Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.9933
Peer-review started: August 27, 2016
First decision: September 6, 2016
Revised: September 30, 2016
Accepted: November 12, 2016
Article in press: November 16, 2016
Published online: December 7, 2016
Processing time: 103 Days and 18.8 Hours
Hepatocellular carcinoma (HCC) is one of the most lethal cancers, and its rate of incidence is rising annually. Despite the progress in diagnosis and treatment, the overall prognoses of HCC patients remain dismal due to the difficulties in early diagnosis and the high level of tumor invasion, metastasis and recurrence. It is urgent to explore the underlying mechanism of HCC carcinogenesis and progression to find out the specific biomarkers for HCC early diagnosis and the promising target for HCC chemotherapy. Recently, the reprogramming of cancer metabolism has been identified as a hallmark of cancer. The shift from the oxidative phosphorylation metabolic pathway to the glycolysis pathway in HCC meets the demands of rapid cell proliferation and offers a favorable microenvironment for tumor progression. Such metabolic reprogramming could be considered as a critical link between the different HCC genotypes and phenotypes. The regulation of metabolic reprogramming in cancer is complex and may occur via genetic mutations and epigenetic modulations including oncogenes, tumor suppressor genes, signaling pathways, noncoding RNAs, and glycolytic enzymes etc. Understanding the regulatory mechanisms of glycolysis in HCC may enrich our knowledge of hepatocellular carcinogenesis and provide important foundations in the search for novel diagnostic biomarkers and promising therapeutic targets for HCC.
Core tip: The reprogramming of glucose metabolism is one of the peculiar characteristics of cancer cells. This paper addresses the regulatory mechanism of glucose metabolism in hepatocellular carcinoma (HCC) and prospects its future application for HCC treatment.
- Citation: Shang RZ, Qu SB, Wang DS. Reprogramming of glucose metabolism in hepatocellular carcinoma: Progress and prospects. World J Gastroenterol 2016; 22(45): 9933-9943
- URL: https://www.wjgnet.com/1007-9327/full/v22/i45/9933.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i45.9933
Hepatocellular carcinoma is the second leading cause of cancer-related death in the world, responsible for approximately 700000 deaths annually[1]. Although many treatment options have been developed and used in the clinic, including hepatic resection, local ablation, liver transplantation and molecular targeted therapies, patients’ prognoses remain poor[2]. Etiological studies of HCC revealed that hepatitis viruses, alcohol and aflatoxin might be the main risk factors for HCC[3]. In different areas of the world, HCC caused by these risk factors alone or together exhibits great diversity in genotype and phenotype, which impede the research of HCC. One remarkable feature of HCC is the alteration of glucose metabolism, which may be a critical link between the different HCC genotypes and phenotypes. Thus, a thorough understanding of cancer metabolism may offer promising therapeutic strategies for HCC in the future.
As early as the 1950s, Otto Heinrich Warburg first characterized cancer cell metabolism. Cancer cells principally use the glycolysis pathway to metabolize glucose and generate ATP whether there is sufficient oxygen present. This phenomenon now referred to as the “Warburg effect” was described and lead to a wave of investigation of cancer metabolism over several decades[4]. In the 1980s, the availability of 18F-deoxyglucose positron emission tomography (FDG-PET) pushed the study of tumor metabolism to the climax[5]. Observations from FDG-PET scanning revealed that approximately 50%-70% ATP was generated by glycolysis in different tumor types[6-8]. The application of FDG-PET was also recently involved in the detection and monitoring of metastasis and the recurrence of HCC and for prediction of patient’s prognosis[9-12]. Moreover, recent studies of metabolomics offer new mechanistic insights into aerobic glycolysis and provide promising individualized therapeutic strategies by targeting the Warburg effect for treatment of HCC[13,14].
In this article, we will review the recent investigations of glucose metabolism in HCC and summarize the regulation methods of metabolic reprogramming. Moreover, we will describe the development of therapy by targeting cancer metabolism.
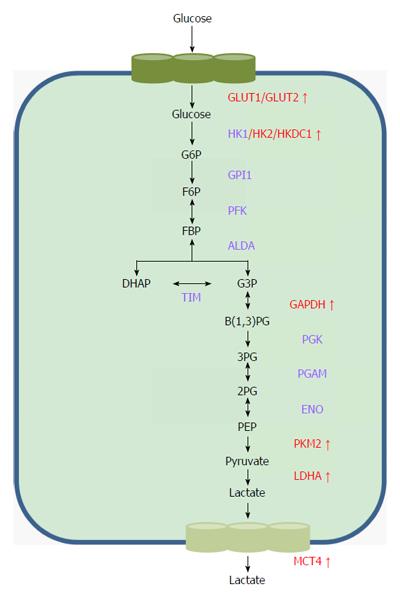
As previously described, tumor cells rely on the aerobic glycolysis pathway to consume glucose and generate ATP, which is a rapid but low-efficiency metabolic process[15]. To meet the demands of energy, biosynthesis and redox for tumor progression, cancer cells reprogram their metabolic related enzymes and transporting proteins to facilitate increased glucose uptake, acceleration of glycolysis and metabolic end-product excretion (Figure 1).
The initial step of glycolysis is the transportation of glucose across the plasma membrane into the cytoplasm, which depends on the family of glucose transporters (GLUTs)[16]. Much evidence has shown that GLUT1-4, particularly GLUT1, are often aberrantly expressed in different cancer types and significantly influence cancer glucose metabolism[17-21]. Amann et al[22] observed that both mRNA and protein expression levels of GLUT1 were significantly up-regulated in HCC, and this plays a critical role in glucose transport, glycolysis and tumor progression in HCC cells. Daskalow et al[23] analyzed GLUT2 expression in 60 HCC samples and revealed the over-expression of GLUT2 in HCC. Another study demonstrated that positive GLUT2 predicts worse prognosis in HCC patients[24]. To the best of our knowledge, studies of GLUT3 and GLUT4 in HCC have not been conducted.
Several glycolysis-related key enzymes have been demonstrated to participate glycolysis and carcinogenesis in HCC. Hexokinase (HK) family members catalyze the first key step of glycolysis in which glucose is phosphorylated to become glucose 6-phosphate (G-6-P). In the HK family, HK2 shows the highest affinity for glucose and is up-regulated in HCC and correlated with poor prognosis[25]. PET-CT scans showed that over-expression of HK2 promotes the uptake of 18FDG in HCC cells[26], which suggested that HK2 has a critical role in HCC glycolysis. The latest study showed that HKDC1, a newly discovered HK family member, was up-regulated in HCC with poorer prognosis and inhibited HCC cellular proliferating and migration in vitro, probably by repression of the Wnt/beta-catenin pathway[27]. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) may also play an important role in HCC glycolysis. GAPDH used to be regarded as a stably expressed gene and was commonly used as a reference gene in the past. Recent studies have reported the aberrant expression of GAPDH in malignancies and raised the concern that it may play a role in tumor glycolysis[28]. Gong et al[29] showed that increased expression of GAPDH promoted glycolysis and tumor progression in HCC. Moreover, GAPDH was able to affect glycolysis via regulating metabolism-related pathways such as the mammalian target of rapamycin (mTOR)-complex1 (mTOR-C1) signaling pathway[30]. Pyruvate kinases (PKs) catalyze the last step of glycolysis to produce ATP and pyruvate, which regulates the influx of the glycolysis pathway together with HK and phosphofructokinase-1. PKs contain 4 isoforms (PKL, PKR, PKM1 and PKM2) that are encoded by the PKL and PKM genes. PKL and PKR are mainly expressed in liver cells and erythrocytes, respectively, whereas PKM1 is constitutively expressed in normal cells. The over-expression of PKM2 was frequently observed in malignances and predicts worse prognosis[31,32]. A recent study demonstrated that the expression of PKM2 is up-regulated in HCC and is a predictor of survival and recurrence[33]. Dong et al[34] revealed the oncogenic role of PKM2 in HCC proliferation by its regulation of the expression of HIF-1α and Bcl-xL. Another study further observed that PKM2 effects on cell growth depend on a glucose rather than glutaminolysis pathways by using PKM2 knockdown-sensitive HCC cells. Additionally, the switching from PKL to PKM2 was reported to promote the rate of glucose uptake and increase the oxidative stress in hepatocarcinogenesis[35]. Lactate dehydrogenase (LDH) catalyzes the conversion of pyruvate to lactate. Up-regulation of the LDHA subunit in cancers has been noticed due to its role in promoting glycolysis and reducing the oxygen dependency of cancer cells[36,37]. A recent study has indicated that LDHA is up-regulated in HCC cells and promotes tumor growth and metastasis[38]. A series of clinical studies assessed the serum levels of LDH in HCC patients who were treated with hepatic resection[39,40], transarterial chemoembolization[41,42] and sorafenib[43,44] and found a similar conclusion that LDH may be an easily obtained biomarker for prognosis prediction and treatment selection for HCC patients.
Activation of the glycolysis pathway in cancer cells not only provides sufficient ATP for tumor progression but also produces acid by-products such as lactate. To avoid apoptosis caused by the accumulation of acids in cells, the monocarboxylate transporters (MCTs) are up-regulated in cancer cells to speed up the export of lactate into the extracellular milieu. Aberrant expression of isoforms MCT1, MCT2 and MCT4 was frequently observed in many cancers including colorectal carcinoma[45], glioblastoma[46] and gallbladder cancer[47]. The role of over-expressed MCT4 in HCC has been illustrated. It is associated with HCC progression and poor prognosis[48,49]. The latest study observed the reduced expression of MCT1 and MCT2 in HCC[50]. However, the data from another study showed that MCT1 was over-expressed in HCC cells which facilitates the lactate exporting and promotes HCC glycolysis[51]. Therefore, further studies are still needed to illuminate the specific role of MCT1 and MCT2 in HCC glycolysis and progression.
Oncogenes are a number of important genes which are over-expressed or mutated in cancer cells that triggered the tumor initiation and maintained the tumor progression. Based on the biological functions, oncogenes are usually classified as growth factors, receptor tyrosine kinases, cytoplasmic tyrosine kinase, regulatory GTPase and transcription factors. The activation of oncogenes is complex and may be attributed to the genetic mutations and the tumor microenvironment. Hypoxic microenvironment is a crucial factor in the activation of some oncogenes. The lack of sufficient blood supply in rapidly proliferating tumor cells leads to hypoxia. HIF-1 is a key transcription factor that is activated in response to oxygen deprivation. In cancer cells, HIF-1 promotes glycolysis by activating glycolytic enzymes[52]. Over-expression of HIF-1 was observed in HCC samples[53] and was shown to promote cell proliferation and resistance to apoptosis by up-regulating FOXM1 expression[54]. Hamaguchi et al[55] analyzed 22 glycolysis-related genes in HCC samples and identified 10 potential transcriptional targets of HIF-1α including HK1, HK2, GAPDH and PKM. Interestingly, several studies showed that HIF-1 could be activated by Ras[56] and membrane type-1 matrix metalloproteinase[57] under normoxic conditions, which may provide new insights into cancer glycolysis regulation beyond the hypoxic microenvironment. Myc is another crucial oncogene involved in the Warburg effect. As a vital transcription factor, Myc was first linked with glucose metabolism through its transactivation of LDHA expression[58]. A series of glycolytic enzymes were subsequently identified as direct targets of Myc, including GLUT1 and HK2[59,60]. Moreover, the interplay between Myc and HIF-1 has also been observed, which indicates that Myc may play a complementary role in cancer metabolism under non-hypoxic conditions[61,62]. CD147 (Basigin) is a transmembrane protein that is highly expressed in tumors. A number of studies have shown that CD147 is a “Warburg oncogene” due to its pivotal role in promoting glycolysis and inhibiting oxidative phosphorylation in cancer cells[63,64]. In HCC, CD147 was reported to reprogram glucose metabolism by facilitating lactate export, mediated by MCT1, and promoting glucose uptake by up-regulating GLUT1 expression[51]. Recently, some newly discovered oncogenes were also reported to play important roles in HCC glycolysis. For instance, PIM1 is involved in both aerobic and anaerobic glycolysis by targeting GLUT1 and PKM2[65].
Likewise, tumor suppressor genes also have a great influence on cancer glycolysis. The role of the p53 tumor suppressor gene in cancer metabolism could be summarized as promoting oxidative phosphorylation and reducing glycolysis. The effect of p53 on glycolysis mainly depends on the reduced expression of glucose transporters[66]. Recently, we investigated the role of the tumor suppressor gene Ras-related associated with diabetes (RRAD) in HCC. We found RRAD could suppress the invasion, migration and aerobic glycolysis in HCC cells and identified GLUT1 and HK2 as potential targets for RRAD[67]. Our results were recently verified by Yan et al[68] (Table 1).
| Genes | Targets | Ref. | |
| Oncogenes | HIF-1 | HK1 | [55] |
| HK2 | [55] | ||
| GAPDH | [55] | ||
| PKM | [55] | ||
| Myc | LDHA | [58] | |
| GLUT1 | [59] | ||
| HK2 | [60] | ||
| CD147 | MCT1 | [62] | |
| GLUT1 | [62] | ||
| PIM1 | GLUT1 | [65] | |
| PKM2 | [65] | ||
| Tumor suppressor | P53 | GLUTs | [66] |
| genes | RRAD | GLUT1 | [67,68] |
| HK2 | [67] |
AMPK pathway: The AMP-activated protein kinase (AMPK) is ubiquitously expressed in eukaryotes and acts as an energy status sensor and regulator of energy homeostasis[69]. The activation of AMPK by energetic stress promotes the switching from glycolysis to oxidative phosphorylation. This switching inhibits the “Warburg effect” in rapidly proliferating cells, including tumor cells to spare glucose and restore energy homeostasis[70]. At the same time, the activation of AMPK shuts down the synthesis of RNA, DNA, protein and lipid to inhibit the cell proliferation and growth. The downstream effect of AMPK activation on cancer metabolism has been well established. mTOR is a crucial downstream modulator of AMPK signaling in cancer cells. AMPK inhibits the activity of mTOR either directly or by reducing the activity of the mTOR-activating GTP-binding protein, Rheb, via activation of the Tuberous sclerosis complex 2[71-73]. Inactivation of mTOR suppresses the expression of HIF-1α, a key regulator of glycolysis, as mentioned previously[52,74]. Recently, several reviews highlighted the regulatory role of AMPK on GLUT4 membrane translocation and GLUT1 activation in skeletal muscle cells and other normal cells[69,75]. In HCC, AMPK signaling pathway was reported to participate in the ciliary neurotrophic factor induced GLUT4 translocation and glucose uptake[76]. Considering the effect of AMPK on the inhibition of glucose uptake in transformed cells, further investigations are greatly needed to clarify the role of AMPK on glucose transporters and glycolytic enzymes in cancer cells.
PI3K/Akt/mTOR pathway: The PI3K/Akt pathway is one of the most frequently activated signaling pathways in human cancers including HCC. The PI3K/Akt pathway can be activated by mutated tumor suppressor genes, signaling from receptor tyrosine kinases, or by the PI3K components[77]. The activation of the PI3K/Akt pathway is involved in cell proliferation, cell survival, cell cycle progression and cancer metabolism[78]. Regulation of glucose metabolism by PI3K/Akt signaling is mediated by glycolytic enzymes. Firstly, PI3K/Akt promotes glucose uptake in cells by increasing the membrane translocation and expression of GLUT4[79,80]. In addition, PI3K/Akt promotes glycolysis by activating HK and by the binding of HK2 to the voltage-dependent anion channel in mitochondria[81,82]. Moreover, PI3K/Akt could regulate glycolytic enzymes indirectly by regulating the expression of AMPK and HIF-1[83,84].
Noncoding RNAs are functional RNAs that are not transcribed into proteins. In the past, noncoding RNAs have been regarded as the “noise” in transcription processes. However, accumulating evidence has suggested the indispensable role of noncoding RNAs in various biological processes including gene transcription and translation. Noncoding RNAs, especially microRNAs (miRNAs) and long noncoding RNAs (lncRNAs) are also reported to be involved in the Warburg effect. The regulatory mechanism of noncoding RNAs in aerobic glycolysis consists of the following two aspects: the regulation of glycolytic enzyme expression and the activation of glycolysis-related oncogenic pathways (Table 2).
| Targets | noncoding RNAs | Ref. | |
| Enzymes | GLUT1 | miR-340 | [85] |
| miR-1291 | [86] | ||
| miR-22 | [87] | ||
| miR-144 | [88,89] | ||
| GLUT3 | miR-195-5p | [90] | |
| GLUT4 | miR-133 | [91] | |
| miR-223 | [92] | ||
| HK2 | miR-143 | [93-95] | |
| miR-155/ miR-143 | [96] | ||
| miR-34a | [102,103] | ||
| PKM2 | miR-122 | [98] | |
| miR-133-a/b | [99] | ||
| miR-326 | [100] | ||
| PFK | miR-52s | [101] | |
| LDHA | miR-34a | [102,103] | |
| MCT1 | miR-199a-3p | [104] | |
| Pathways | AMPK | miR-451 | [111-113] |
| miR-195 | [112] | ||
| PI3K/Akt/mTOR | miR-125a | [114] | |
| miR-7 | [115] |
Noncoding RNAs were reported to regulate glucose uptake in cancer cells by targeting expression of GLUTs. MicroRNA-340, which increases the glucose uptake and lactate secretion by increasing the expression of GLUT1, was decreased in oral squamous cell carcinoma[85]. Yamasaki et al[86] evaluated the role of microRNA-1291 in renal cell carcinoma and found that reduced expression of miR-1291 promotes cancer cell proliferation and invasion and migration by direct targeting of SLCA1/GLUT1. Chen et al[87] demonstrated that miR-22 regulates GLUT1 expression and inhibits the proliferation and invasion of breast cancer. MicroRNA-144 was also reported to mediate the metabolic shift by regulating GLUT1 expression in lung and ovarian cancers[88,89]. Moreover, miR-195-5p inhibits the glucose uptake by down-regulating GLUT3 expression and thus reduces proliferation in bladder cancer cells[90]. The expression of GLUT4 is also regulated by microRNAs, including miR-113[91] and miR-223[92]. MicroRNA-143 is a key regulator of HK2 in cancer. Studies have shown that miR-143 negatively regulates the expression of HK2 and thus modulates glycolysis in colon cancer[93], lung cancer[94] and head and neck squamous cell carcinoma[95]. In breast cancer cells, HK2 was regulated by the miR-155/miR-143 cascade at the post-transcriptional level[96]. Burchard et al[97] showed the up-regulation of miR-122 reduced lactate production and increased oxygen consumption in HCC. A subsequent study further demonstrated that miR-122 reduced the expression of PKM2 and thus repressed glycolytic activities[98]. Other microRNAs, including miR-133a/b and miR-326, were reported to regulate PKM2 expression in cancers[99,100]. PFK catalyzes the conversion from fructose-6-phosphate to fructose-1, 6-bisphosphate and is over-expressed in cancers. A recent study showed that the miR-52 family mediated the regulation of Tat-activating regulatory DNA-binding protein on PFK in HCC[101]. Some microRNAs were able to regulate multiple glycolytic enzymes. For instance, miR-34a was reported to regulate key enzymes including HK1, HK2, GPI, LDHA and PDK1[102,103]. Additionally, miR-199a-3p serves an important role in the aerobic glycolysis of testicular germ cell tumors by targeting MCT1 and PGK1[104].
Noncoding RNAs were able to regulate cancer metabolism by interactions with oncogenes (tumor suppressor genes) and oncogenic pathways. LncRNA-p21 was first discovered as a p53-inducible lncRNA that mediates p53-related apoptosis in mouse cells[105]. In cancer cells, the hypoxia-induced LncRNA-p21 was shown to be a direct transcriptional target of HIF-1α and in turn promoted the stability of HIF-1α by interfering with the VHL-HIF-1α association. The hypoxic microenvironment and the reciprocal regulation of p21 and HIF-1α constructs a positive-feedback loop leading to continual activation of GLUT1 and LDHA expression thus accelerating glycolysis in cancer cells[106]. In another study published in 2011, Bruning et al[107] evaluated the interaction between HIF-1α and miR-155 and proposed that miR-155 contributes to a negative-feedback loop for the degradation of HIF-1α-dependent transcription, under continuous hypoxic conditions. The tumor suppressor gene p53 is one the most frequent targets of microRNAs and LncRNAs. MicroRNAs including miR-125b, miR-504 and miR-1228 can regulate p53 expression by directly binding to sites in p53 3’-UTR[108,109]. It is worth noting that the over-expression of miR-1228 can negatively regulate p53 expression, and the down-regulation of p53, in turn, increases miR-1228 expression. This positive-feedback loop contributes to the progression of HCC[110]. Studies also showed that oncogenic pathways are regulated by noncoding-RNAs. Down-regulation of miR-451 was originally linked with cancer glycolysis through its contributions to the adaptation to glucose deprivation and its effect on the LKB1/AMPK pathway in glioma cells[111]. A further study confirmed that the regulation of the LKB1/AMPK pathway by miR-451 is mediated by MO25 (an upstream modulator of AMPK)[112]. Another study discovered a novel reciprocal negative-feedback loop that consists of OCT1, AMPK and miR-451 in glioblastoma multiforme. Briefly, under the conditions of glucose deprivation, the activation of AMPK inactivated OCT1, which subsequently reduced the level of miR-451, and conversely, sufficient glucose supply significantly increased miR-451 expression, which in turn impaired the activity of the AMPK pathway[113]. Moreover, microRNAs can regulate the PI3K/Akt/mTOR pathway in HCC. Tang et al[114] reported that miR-125a suppress HCC progression by inhibiting the PI3K/Akt pathway. Fang et al[115] investigated the molecular mechanism of miR-7 in HCC growth and metastasis and revealed the regulatory role of miR-1 in the PI3K/Akt pathway via targeting PIK3CD, mTOR and p70S6K.
The metabolic shift from oxidative phosphorylation to aerobic glycolysis in HCC not only provides abundant ATP for sustaining tumor survival but also offers a favorable microenvironment for tumor progression. As one of the “hallmarks” of cancer, metabolic reprogramming relies on metabolic enzymes, thus providing many potential targets that could be exploited in HCC therapy.
Flavonoids are safe and reliable agents that are extracted from natural products, which show a broad spectrum of biological activities with fewer side effects[116]. Phloretin is a natural phenol which could be extracted from manchurian apricot and apple tree leaves. Studies showed the ability of phloretin to suppress cell proliferation and induce apoptosis by inhibiting glucose uptake in cancers[117,118]. Wu et al[117] showed that the inhibition of GLUT2 by phloretin leads to apoptosis in HCC cells. Another study demonstrated that phloretin strengthens the anticancer effects of paclitaxel in HCC[119]. Another natural compound, silybin, was identified as a GLUT inhibitor and showed a significant inhibitory effect on HCC growth in vivo[120,121]. Moreover, a phase I clinical study of silybin-phosphatidylcholine has been conducted in advanced HCC[122]. Quercetin is another bioactive flavonoid which has been proposed as a promising anticancer agent[123]. The latest study showed that quercetin might be a potential agent in HCC therapy that induced apoptosis and metabolic inhibition by competitively inhibiting GLUT1[124].
2-deoxy-D-glucose (2-DG) is a glucose analog that is frequently used in inhibiting glycolysis. The phosphorylation of 2-DG catalyzed by HK2 in turn noncompetitively inhibits the activity of HK2 and leads to the reduction of the glycolytic rate. Several studies showed increased apoptosis induced by 2-DG in cancer, including HCC[125,126]. However, normal cells are only arrested in G1 phase of mitosis when treated with 2-DG[127]. 3-bromopyruvate (3-BP) is a halogenated analog that suppresses the glycolytic pathway by directly inhibiting HK2 activity. A study performed on a rabbit VX2 tumor model of liver cancer showed that 3-BP induced more efficient damage to hepatoma cells compared with 2-DG. Apart from the inhibition of HK, this study also revealed that 3-BP inhibits HCC glycolysis by suppressing mitochondrial ATP synthesis[128]. Based on these promising research achievements in vitro and in vivo, the orphan drug, 3-BP, has been designated for HCC by the FDA[13] and was reported to prolong the lifetime and improved the quality of life of a patient with HCC[14].
Metformin, a first-line anti-diabetic drug, was linked to cancer prevalence and therapy because diabetes mellitus is a risk factor for cancer death in some cancer types. The association between diabetes and HCC was evaluated in large populations in the 1990s[129,130]. Recent studies demonstrated that diabetes mellitus is an independent risk factor for HCC[131,132]. The preventive effect of metformin in HCC has been established. Studies showed a decreased incidence of HCC in the type 2 diabetic patients who received metformin therapy[133-135]. The results of a systematic review showed a direct anti-HCC effect of metformin in animal models[136]. The mechanism of metformin in HCC prevention and therapy in type 2 diabetic patients is closely linked with the AMPK pathway. Metformin activates the expression of LKB1 and AMPK by increasing the energy stress levels inside cells. The activated AMPK pathway reduced IRS-1 and caused the inhibition of insulin/IGF-1 signaling, which is involved in carcinogenesis and cancer glycolysis regulation[137]. Additionally, AMPK inactivated the downstream modulator, mTOR, which indirectly regulates glycolysis by targeting HIF-1α, as previously described.
The reprogramming of glucose metabolism in cancer is a multi-factor and multi-step process, which can be regulated by oncogenes, oncogenic signaling pathways, and even noncoding RNAs. The developments in the study of cancer metabolism greatly enriched the understanding of carcinogenesis and afforded numerous potential targets to hit the Achilles’ heel of cancer[138]. The agents that target glycolytic enzymes directly and glycolysis-related pathways indirectly showed some promising effects in HCC prevention and therapy in the laboratory. However, the limitation of glycolysis targeted anti-cancer therapy should be noted. As multiple enzymes catalyze multiple steps in the process, there is a complex compensatory mechanism in cancer metabolism. Therefore, the inhibitors that specifically target a single modulator of glycolysis may not have a prominent or persistent effect on cancer metabolism in the human body. In the future, the effects of combination drug therapy should be evaluated. Moreover, noncoding-RNAs, which target multiple glycolysis-related enzymes and pathways, are also needed to be carefully considered in future studies.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Chiu KW, Kobayashi Y, Zhang KZ S- Editor: Gong ZM L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25538] [Article Influence: 1824.1] [Reference Citation Analysis (7)] |
| 2. | Poon RT, Ng IO, Fan ST, Lai EC, Lo CM, Liu CL, Wong J. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001;19:3037-3044. [PubMed] |
| 3. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [PubMed] |
| 4. | Upadhyay M, Samal J, Kandpal M, Singh OV, Vivekanandan P. The Warburg effect: insights from the past decade. Pharmacol Ther. 2013;137:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 5. | Lemasters JJ, Holmuhamedov EL, Czerny C, Zhong Z, Maldonado EN. Regulation of mitochondrial function by voltage dependent anion channels in ethanol metabolism and the Warburg effect. Biochim Biophys Acta. 2012;1818:1536-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1235] [Cited by in RCA: 1162] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 7. | Mathupala SP, Ko YH, Pedersen PL. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin Cancer Biol. 2009;19:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 450] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 8. | Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029-1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12491] [Cited by in RCA: 11782] [Article Influence: 736.4] [Reference Citation Analysis (0)] |
| 9. | Choi SH, Chang JS, Jeong YH, Lee Y, Yun M, Seong J. FDG-PET predicts outcomes of treated bone metastasis following palliative radiotherapy in patients with hepatocellular carcinoma. Liver Int. 2014;34:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Hayakawa N, Nakamoto Y, Nakatani K, Hatano E, Seo S, Higashi T, Saga T, Uemoto S, Togashi K. Clinical utility and limitations of FDG PET in detecting recurrent hepatocellular carcinoma in postoperative patients. Int J Clin Oncol. 2014;19:1020-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Kamaleshwaran KK, Kashyap R, Bhattacharya A, Mittal BR. Solitary sternal metastasis from hepatocellular carcinoma detected by F-18 FDG PET/CT. Indian J Nucl Med. 2013;28:28-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Pant V, Sen IB, Soin AS. Role of 18F-FDG PET CT as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Geschwind JF, Georgiades CS, Ko YH, Pedersen PL. Recently elucidated energy catabolism pathways provide opportunities for novel treatments in hepatocellular carcinoma. Expert Rev Anticancer Ther. 2004;4:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Ko YH, Verhoeven HA, Lee MJ, Corbin DJ, Vogl TJ, Pedersen PL. A translational study “case report” on the small molecule “energy blocker” 3-bromopyruvate (3BP) as a potent anticancer agent: from bench side to bedside. J Bioenerg Biomembr. 2012;44:163-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3445] [Cited by in RCA: 3711] [Article Influence: 265.1] [Reference Citation Analysis (0)] |
| 16. | Macheda ML, Rogers S, Best JD. Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J Cell Physiol. 2005;202:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 896] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 17. | Szablewski L. Expression of glucose transporters in cancers. Biochim Biophys Acta. 2013;1835:164-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 266] [Article Influence: 22.2] [Reference Citation Analysis (1)] |
| 18. | Shimada Y, Sawada S, Hojo S, Okumura T, Nagata T, Nomoto K, Tsukada K. Glucose transporter 3 and 1 may facilitate high uptake of 18F-FDG in gastric schwannoma. Clin Nucl Med. 2013;38:e417-e420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Shen YM, Arbman G, Olsson B, Sun XF. Overexpression of GLUT1 in colorectal cancer is independently associated with poor prognosis. Int J Biol Markers. 2011;26:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Ozerlat I. Kidney cancer: targeted therapy of glucose uptake via GLUT1 kills RCC cells. Nat Rev Urol. 2011;8:471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Younes M, Juarez D, Lechago LV, Lerner SP. Glut 1 expression in transitional cell carcinoma of the urinary bladder is associated with poor patient survival. Anticancer Res. 2001;21:575-578. [PubMed] |
| 22. | Amann T, Maegdefrau U, Hartmann A, Agaimy A, Marienhagen J, Weiss TS, Stoeltzing O, Warnecke C, Schölmerich J, Oefner PJ. GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am J Pathol. 2009;174:1544-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 289] [Article Influence: 18.1] [Reference Citation Analysis (1)] |
| 23. | Daskalow K, Pfander D, Weichert W, Rohwer N, Thelen A, Neuhaus P, Jonas S, Wiedenmann B, Benckert C, Cramer T. Distinct temporospatial expression patterns of glycolysis-related proteins in human hepatocellular carcinoma. Histochem Cell Biol. 2009;132:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Paudyal B, Paudyal P, Oriuchi N, Tsushima Y, Nakajima T, Endo K. Clinical implication of glucose transport and metabolism evaluated by 18F-FDG PET in hepatocellular carcinoma. Int J Oncol. 2008;33:1047-1054. [PubMed] |
| 25. | Gong L, Cui Z, Chen P, Han H, Peng J, Leng X. Reduced survival of patients with hepatocellular carcinoma expressing hexokinase II. Med Oncol. 2012;29:909-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Ahn KJ, Hwang HS, Park JH, Bang SH, Kang WJ, Yun M, Lee JD. Evaluation of the role of hexokinase type II in cellular proliferation and apoptosis using human hepatocellular carcinoma cell lines. J Nucl Med. 2009;50:1525-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Zhang Z, Huang S, Wang H, Wu J, Chen D, Peng B, Zhou Q. High expression of hexokinase domain containing 1 is associated with poor prognosis and aggressive phenotype in hepatocarcinoma. Biochem Biophys Res Commun. 2016;474:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Guo C, Liu S, Sun MZ. Novel insight into the role of GAPDH playing in tumor. Clin Transl Oncol. 2013;15:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Gong Y, Cui L, Minuk GY. Comparison of glyceraldehyde-3-phosphate dehydrogenase and 28s-ribosomal RNA gene expression in human hepatocellular carcinoma. Hepatology. 1996;23:734-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Ganapathy-Kanniappan S, Kunjithapatham R, Geschwind JF. Glyceraldehyde-3-phosphate dehydrogenase: a promising target for molecular therapy in hepatocellular carcinoma. Oncotarget. 2012;3:940-953. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | Mazurek S, Boschek CB, Hugo F, Eigenbrodt E. Pyruvate kinase type M2 and its role in tumor growth and spreading. Semin Cancer Biol. 2005;15:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 616] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 32. | Wu J, Hu L, Chen M, Cao W, Chen H, He T. Pyruvate kinase M2 overexpression and poor prognosis in solid tumors of digestive system: evidence from 16 cohort studies. Onco Targets Ther. 2016;9:4277-4288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Chen Z, Lu X, Wang Z, Jin G, Wang Q, Chen D, Chen T, Li J, Fan J, Cong W. Co-expression of PKM2 and TRIM35 predicts survival and recurrence in hepatocellular carcinoma. Oncotarget. 2015;6:2538-2548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 34. | Dong T, Yan Y, Chai H, Chen S, Xiong X, Sun D, Yu Y, Deng L, Cheng F. Pyruvate kinase M2 affects liver cancer cell behavior through up-regulation of HIF-1α and Bcl-xL in culture. Biomed Pharmacother. 2015;69:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 35. | Wong CC, Au SL, Tse AP, Xu IM, Lai RK, Chiu DK, Wei LL, Fan DN, Tsang FH, Lo RC. Switching of pyruvate kinase isoform L to M2 promotes metabolic reprogramming in hepatocarcinogenesis. PLoS One. 2014;9:e115036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Le A, Cooper CR, Gouw AM, Dinavahi R, Maitra A, Deck LM, Royer RE, Vander Jagt DL, Semenza GL, Dang CV. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci USA. 2010;107:2037-2042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 1137] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 37. | Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1226] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 38. | Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP, Huang G. Knockdown of lactate dehydrogenase A suppresses tumor growth and metastasis of human hepatocellular carcinoma. FEBS J. 2012;279:3898-3910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 211] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 39. | Wang ZX, Jiang CP, Cao Y, Zhang G, Chen WB, Ding YT. Preoperative serum liver enzyme markers for predicting early recurrence after curative resection of hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2015;14:178-185. [PubMed] |
| 40. | Hu WJ, Fu SJ, Diao JF, Wan DW, Tan ZJ, Zhi QM, He JM. The prognostic value of serum LDH levels in patients with hepatocellualr carcinoma after hepatic resection. Anticancer Agents Med Chem. 2015; Epub ahead of print. [PubMed] |
| 41. | Kohles N, Nagel D, Jüngst D, Durner J, Stieber P, Holdenrieder S. Prognostic relevance of oncological serum biomarkers in liver cancer patients undergoing transarterial chemoembolization therapy. Tumour Biol. 2012;33:33-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 42. | Scartozzi M, Faloppi L, Bianconi M, Giampieri R, Maccaroni E, Bittoni A, Del Prete M, Loretelli C, Belvederesi L, Svegliati Baroni G. The role of LDH serum levels in predicting global outcome in HCC patients undergoing TACE: implications for clinical management. PLoS One. 2012;7:e32653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Fiume L, Vettraino M, Manerba M, Di Stefano G. Inhibition of lactic dehydrogenase as a way to increase the anti-proliferative effect of multi-targeted kinase inhibitors. Pharmacol Res. 2011;63:328-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 44. | Faloppi L, Scartozzi M, Bianconi M, Svegliati Baroni G, Toniutto P, Giampieri R, Del Prete M, De Minicis S, Bitetto D, Loretelli C. The role of LDH serum levels in predicting global outcome in HCC patients treated with sorafenib: implications for clinical management. BMC Cancer. 2014;14:110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 45. | Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA, Schmitt F, Baltazar F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch. 2008;452:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Mathupala SP, Parajuli P, Sloan AE. Silencing of monocarboxylate transporters via small interfering ribonucleic acid inhibits glycolysis and induces cell death in malignant glioma: an in vitro study. Neurosurgery. 2004;55:1410-149; discussion 1419. [PubMed] |
| 47. | Shang RZ, Dai B, Ruan B, Sun W, Wang JL, Dou KF, Li Y, Wang DS. Expression of monocarboxylate transporters in gallbladder cancer and their prognostic clinical significance. Int J Clin Exp Pathol. 2016;9:1901-1908. |
| 48. | Ohno A, Yorita K, Haruyama Y, Kondo K, Kato A, Ohtomo T, Kawaguchi M, Marutuska K, Chijiiwa K, Kataoka H. Aberrant expression of monocarboxylate transporter 4 in tumour cells predicts an unfavourable outcome in patients with hepatocellular carcinoma. Liver Int. 2014;34:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 49. | Gao HJ, Zhao MC, Zhang YJ, Zhou DS, Xu L, Li GB, Chen MS, Liu J. Monocarboxylate transporter 4 predicts poor prognosis in hepatocellular carcinoma and is associated with cell proliferation and migration. J Cancer Res Clin Oncol. 2015;141:1151-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 50. | Alves VA, Pinheiro C, Morais-Santos F, Felipe-Silva A, Longatto-Filho A, Baltazar F. Characterization of monocarboxylate transporter activity in hepatocellular carcinoma. World J Gastroenterol. 2014;20:11780-11787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (2)] |
| 51. | Huang Q, Li J, Xing J, Li W, Li H, Ke X, Zhang J, Ren T, Shang Y, Yang H. CD147 promotes reprogramming of glucose metabolism and cell proliferation in HCC cells by inhibiting the p53-dependent signaling pathway. J Hepatol. 2014;61:859-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 52. | Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269:23757-23763. [PubMed] |
| 53. | Zhang FJ, Tang WX, Wu CH, Yan W, Gu H. [Expression and significance of hypoxia inducible factor-1 alpha in hepatocellular carcinoma tissues]. Zhonghua Gan Zang Bing Za Zhi. 2006;14:281-284. [PubMed] |
| 54. | Xia L, Mo P, Huang W, Zhang L, Wang Y, Zhu H, Tian D, Liu J, Chen Z, Zhang Y. The TNF-α/ROS/HIF-1-induced upregulation of FoxMI expression promotes HCC proliferation and resistance to apoptosis. Carcinogenesis. 2012;33:2250-2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 55. | Hamaguchi T, Iizuka N, Tsunedomi R, Hamamoto Y, Miyamoto T, Iida M, Tokuhisa Y, Sakamoto K, Takashima M, Tamesa T. Glycolysis module activated by hypoxia-inducible factor 1alpha is related to the aggressive phenotype of hepatocellular carcinoma. Int J Oncol. 2008;33:725-731. [PubMed] |
| 56. | Chen C, Pore N, Behrooz A, Ismail-Beigi F, Maity A. Regulation of glut1 mRNA by hypoxia-inducible factor-1. Interaction between H-ras and hypoxia. J Biol Chem. 2001;276:9519-9525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 577] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 57. | Sakamoto T, Niiya D, Seiki M. Targeting the Warburg effect that arises in tumor cells expressing membrane type-1 matrix metalloproteinase. J Biol Chem. 2011;286:14691-14704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 58. | Shim H, Dolde C, Lewis BC, Wu CS, Dang G, Jungmann RA, Dalla-Favera R, Dang CV. c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci USA. 1997;94:6658-6663. [PubMed] |
| 59. | Kim JW, Gao P, Liu YC, Semenza GL, Dang CV. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol Cell Biol. 2007;27:7381-7393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 508] [Cited by in RCA: 504] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 60. | Osthus RC, Shim H, Kim S, Li Q, Reddy R, Mukherjee M, Xu Y, Wonsey D, Lee LA, Dang CV. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J Biol Chem. 2000;275:21797-21800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 61. | Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8:51-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 462] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 62. | Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479-6483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 710] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 63. | Le Floch R, Chiche J, Marchiq I, Naiken T, Ilc K, Murray CM, Critchlow SE, Roux D, Simon MP, Pouysségur J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci USA. 2011;108:16663-16668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 342] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 64. | Calvisi DF. CD147/Basigin: a Warburg oncogene in hepatocellular carcinoma? Chin J Cancer Res. 2016;28:377-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Leung CO, Wong CC, Fan DN, Kai AK, Tung EK, Xu IM, Ng IO, Lo RC. PIM1 regulates glycolysis and promotes tumor progression in hepatocellular carcinoma. Oncotarget. 2015;6:10880-10892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Watanabe M, Naraba H, Sakyo T, Kitagawa T. DNA damage-induced modulation of GLUT3 expression is mediated through p53-independent extracellular signal-regulated kinase signaling in HeLa cells. Mol Cancer Res. 2010;8:1547-1557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 67. | Shang R, Wang J, Sun W, Dai B, Ruan B, Zhang Z, Yang X, Gao Y, Qu S, Lv X. RRAD inhibits aerobic glycolysis, invasion, and migration and is associated with poor prognosis in hepatocellular carcinoma. Tumour Biol. 2016;37:5097-5105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 68. | Yan Y, Xie M, Zhang L, Zhou X, Xie H, Zhou L, Zheng S, Wang W. Ras-related associated with diabetes gene acts as a suppressor and inhibits Warburg effect in hepatocellular carcinoma. Onco Targets Ther. 2016;9:3925-3937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2774] [Cited by in RCA: 3434] [Article Influence: 264.2] [Reference Citation Analysis (0)] |
| 70. | Hardie DG. Molecular Pathways: Is AMPK a Friend or a Foe in Cancer? Clin Cancer Res. 2015;21:3836-3840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 71. | Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926-1945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3080] [Cited by in RCA: 3271] [Article Influence: 155.8] [Reference Citation Analysis (0)] |
| 72. | Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577-590. [PubMed] |
| 73. | Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 1278] [Article Influence: 91.3] [Reference Citation Analysis (0)] |
| 74. | Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 687] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 75. | Grahame Hardie D. AMP-activated protein kinase: a key regulator of energy balance with many roles in human disease. J Intern Med. 2014;276:543-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 76. | Hu X, Zhao Y, He X, Li J, Wang T, Zhou W, Wan D, Wang H, Gu J. Ciliary neurotrophic factor receptor alpha subunit-modulated multiple downstream signaling pathways in hepatic cancer cell lines and their biological implications. Hepatology. 2008;47:1298-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 77. | Wong KK, Engelman JA, Cantley LC. Targeting the PI3K signaling pathway in cancer. Curr Opin Genet Dev. 2010;20:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 433] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 78. | Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892-3899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1033] [Cited by in RCA: 1107] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 79. | Kohn AD, Summers SA, Birnbaum MJ, Roth RA. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem. 1996;271:31372-31378. [PubMed] |
| 80. | Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 860] [Cited by in RCA: 874] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 81. | Gottlob K, Majewski N, Kennedy S, Kandel E, Robey RB, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 724] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 82. | Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683-4696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 410] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 83. | Hahn-Windgassen A, Nogueira V, Chen CC, Skeen JE, Sonenberg N, Hay N. Akt activates the mammalian target of rapamycin by regulating cellular ATP level and AMPK activity. J Biol Chem. 2005;280:32081-32089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 437] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 84. | Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 766] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 85. | Xu P, Li Y, Zhang H, Li M, Zhu H. MicroRNA-340 Mediates Metabolic Shift in Oral Squamous Cell Carcinoma by Targeting Glucose Transporter-1. J Oral Maxillofac Surg. 2016;74:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 86. | Yamasaki T, Seki N, Yoshino H, Itesako T, Yamada Y, Tatarano S, Hidaka H, Yonezawa T, Nakagawa M, Enokida H. Tumor-suppressive microRNA-1291 directly regulates glucose transporter 1 in renal cell carcinoma. Cancer Sci. 2013;104:1411-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 87. | Chen B, Tang H, Liu X, Liu P, Yang L, Xie X, Ye F, Song C, Xie X, Wei W. miR-22 as a prognostic factor targets glucose transporter protein type 1 in breast cancer. Cancer Lett. 2015;356:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 88. | Liu M, Gao J, Huang Q, Jin Y, Wei Z. Downregulating microRNA-144 mediates a metabolic shift in lung cancer cells by regulating GLUT1 expression. Oncol Lett. 2016;11:3772-3776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 89. | Fan JY, Yang Y, Xie JY, Lu YL, Shi K, Huang YQ. MicroRNA-144 mediates metabolic shift in ovarian cancer cells by directly targeting Glut1. Tumour Biol. 2016;37:6855-6860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Fei X, Qi M, Wu B, Song Y, Wang Y, Li T. MicroRNA-195-5p suppresses glucose uptake and proliferation of human bladder cancer T24 cells by regulating GLUT3 expression. FEBS Lett. 2012;586:392-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 91. | Horie T, Ono K, Nishi H, Iwanaga Y, Nagao K, Kinoshita M, Kuwabara Y, Takanabe R, Hasegawa K, Kita T. MicroRNA-133 regulates the expression of GLUT4 by targeting KLF15 and is involved in metabolic control in cardiac myocytes. Biochem Biophys Res Commun. 2009;389:315-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 92. | Lu H, Buchan RJ, Cook SA. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc Res. 2010;86:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 93. | Gregersen LH, Jacobsen A, Frankel LB, Wen J, Krogh A, Lund AH. MicroRNA-143 down-regulates Hexokinase 2 in colon cancer cells. BMC Cancer. 2012;12:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 94. | Fang R, Xiao T, Fang Z, Sun Y, Li F, Gao Y, Feng Y, Li L, Wang Y, Liu X. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol Chem. 2012;287:23227-23235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 95. | Peschiaroli A, Giacobbe A, Formosa A, Markert EK, Bongiorno-Borbone L, Levine AJ, Candi E, D’Alessandro A, Zolla L, Finazzi Agrò A. miR-143 regulates hexokinase 2 expression in cancer cells. Oncogene. 2013;32:797-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 96. | Jiang S, Zhang LF, Zhang HW, Hu S, Lu MH, Liang S, Li B, Li Y, Li D, Wang ED. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012;31:1985-1998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 296] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 97. | Burchard J, Zhang C, Liu AM, Poon RT, Lee NP, Wong KF, Sham PC, Lam BY, Ferguson MD, Tokiwa G. microRNA-122 as a regulator of mitochondrial metabolic gene network in hepatocellular carcinoma. Mol Syst Biol. 2010;6:402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 98. | Liu AM, Xu Z, Shek FH, Wong KF, Lee NP, Poon RT, Chen J, Luk JM. miR-122 targets pyruvate kinase M2 and affects metabolism of hepatocellular carcinoma. PLoS One. 2014;9:e86872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 99. | Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing Yuen A, Wai-Man Ng R, Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int J Cancer. 2008;123:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 100. | Kefas B, Comeau L, Erdle N, Montgomery E, Amos S, Purow B. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro Oncol. 2010;12:1102-1112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 101. | Park YY, Kim SB, Han HD, Sohn BH, Kim JH, Liang J, Lu Y, Rodriguez-Aguayo C, Lopez-Berestein G, Mills GB. Tat-activating regulatory DNA-binding protein regulates glycolysis in hepatocellular carcinoma by regulating the platelet isoform of phosphofructokinase through microRNA 520. Hepatology. 2013;58:182-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 102. | Kaller M, Liffers ST, Oeljeklaus S, Kuhlmann K, Röh S, Hoffmann R, Warscheid B, Hermeking H. Genome-wide characterization of miR-34a induced changes in protein and mRNA expression by a combined pulsed SILAC and microarray analysis. Mol Cell Proteomics. 2011;10:M111.010462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 103. | Kim HR, Roe JS, Lee JE, Cho EJ, Youn HD. p53 regulates glucose metabolism by miR-34a. Biochem Biophys Res Commun. 2013;437:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 104. | Liu X, Duan H, Zhou S, Liu Z, Wu D, Zhao T, Xu S, Yang L, Li D. microRNA-199a-3p functions as tumor suppressor by regulating glucose metabolism in testicular germ cell tumors. Mol Med Rep. 2016;14:2311-2320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 105. | Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1745] [Cited by in RCA: 1704] [Article Influence: 113.6] [Reference Citation Analysis (0)] |
| 106. | Yang F, Zhang H, Mei Y, Wu M. Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the Warburg effect. Mol Cell. 2014;53:88-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 432] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 107. | Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol. 2011;31:4087-4096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 244] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 108. | Le MT, Teh C, Shyh-Chang N, Xie H, Zhou B, Korzh V, Lodish HF, Lim B. MicroRNA-125b is a novel negative regulator of p53. Genes Dev. 2009;23:862-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 527] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 109. | Hu W, Chan CS, Wu R, Zhang C, Sun Y, Song JS, Tang LH, Levine AJ, Feng Z. Negative regulation of tumor suppressor p53 by microRNA miR-504. Mol Cell. 2010;38:689-699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 245] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 110. | Zhang Y, Dai J, Deng H, Wan H, Liu M, Wang J, Li S, Li X, Tang H. miR-1228 promotes the proliferation and metastasis of hepatoma cells through a p53 forward feedback loop. Br J Cancer. 2015;112:365-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 111. | Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37:620-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 348] [Cited by in RCA: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 112. | Chen H, Untiveros GM, McKee LA, Perez J, Li J, Antin PB, Konhilas JP. Micro-RNA-195 and -451 regulate the LKB1/AMPK signaling axis by targeting MO25. PLoS One. 2012;7:e41574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 113. | Ansari KI, Ogawa D, Rooj AK, Lawler SE, Krichevsky AM, Johnson MD, Chiocca EA, Bronisz A, Godlewski J. Glucose-based regulation of miR-451/AMPK signaling depends on the OCT1 transcription factor. Cell Rep. 2015;11:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 114. | Tang H, Li RP, Liang P, Zhou YL, Wang GW. miR-125a inhibits the migration and invasion of liver cancer cells via suppression of the PI3K/AKT/mTOR signaling pathway. Oncol Lett. 2015;10:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 115. | Fang Y, Xue JL, Shen Q, Chen J, Tian L. MicroRNA-7 inhibits tumor growth and metastasis by targeting the phosphoinositide 3-kinase/Akt pathway in hepatocellular carcinoma. Hepatology. 2012;55:1852-1862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 346] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 116. | Liao CY, Lee CC, Tsai CC, Hsueh CW, Wang CC, Chen IH, Tsai MK, Liu MY, Hsieh AT, Su KJ. Novel Investigations of Flavonoids as Chemopreventive Agents for Hepatocellular Carcinoma. Biomed Res Int. 2015;2015:840542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 117. | Wu CH, Ho YS, Tsai CY, Wang YJ, Tseng H, Wei PL, Lee CH, Liu RS, Lin SY. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int J Cancer. 2009;124:2210-2219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 118. | Kobori M, Shinmoto H, Tsushida T, Shinohara K. Phloretin-induced apoptosis in B16 melanoma 4A5 cells by inhibition of glucose transmembrane transport. Cancer Lett. 1997;119:207-212. [PubMed] |
| 119. | Yang KC, Tsai CY, Wang YJ, Wei PL, Lee CH, Chen JH, Wu CH, Ho YS. Apple polyphenol phloretin potentiates the anticancer actions of paclitaxel through induction of apoptosis in human hep G2 cells. Mol Carcinog. 2009;48:420-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 120. | Zhan T, Digel M, Küch EM, Stremmel W, Füllekrug J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J Cell Biochem. 2011;112:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 121. | Cui W, Gu F, Hu KQ. Effects and mechanisms of silibinin on human hepatocellular carcinoma xenografts in nude mice. World J Gastroenterol. 2009;15:1943-1950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 50] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Siegel AB, Narayan R, Rodriguez R, Goyal A, Jacobson JS, Kelly K, Ladas E, Lunghofer PJ, Hansen RJ, Gustafson DL. A phase I dose-finding study of silybin phosphatidylcholine (milk thistle) in patients with advanced hepatocellular carcinoma. Integr Cancer Ther. 2014;13:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 123. | Chen C, Zhou J, Ji C. Quercetin: a potential drug to reverse multidrug resistance. Life Sci. 2010;87:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 124. | Brito AF, Ribeiro M, Abrantes AM, Mamede AC, Laranjo M, Casalta-Lopes JE, Gonçalves AC, Sarmento-Ribeiro AB, Tralhão JG, Botelho MF. New Approach for Treatment of Primary Liver Tumors: The Role of Quercetin. Nutr Cancer. 2016;68:250-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 125. | Aft RL, Zhang FW, Gius D. Evaluation of 2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell death. Br J Cancer. 2002;87:805-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 227] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 126. | Zhang Y, Huang F, Wang J, Luo H, Wang Z. 2-DG-Regulated RIP and c-FLIP Effect on Liver Cancer Cell Apoptosis Induced by TRAIL. Med Sci Monit. 2015;21:3442-3448. [PubMed] |
| 127. | Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68-72. [PubMed] |
| 128. | Ko YH, Pedersen PL, Geschwind JF. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001;173:83-91. [PubMed] |
| 129. | Adami HO, Chow WH, Nyrén O, Berne C, Linet MS, Ekbom A, Wolk A, McLaughlin JK, Fraumeni JF. Excess risk of primary liver cancer in patients with diabetes mellitus. J Natl Cancer Inst. 1996;88:1472-1477. [PubMed] |
| 130. | Wideroff L, Gridley G, Mellemkjaer L, Chow WH, Linet M, Keehn S, Borch-Johnsen K, Olsen JH. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360-1365. [PubMed] |
| 131. | El-Serag HB, Hampel H, Javadi F. The association between diabetes and hepatocellular carcinoma: a systematic review of epidemiologic evidence. Clin Gastroenterol Hepatol. 2006;4:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 581] [Cited by in RCA: 601] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 132. | Koh WP, Wang R, Jin A, Yu MC, Yuan JM. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer. 2013;108:1182-1188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 133. | Donadon V, Balbi M, Ghersetti M, Grazioli S, Perciaccante A, Della Valentina G, Gardenal R, Dal Mas M, Casarin P, Zanette G. Antidiabetic therapy and increased risk of hepatocellular carcinoma in chronic liver disease. World J Gastroenterol. 2009;15:2506-2511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 109] [Cited by in RCA: 117] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 134. | Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int. 2010;30:750-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 135. | Hassan MM, Curley SA, Li D, Kaseb A, Davila M, Abdalla EK, Javle M, Moghazy DM, Lozano RD, Abbruzzese JL. Association of diabetes duration and diabetes treatment with the risk of hepatocellular carcinoma. Cancer. 2010;116:1938-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 241] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 136. | Li J, Hernanda PY, Bramer WM, Peppelenbosch MP, van Luijk J, Pan Q. Anti-tumor effects of metformin in animal models of hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One. 2015;10:e0127967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 137. | Takano A, Usui I, Haruta T, Kawahara J, Uno T, Iwata M, Kobayashi M. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol Cell Biol. 2001;21:5050-5062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 203] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 138. | Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1553] [Cited by in RCA: 1702] [Article Influence: 100.1] [Reference Citation Analysis (0)] |