasion. BMJ Open. 2013;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Billmann-Born S, Lipinski S, Böck J, Till A, Rosenstiel P, Schreiber S. The complex interplay of NOD-like receptors and the autophagy machinery in the pathophysiology of Crohn disease. Eur J Cell Biol. 2011;90:593-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Wiese RJ, Uhland-Smith A, Ross TK, Prahl JM, DeLuca HF. Up-regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization. J Biol Chem. 1992;267:20082-20086. [PubMed] |
| 31. | Mangelsdorf DJ, Pike JW, Haussler MR. Avian and mammalian receptors for 1,25-dihydroxyvitamin D3: in vitro translation to characterize size and hormone-dependent regulation. Proc Natl Acad Sci USA. 1987;84:354-358. [PubMed] |
| 32. | Sun J, Mustafi R, Cerda S, Chumsangsri A, Xia YR, Li YC, Bissonnette M. Lithocholic acid down-regulation of NF-kappaB activity through vitamin D receptor in colonic cancer cells. J Steroid Biochem Mol Biol. 2008;111:37-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Li YC, Chen Y, Du J. Critical roles of intestinal epithelial vitamin D receptor signaling in controlling gut mucosal inflammation. J Steroid Biochem Mol Biol. 2015;148:179-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Kong J, Zhang Z, Musch MW, Ning G, Sun J, Hart J, Bissonnette M, Li YC. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008;294:G208-G216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 503] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 35. | Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 36. | Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648-2652. [PubMed] |
| 37. | Meeker S, Seamons A, Maggio-Price L, Paik J. Protective links between vitamin D, inflammatory bowel disease and colon cancer. World J Gastroenterol. 2016;22:933-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 90] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 38. | Zella LA, Kim S, Shevde NK, Pike JW. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol. 2006;20:1231-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Chen Y, Liu W, Sun T, Huang Y, Wang Y, Deb DK, Yoon D, Kong J, Thadhani R, Li YC. 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J Immunol. 2013;190:3687-3695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 177] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 40. | Sorbara MT, Ellison LK, Ramjeet M, Travassos LH, Jones NL, Girardin SE, Philpott DJ. The protein ATG16L1 suppresses inflammatory cytokines induced by the intracellular sensors Nod1 and Nod2 in an autophagy-independent manner. Immunity. 2013;39:858-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 41. | Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1664] [Article Influence: 97.9] [Reference Citation Analysis (0)] |
| 42. | Plantinga TS, Crisan TO, Oosting M, van de Veerdonk FL, de Jong DJ, Philpott DJ, van der Meer JW, Girardin SE, Joosten LA, Netea MG. Crohn’s disease-associated ATG16L1 polymorphism modulates pro-inflammatory cytokine responses selectively upon activation of NOD2. Gut. 2011;60:1229-1235. [PubMed] |
| 43. | McAlindon ME, Hawkey CJ, Mahida YR. Expression of interleukin 1 beta and interleukin 1 beta converting enzyme by intestinal macrophages in health and inflammatory bowel disease. Gut. 1998;42:214-219. [PubMed] |
| 44. | Ludwiczek O, Vannier E, Borggraefe I, Kaser A, Siegmund B, Dinarello CA, Tilg H. Imbalance between interleukin-1 agonists and antagonists: relationship to severity of inflammatory bowel disease. Clin Exp Immunol. 2004;138:323-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Cominelli F, Nast CC, Clark BD, Schindler R, Lierena R, Eysselein VE, Thompson RC, Dinarello CA. Interleukin 1 (IL-1) gene expression, synthesis, and effect of specific IL-1 receptor blockade in rabbit immune complex colitis. J Clin Invest. 1990;86:972-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 260] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Liu W, Chen Y, Golan MA, Annunziata ML, Du J, Dougherty U, Kong J, Musch M, Huang Y, Pekow J. Intestinal epithelial vitamin D receptor signaling inhibits experimental colitis. J Clin Invest. 2013;123:3983-3996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 270] [Article Influence: 22.5] [Reference Citation Analysis (0)] |