Published online Dec 7, 2016. doi: 10.3748/wjg.v22.i45.10071
Peer-review started: April 27, 2016
First decision: June 20, 2016
Revised: July 6, 2016
Accepted: August 5, 2016
Article in press: August 5, 2016
Published online: December 7, 2016
Processing time: 223 Days and 11.7 Hours
Commercial dietary supplements are marketed as a panacea for the morbidly obese seeking sustainable weight-loss. Unfortunately, many claims cited by supplements are unsupported and inadequately regulated. Most concerning, however, are the associated harmful side effects, often unrecognized by consumers. Garcinia cambogia extract and Garcinia cambogia containing products are some of the most popular dietary supplements currently marketed for weight loss. Here, we report the first known case of fulminant hepatic failure associated with this dietary supplement. One active ingredient in this supplement is hydroxycitric acid, an active ingredient also found in weight-loss supplements banned by the Food and Drug Administration in 2009 for hepatotoxicity. Heightened awareness of the dangers of dietary supplements such as Garcinia cambogia is imperative to prevent hepatoxicity and potential fulminant hepatic failure in additional patients.
Core tip: The current regulatory practice for over-the-counter dietary supplements in addition to celebrity endorsements of these products unfounded claims has resulted in a significant increase in the use of dietary supplements for weight loss. Unfortunately, several such products have previously been demonstrated to be serious health risks. Here we present one of the first known cases of fulminant hepatic failure associated with one such popular weight loss supplement, Garcinia cambogia.
- Citation: Lunsford KE, Bodzin AS, Reino DC, Wang HL, Busuttil RW. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J Gastroenterol 2016; 22(45): 10071-10076
- URL: https://www.wjgnet.com/1007-9327/full/v22/i45/10071.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i45.10071
Dietary supplements are an increasingly recognized cause of acute liver injury and fulminant hepatic failure. Under the Dietary Supplement Health and Education Act of 1994, supplements, unlike prescription and over-the-counter medications, require proven toxicity prior to FDA sanctions[1]. The Drug Induced Liver Injury Network (DILIN) identifies dietary supplements among the most common causes of drug-induced hepatotoxicity. Nearly a quarter of cases suffer irreversible liver damage, resulting in potential liver transplant (4%) and death (6%)[2]. Evaluation of dietary supplement-induced hepatoxicity is difficult due to wide formulaic variations, and ineffectual federal manufacturing oversight allows contamination by alfatoxins, microorganisms, pesticides, heavy metals, and synthetic drugs. These contaminants have known hepatotoxicity and may contribute to detrimental effects[3]. In addition, following formal FDA citation, a supplement may be remarketed after minor reformulation and/or rebranding.
Of particular note has been hepatotoxicity associated with several different brands of “fat busters”. Commercial fat-burning dietary supplements are widely marketed as “miracle-cures” for obesity on major network television shows with celebrity endorsements. Supplements are advertised to stimulate weight loss by increasing the body’s basal metabolic rate; however, campaigns are bereft of associated side effects (reviewed in[3,4]). Multiple companies manufacture supplements of the same name with different composition, contaminants, and concentrations of active ingredients, potentially resulting in variable hepatotoxicity. Effort made by the FDA to collect data regarding toxicities through “Safety Reporting Portal” in the MedWatch system[5] is reliant upon consumer and industry reporting compliance. As a result, recognition of toxicities may arise slowly.
Garcinia cambogia (G. cambogia) containing products are currently one of the most highly marketed group of weight-loss supplements commercially available. The supplement is derived from the rind of the fruit of the Garcinia cambogia tree, which is native to southwestern India. It has gained significant acclaim for its weight-loss benefits through mainstream talk shows and medical media celebrity spokespeople. Here, we report the first known case of fulminant hepatic failure associated with dietary intake of a “pure”Garcinia cambogia supplement.
A 34-year old Hispanic male presented with nausea, vomiting, abdominal pain, and dark urine. Testing revealed elevated transaminases and bilirubin; however, imaging failed to demonstrate cirrhosis or anatomic abnormality. Hepatitis work-up, including testing for viral hepatitis, hemochromatosis, Wilson’s disease, and autoimmune hepatitis, was unremarkable with exception of an elevated Ferritin level of 7089 mg/dL. Genetic testing for hemochromatosis was negative. Medical history was only positive for occasional social alcohol use, and drug toxicology testing was negative. He denied use of energy drinks, herbs, Chinese teas, or muscle milk. He was advised to discontinue alcohol use, which he did, and his symptoms initially seemed to abate.
Six weeks later, the patient developed asterixis, jaundice, and confusion. Follow-up imaging was concerning for rapid onset of cirrhosis or infiltrative hepatocellular carcinoma. He was transferred to our center for further evaluation. On admission, transaminases were elevated with aspartate aminotransferase (AST) 624 U/L, alanine aminotransferase (ALT) 520 U/L and total bilirubin of 34.7 mg/dL. International normalized ratio (INR) remained elevated despite multiple infusions of fresh frozen plasma and vitamin K. Factor Vand VII activities were 18% and < 6%, respectively. Magnetic resonance imaging (MRI) with Eovist contrast demonstrated interval development of heterogeneous, enhancing nodularity with portal venous washout, unlikely to be an infiltrative tumor process.
A full repeat hepatitis work-up was performed (Table 1). No definitive cause of acute liver failure could be identified; however, some findings were equivocal. Autoantibody titers demonstrated a positive antinuclear antibody, but no other positive autoantibodies. Evaluation of Wilson’s disease demonstrated normal ceruloplasmin and copper levels; however, 24-h urine copper was elevated. Serum ferritin but not transferrin was elevated.
| Laboratory test | Result | Reference range |
| Hepatic function panel | ||
| Aspartate aminotransferase | 624 U/L (H) | 7-36 U/L |
| Alanine aminotransferase | 520 U/L (H) | 4-45 U/L |
| Alkaline phosphatase | 156 U/L (H) | 31-103 U/L |
| Bilirubin, total | 34.7 mg/dL (H) | 0.2-1.1 mg/dL |
| Bilirubin, conjugated | 14.8 mg/dL (H) | 0.0-0.2 mg/dL |
| Albumin | 3.6 g/dL (L) | 3.7-5.1 g/dL |
| Total Protein | 5.8 g/dL (L) | 6.2-8.6 g/dL |
| Coagulation factors | ||
| Prothombin time | 37.9 s (H) | 9.1-11.9 s |
| INR | 3.5 (H) | < 1.2 |
| Factor VII activity | < 6% (L) | > 50% activity |
| Factor V activity | 18% (L) | > 50% activity |
| Tumor markers | ||
| CEA | 2.3 ng/mL | < 3.1 ng/mL |
| CA 19-9 | 235 U/mL (H) | 0-35 U/mL |
| AFP | 51.1 ng/mL (H) | 1.6-4.5 ng/mL |
| AFP-L3 | 19.0% (H) | 0.5%-9.9% |
| PIVKA | 4.4 ng/mL | < 6.3 ng/mL |
| Viral serologies | ||
| Hepatitis A, IgM | Nonreactive | Nonreactive |
| Hepatitis A, IgG | Reactive1 | Nonreactive |
| Hepatitis B surface antigen | Nonreactive | Nonreactive |
| Hepatitis B surface antibody, quantitative | < 10 IU/L | < 10 IU/L |
| Hepatitis B core antibody, total | Nonreactive | Nonreactive |
| Hepatitis C antibody screen | Nonreactive | Nonreactive |
| Hepatitis C RNA quantitative PCR | Not Detected | Not detected |
| Hepatitis E antibody, IgG | Not Detected | Not detected |
| Hepatitis E antibody, IgM | Not Detected | Not detected |
| CMV antibody immune status | Positive1 | Negative |
| CMV DNA quantitative PCR | Not Detected | Not detected |
| Liver tissue CMV in situ hybridization | Negative | Negative |
| EBV-VCA IgM | Negative | Negative |
| EBV-VCA IgG | Positive1 | Negative |
| EBV DNA quantitative PCR | Not Detected | Not detected |
| Liver tissue EBV in situ Hybridization | Negative | Negative |
| Adenovirus DNA Quantitative PCR | Not Detected | Not Detected |
| Liver tissue adenovirus in situ hybridization | Negative | Negative |
| Herpes Simplex 1 IgM screen | Negative | Negative |
| Herpes Simplex 2 IgM screen | Negative | Negative |
| Liver Tissue HSV 1 and 2 in situ hybridization | Negative | Negative |
| RPR | Nonreactive | Nonreactive |
| Autoantibody titer | ||
| Antinuclear antibody | Positive1 | Negative |
| Antinuclear antibody titer | 1:401 | < 1:20 |
| Smooth muscle antibody | < 1:20 | < 1:20 |
| Liver kidney microsome antibody IgG | < 20.0 U | < 20.0 U |
| Soluble liver antigen autoantibody | < 20.1 U | < 20.1 U |
| Wilson’s disease evaluation | ||
| Copper, RBC | 0.71 mg/L | 0.53-0.91 mg/L |
| Copper, serum | 95 μg/dL | 70-140 μg/dL |
| Ceruloplasmin | 22 mg/dL | 17-48 mg/dL |
| Copper, 24-h urine | 1055 μg/d (H) | 3-50 μg/day |
| Quantitative liver copper | 47 μg/g tissue | 10-55 μg/g tissue |
| Hemochromatosis evaluation | ||
| Total iron | 243 μg/dL (H)2 | 23-202 μg/dL |
| Iron binding capacity | < 308 μg/dL (L)3 | 240-520 μg/dL |
| Transferrin | 163 mg/dL (L) | 198-386 mg/dL |
| Ferritin | 3254 ng/mL (H) | 8-350 ng/mL |
| Alpha-1-antitrypsin | 91 mg/dL | 83-199 mg/dL |
| Acetaminophen | < 10 μg/mL | 10-20 μg/mL |
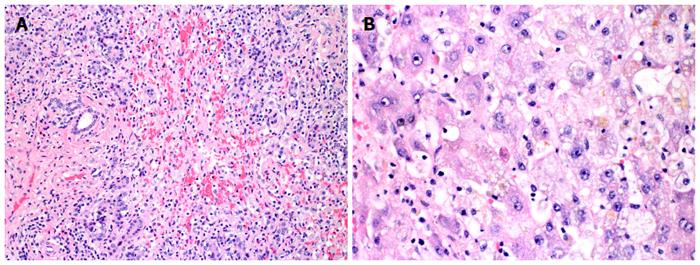
Liver biopsy was performed and demonstrated submassive necrosis with collapse of the hepatic architecture involving about 70% of the liver parenchyma. Mild lymphocytic inflammatory infiltration and minimal canalicular cholestasis were seen. No viral inclusions or other infectious agents were identified by histology or immunohistochemistry. No evidence of granuloma, tumor, or features of cirrhosis were demonstrated. Periodic acid-Schiff (PAS) stain with diastase was negative for alpha-1 antitrypsin globules. Iron stain showed only mild iron deposition in Kupffer cells and hepatocytes. Quantitative tissue copper was within normal limits (Table 1). Findings were felt to be potentially related to drug-induced liver injury.
After extensive questioning, the patient divulged intake of Garcinia cambogia, purchased through the Internet retailor Swanson Vitamins. He imbibed two 80 mg capsules of “Garcinia Cambogia 5:1 Extract” three times daily before meals for five months preceding initial presentation. Since not advised against intake, he continued the supplement after initial presentation. He denied any other medications or supplements and reported no alcohol intake for two months.
The patient’s status declined and his mental status deteriorated. He was listed status 1A for liver transplantation. He received an orthotopic liver transplant from an ABO-identical brain dead donor and has recovered without incident. Histopathologic examination of the explanted liver demonstrated near total hepatic necrosis with massive hepatocellular dropout and mixed inflammatory cell infiltrates, consistent with severe drug-induced liver injury (Figure 1).
Americans spent approximately 59.8 billion dollars on weight loss products in 2014[6], and an estimated 10.1% of obese Americans have purchased over-the-counter supplements for weight-loss. Unfortunately, utilization does not correlate with sustained weight-loss[7]. One product that has been heavily marketed as a “revolutionary fat buster” and a “magical ingredient” to promote weight loss is G. cambogia. This extract from the rind of the G. cambogia fruit is currently contained in 655 currently marketed products according to the Natural Medicine Comprehensive Database[8]. These include “purified” supplement pills, multivitamins, and even energy drinks with widely disparate compositions, dosage, and potential contaminants.
The particular brand of Garcinia cambogia in this case was “Swanson Premium Brand Garcinia Cambogia 5:1 Extract,” reported to contain 80mg of a 5:1 concentrate of G. cambogia (equivalent to 400mg of standard preparation). Other listed ingredients include rice flour, gelatin, magnesium stearate, and silica. The company reports that they do no assay for the hydroxycitric acid concentration, the fruit derivative reportedly responsible for weight-loss benefits of Garcinia cambogia[9]. This is of note since hydroxycitric acid is the main derivative thought to be responsible for the weight-loss benefits of Garcinia cambogia. Mechanistically, it acts to prevents citric acid metabolism resulting inhibition of de novo fatty acid synthesis[10].
G. cambogia was also a main active ingredient in the weight-loss supplement Hydroxycut® (Iovate Health Sciences, Inc., Oakville, ON), which has known hepatoxicity[11-16]. In May 2009, the FDA issued a consumer warning recalling all Hydroxycut® products due to 23 hepatotoxicity cases. Prior to 2004, the formulation also contained ephedra, which was removed following the FDA ban. However, ten of 23 cases of hepatotoxicity, including the patient death, occurred after the 2004 reformulation to remove ephedra[17]. G. cambogia was present in Hydroxycut® following the 2004 reformulation, but additional cases of hepatotoxicity occurred and a second FDA warning resulted in a second recall in 2009. The supplement was again reformulated and remarketed. G. cambogia is absent from the currently marketed formulations of Hydroxycut®.
Although G.cambogia has been suggested as the putative cause of the banned supplement’s hepatotoxic effects[16], there is no definitive evidence. The majority of G. cambogia formulation associated with hepatotoxicity have been mixed supplements were a definitive causal relation could not be drawn. However, in the past several months, several cases of G. cambogia associated acute liver failure have been reported[18,19], reinforcing the toxic potential of this particular supplement. Agreement upon the actual liver toxicity of G. cambogia has been mixed, and the majority of evidence is drawn from rodent models[20]. The product can induce liver inflammation, fibrosis, and oxidative stress in mice. In one such study, supplement intake increased collagen deposition, elevated liver function tests, induced inflammatory cytokines, and stimulated oxygen free-radicals[21]. However, supplement advocates cite rodent models in which G. cambogia demonstrates hepatoprotective effects, including decreased hyperlipidemia and hepatic oxidative stress in rats fed with a high-fat diet[22].
This is one of the first reported cases of acute liver failure specifically associated with a “purified” supplement of G. cambogia. The patient had histologic evidence of drug-induced liver injury in the absence of other medication or alcohol use. Viral, autoimmune, and genetic (i.e., hemochromatosis and Wilson’s disease) causes of acute liver failure were definitively ruled-out, and G. cambogia intake was the only apparent risk factor. Unfortunately, independent laboratory evaluation of the supplement was not performed, which could have identified potential contaminants and verified manufacturer reported composition. While evidence from a case report rarely offers indisputable proof of causality, this case, in conjunction with known cases of hepatotoxicity and liver failure associated with other G. cambogia-containing supplements warrants a high index of suspicion.
Conditions predisposing patients to liver toxicity associated with Garcinia cambogia and like products remain unidentified. Acute liver failure from supplement ingestion appears relatively rare compared to their widespread use. Certain patients may have genetic predisposition or pre-existing liver damage, compounding hepatotoxicity. Cytochrome P450 is most commonly responsible for hepatic metabolism of drugs, and genetic polymorphisms in cytochrome P450 genes have previously be shown to result in toxic accumulation of certain drugs or metabolites. For example, toxicity associated with weight-loss supplements containing N-nitrofenfluramine has been associated with cytochrome CYP2C19 phenotypes[23]. Mitochondrial injury, suggesting of toxic accumulation of N-nitrofenfluramine, was associated with a poor metabolizer phenotype; while, mitochondrial injury was absent in extensive metabolizers of the drug. One extensive metabolizer developed hypersensitivity-associated hepatitis related to drug ingestion; however, mitochondrial injury was absent. Toxicity to G. cambogia may have incomplete penetrance due to a similar dependence upon genetic polymorphisms. Alternatively, injury may be more likely as a second hit in the setting of pre-existing liver damage. At our institution, a second case of G. cambogia-associated acute liver failure was identified; however, the patient had a remote history of heavy binge drinking with final pathology suggestive of early fibrosis. Drug-induced hepatotoxicity could not be definitively diagnosed due to this history, but the association with ingestion of large quantities of G. cambogia was suspicious, given that the degree chronic liver disease insufficiently accounted for her acute hepatic failure.
While additional research is necessary to further identify the link between Garcinia Cambogia and severe liver damage, public warning to potentially deadly side effects is necessary. This case emphasizes the need for direct questioning regarding dietary supplement intake in any case of acute hepatic injury. Manufacturers compliance with current regulations regarding contaminants is insufficient to preclude consumer toxicity, and increased public awareness of these dangers is crucial. Current regulation and oversight of the dietary supplement market should be scrutinized to improve supplement purity and identification of dangers. Endorsements by medical media celebrities and claims of “miracle results” should be carefully monitored for veracity. This case bears concerning similarity to those of Hydroxycut-associated liver failure, suggesting that although the product name may change, deadly side effects remain the same.
The patient initially presented with symptoms of nausea, vomiting, abdominal pain, and symptoms progressed to include confusion, coagulopathy, and jaundice.
Evaluation for viral, genetic, and antibody mediated causes of hepatitis were largely negative with histological evidence of near total hepatic necrosis on biopsy.
Alternative autoimmune, viral, and genetic causes of acute liver failure must be excluded. In the absence of these, careful questioning regarding medications, herbal supplements, and energy drinks must be undertaken.
Negative laboratory evaluation for autoimmune, viral, and genetic causes of acute liver failure in the presence of elevated liver function tests and coagulopathy should raise clinical suspicion for drug-induced liver injury.
Magnetic resonance imaging with Eovist contrast demonstrated interval development of heterogeneous, enhancing nodularity with portal venous washout, unlikely to be an infiltrative tumor process.
Explant pathology demonstrated near total hepatic necrosis with massive hepatocellular dropout and mixed inflammatory cell infiltrates.
Patient received liver transplantation with complete resolution of symptoms.
Acute liver failure and severe hepatotoxicity has been associated multiple dietary supplements utilized for weight loss. These include the supplement Hydroxycut®, which was removed from the market by the FDA and has undergone several reformulations.
Garcinia cambogia - tree grown in southwestern India. Extracts from the rind of the fruit from this tree are high in hydroxycitric acid and are marketed as weight-loss supplements.
Careful questioning of any patient presenting with liver function abnormalities or acute liver failure should prompt questioning regarding dietary supplement and energy drink consumption. Patients with acute liver failure should be promptly referred to a transplant center for treatment.
This article presents one of the first known cases of hepatotoxicity associated with intake of the dietary supplement Garcinia cambogia. Reviewers felt the article was timely and well written. They requested additional information regarding dosage of the supplement as well as some additional discussion regarding its effects.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Arinc E, Shi YJ, Stawarska A S- Editor: Yu J L- Editor: A E- Editor: Liu WX
| 1. | Cohen PA. Hazards of hindsight--monitoring the safety of nutritional supplements. N Engl J Med. 2014;370:1277-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, Watkins PB, Navarro V, Barnhart H. Features and Outcomes of 899 Patients With Drug-Induced Liver Injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340-52.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 638] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 3. | De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046-2056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 352] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 4. | Chitturi S, Farrell GC. Hepatotoxic slimming aids and other herbal hepatotoxins. J Gastroenterol Hepatol. 2008;23:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | MarketData Enterprises I. The US Weight Loss Market: 2015 Status Report & Forcast. Tampa: FL 2015; 1-64. |
| 7. | Nicklas JM, Huskey KW, Davis RB, Wee CC. Successful weight loss among obese U.S. adults. Am J Prev Med. 2012;42:481-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 8. | GARCINIA: Natural Medicines Comprehensive Database. Access date March 24, 2015. Available from: http://naturaldatabase.therapeuticresearch.com. |
| 9. | Swanson Premium Garcinia Cambogia 5:1 Extract: Swanson Health Products. Access date March 24, 2015. Available from: http://www.swansonvitamins.com/swanson-premium-garcinia-cambogia-5-1-extract-80-mg-60-caps. |
| 10. | Heymsfield SB, Allison DB, Vasselli JR, Pietrobelli A, Greenfield D, Nunez C. Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. JAMA. 1998;280:1596-1600. [PubMed] |
| 11. | Kaswala D, Shah S, Patel N, Raisoni S, Swaminathan S. Hydroxycut-induced Liver Toxicity. Ann Med Health Sci Res. 2014;4:143-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Dara L, Hewett J, Lim JK. Hydroxycut hepatotoxicity: a case series and review of liver toxicity from herbal weight loss supplements. World J Gastroenterol. 2008;14:6999-7004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 101] [Cited by in RCA: 82] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Lobb A. Hepatoxicity associated with weight-loss supplements: a case for better post-marketing surveillance. World J Gastroenterol. 2009;15:1786-1787. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Stevens T, Qadri A, Zein NN. Two patients with acute liver injury associated with use of the herbal weight-loss supplement hydroxycut. Ann Intern Med. 2005;142:477-478. [PubMed] |
| 15. | Shim M, Saab S. Severe hepatotoxicity due to Hydroxycut: a case report. Dig Dis Sci. 2009;54:406-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Actis GC, Bugianesi E, Ottobrelli A, Rizzetto M. Fatal liver failure following food supplements during chronic treatment with montelukast. Dig Liver Dis. 2007;39:953-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Food and Drug Administration. Warning on Hydroxycut Products. : US Department of Health and Human Services 2009; . |
| 18. | Melendez-Rosado J, Snipelisky D, Matcha G, Stancampiano F. Acute hepatitis induced by pure Garcinia cambogia. J Clin Gastroenterol. 2015;49:449-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Corey R, Werner KT, Singer A, Moss A, Smith M, Noelting J, Rakela J. Acute liver failure associated with Garcinia cambogia use. Ann Hepatol. 2016;15:123-126. [PubMed] |
| 20. | Clouatre DL, Preuss HG. Hydroxycitric acid does not promote inflammation or liver toxicity. World J Gastroenterol. 2013;19:8160-8162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Kim YJ, Choi MS, Park YB, Kim SR, Lee MK, Jung UJ. Garcinia Cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J Gastroenterol. 2013;19:4689-4701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 22. | Sripradha R, Sridhar MG, Maithilikarpagaselvi N. Antihyperlipidemic and antioxidant activities of the ethanolic extract of Garcinia cambogia on high fat diet-fed rats. J Complement Integr Med. 2016;13:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Kawaguchi T, Harada M, Arimatsu H, Nagata S, Koga Y, Kuwahara R, Hisamochi A, Hino T, Taniguchi E, Kumemura H. Severe hepatotoxicity associated with a N-nitrosofenfluramine-containing weight-loss supplement: report of three cases. J Gastroenterol Hepatol. 2004;19:349-350. [PubMed] |