Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9803
Peer-review started: August 24, 2016
First decision: September 5, 2016
Revised: September 20, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: November 28, 2016
Processing time: 97 Days and 1.9 Hours
To investigate the clinicopathological features and prognostic implications of combined MYC and fibroblast growth factor receptor 1 (FGFR1) status in esophageal squamous cell carcinomas (ESCCs).
All patients with ESCC (n = 180) underwent surgical resection at Seoul National University Hospital sometime between 2000 and 2013. A tissue microarray was constructed using cores obtained from representative tumor areas of formalin-fixed, paraffin-embedded tissue blocks. FGFR1 and MYC copy numbers were quantified using fluorescence in situ hybridization. The level of MYC expression was determined using immunohistochemistry. FGFR1 and MYC amplification status was compared between primary and metastatic lymph nodes. Univariate and multivariate survival analyses were performed according to adjuvant therapy status.
FGFR1 and MYC amplifications were observed in 21.4% (37/173) and 54.2% (91/168) of patients, respectively, while MYC expression was observed in 58.9% (106/180) of patients. There was a positive correlation between MYC amplification and overexpression (P = 0.002). Although FGFR1 amplification was not associated with MYC amplification or expression, 12.3% (20/163) of patients exhibited both FGFR1 amplification and MYC expression. There was also a correlation in FGFR1 amplification status between matched primary tumors and metastatic lymph nodes (P < 0.001). MYC expression was higher in ESCCs with pT1 (P < 0.001) and in those with no lymph node metastasis (P = 0.023). MYC expression was associated with prolonged disease-free survival (P = 0.036) and overall survival (OS) (P = 0.017) but was not an independent prognostic factor. FGFR1 amplification was an independent predictor for prolonged OS in all patients (P = 0.029) and in those who did not receive adjuvant therapy (P = 0.013). Combined FGFR1 amplification and MYC expression predicted better OS in patients who did not receive adjuvant therapy (P = 0.034) but not in those who did receive adjuvant therapy.
FGFR1 amplification and MYC expression have prognostic implications in resected ESCCs with respect to adjuvant therapy. The role of FGFR1-targeted therapy in ESCC remains to be explored.
Core tip: MYC expression, together with fibroblast growth factor receptor 1 (FGFR1) amplification, was reported to modulate oncogenic transformation. We evaluated both FGFR1 and MYC statuses in patients with resected esophageal squamous cell carcinoma (ESCC). FGFR1 and MYC amplifications were observed in 21.4% and 54.2% of patients with ESCC, respectively, while 12.3% exhibited both FGFR1 amplification and MYC expression. MYC expression and FGFR1 amplification were significantly associated with prolonged survival. Combined FGFR1 amplification and MYC expression was a predictor of better survival in patients who did not receive adjuvant therapy, but not in those who did. As such, FGFR1 and MYC might have prognostic implications in resected ESCCs with respect to adjuvant therapy.
- Citation: Kwon D, Yun JY, Keam B, Kim YT, Jeon YK. Prognostic implications of FGFR1 and MYC status in esophageal squamous cell carcinoma. World J Gastroenterol 2016; 22(44): 9803-9812
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9803.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9803
Esophageal cancer is the eighth most common and the sixth leading cause of cancer-related mortality worldwide[1]. Esophageal squamous cell carcinoma (ESCC) accounts for the majority of esophageal cancers. Recently, genomic and molecular alterations have been discovered in ESCC, including activation of the receptor tyrosine kinase (RTK) pathway, cell cycle dysregulation, activation of Wnt and Notch signaling pathways and epigenetic modifications[2,3]. However, molecular targeted therapy for ESCC remains to be established[4].
Fibroblast growth factor receptors (FGFRs) are RTKs expressed in many different cell types and regulate cell proliferation, differentiation and survival. FGFRs also have oncogenic roles in many cancers[5,6]. In contrast, the FGFR signaling pathway can act as a tumor suppressor by promoting cell differentiation, regulating other oncogenic pathways, protecting cells from injury, or mediating immune surveillance[5,7]. FGFR1 is one of the most frequently amplified genes in ESCC[2,8,9]. Additionally, new drugs targeting FGFR and its related pathways, including multi-kinase inhibitors and selected FGFR inhibitors, have been introduced for cancer treatment[5,10]. However, the prognostic significance of FGFR1 amplification in patients with ESCC remains controversial[11,12].
Pulmonary squamous cell carcinoma (SCC) is another cancer frequently showing FGFR1 amplification. Reportedly, MYC expression together with FGFR1 amplification regulates oncogenic transformation of FGFR1 and modulates responses to FGFR inhibitors in pulmonary SCC[13,14]. Those studies showed that FGFR1, located at 8p12, and MYC, located at 8q24, were frequently co-amplified in pulmonary SCC[13]. MYC plays an important role in cell proliferation and carcinogenesis in many types of cancer[15,16]. Additionally, a potential role of MYC as a predictor of the sensitivity to FGFR inhibitors in pulmonary SCC requires further investigation. However, the prevalence of FGFR1 and MYC alterations and their relationship have not been addressed in patients with ESCC. Thus, we investigated FGFR1 amplification and MYC amplification and expression in patients with resected ESCC and analyzed their clinicopathological features and prognostic significance.
Patients who underwent surgical resection for ESCC at Seoul National University Hospital (SNUH) from 2000 to 2013 were reviewed. Patients who received neoadjuvant chemo- and/or radiotherapy and those who had distant metastasis at the time of surgery were excluded. Finally, 180 total patients participated in this study. Clinical data including demographic features, treatment modalities and outcomes were obtained from medical records by an oncologist (B.K.). Overall survival (OS) was calculated from the date of diagnosis to the date of death from any cause or the last follow-up, and disease-free survival (DFS) was calculated from the date of diagnosis to the date of disease recurrence. Pathological tumor-node-metastasis (TNM) stage was based on the 7th American Joint Committee on Cancer. A tissue microarray was constructed from 2-mm diameter cores obtained from representative tumor areas of formalin-fixed paraffin-embedded tissue blocks and submitted for fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC). This study followed the World Medical Association Declaration of Helsinki recommendations and was approved by the Institutional Review Board of SNUH (H-1405-055-579).
To evaluate FGFR1 and MYC gene copies, FISH was performed using Vysis LSI FGFR1 SpectrumRed probe, Vysis LSI c-MYC (8q24.12-q24.13) SpectrumOrange probe and Vysis CEP8 (D8Z2) SpectrumGreen probe as a chromosome enumeration probe (Abbott Molecular, Abbott Park, IL, United States), according to the manufacturer’s protocol and as reported previously[17]. The entire tumor area was scanned for hot spots representing increased FGFR1 copy numbers. Random areas were selected for evaluation if the signals were distributed homogeneously. A minimum of 60 non-overlapping tumor nuclei were counted for the number of FGFR1 and CEP8 signals. FGFR1 gene copy status was classified according to the criteria proposed by Schildhaus et al[18]. In brief, high-level FGFR1 amplification was defined as follows: (1) an FGFR1/CEP8 ratio ≥ 2; (2) ≥ 15 FGFR1 signals in ≥10% of tumor cells; or (3) average number of FGFR1 signals/cell ≥ 6. Low-level FGFR1 amplification was defined as ≥ 5 FGFR1 signals/cell in ≥ 50% of tumor cells. The same methods and criteria were applied to evaluate MYC gene status.
To evaluate MYC expression, IHC was performed using a rabbit monoclonal anti-c-MYC antibody (EP121, Cell Marque, Rocklin, CA, United States) and the Benchmark XT autostainer (Ventana Medical Systems, Tucson, AZ, United States). MYC expression was evaluated using a four-tier scoring system as follows: 0, none or any staining in < 10% of cells; 1, weak; 2, moderate; and 3, strong staining in ≥ 10% of tumor cells. Cases with a score of 1-3 were considered to express MYC.
Statistical analysis was performed using SPSS (version 22.0; IBM Corp., Armonk, NY, United States). Differences between FGFR1 or MYC status and clinicopathological variables were determined using Fisher’s exact test or Student’s t-test. The Kaplan-Meier method with the log-rank test and Cox proportional hazards regression analysis were used for survival analyses. Two-sided P-values < 0.05 were considered to indicate statistical significance.
The clinicopathological features of 180 patients with resected ESCC are summarized in Table 1. Briefly, the median age of the patients was 64.76 years (range, 41-83 years), and 74.4% were older than 60 years of age. Most patients were males (93.9%) and former or current smokers (84.4%). pT1b (36.1%) and pT3 (43.3%) diseases were common, and lymph node metastasis was observed in 48.9% of patients. Tumors were frequently localized in the lower esophagus (65.5%).
| Variables | n (%) | |
| Age (yr) | ≤ 60 | 46 (25.6) |
| > 60 | 134 (74.4) | |
| Sex | Male | 169 (93.9) |
| Female | 11 (6.1) | |
| Smoking | No | 28 (15.6) |
| Yes | 151 (84.4) | |
| Histological grade | WD | 35 (19.4) |
| MD | 119 (66.1) | |
| PD and basaloid | 26 (14.4) | |
| Localization | Upper | 7 (3.9) |
| Middle | 44 (24.9) | |
| Lower | 116 (65.5) | |
| EGJ | 10 (5.6) | |
| T | 1a | 16 (8.9) |
| 1b | 65 (36.1) | |
| 2 | 17 (9.4) | |
| 3 | 78 (43.3) | |
| 4 | 4 (2.2) | |
| N | 0 | 92 (51.1) |
| 1 | 52 (28.9) | |
| 2 | 29 (16.1) | |
| 3 | 7 (3.9) | |
| Stage | IA | 14 (7.8) |
| IB | 49 (27.2) | |
| IIA | 21 (11.7) | |
| IIB | 32 (17.8) | |
| IIIA | 37 (20.6) | |
| IIIB | 19 (10.6) | |
| IIIC | 8 (4.4) | |
| Adjuvant therapy | No | 112 (67.8) |
| Yes | 58 (32.2) | |
| FGFR1 amplification | No amplification | 136 (78.6) |
| Low amplification | 3 (1.7) | |
| High amplification | 34 (19.7) | |
| MYC amplification | No amplification | 77 (45.9) |
| Low amplification | 20 (11.9) | |
| High amplification | 71 (42.3) | |
| MYC expression | 0 (none) | 74 (41.1) |
| 1 (weak) | 54 (30.0) | |
| 2 (moderate) | 41 (22.8) | |
| 3 (strong) | 11 (6.1) |
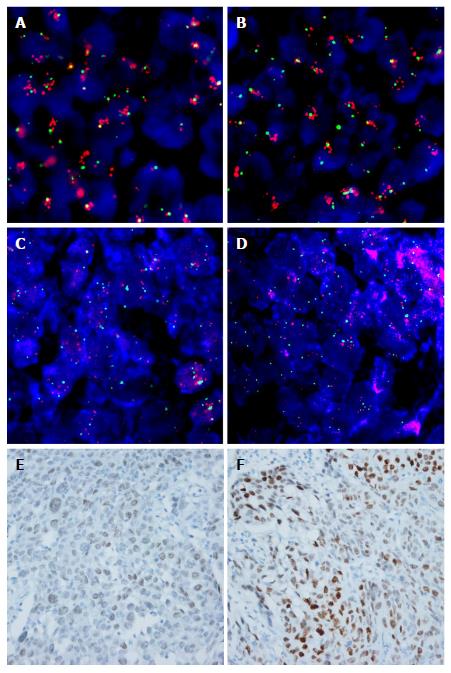
In ESCCs, FGFR1 amplification was detected in 21.4% (37/173) of patients (high amplification in 19.7%, n = 34 and low amplification in 1.7%, n = 3; Figure 1A and B). MYC amplification was found in 54.2% (91/168) of patients (high amplification in 42.3%, n = 71 and low amplification in 11.9%, n = 20; Figure 1C and D). MYC expression was observed in 58.9% (106/180) of patients (weak expression in 30%, moderate expression in 22.8% and strong expression in 6.1%; Figure 1E and F). MYC amplification was positively correlated with MYC expression (P = 0.002; data not shown). FGFR1 amplification status was not associated with MYC amplification or protein expression.
The relationships between FGFR1 or MYC status and clinicopathological features are summarized in Table 2. ESCC patients with FGFR1 amplification were younger than those without FGFR1 amplification (mean ± SD, 62.3 ± 8.4 years versus 65.6 ± 7.4 years, P = 0.022). Other clinicopathological parameters including sex, histological differentiation, smoking status and TNM stage were not significantly correlated with FGFR1 amplification. In contrast, MYC expression was higher in patients with early pT stage disease (P < 0.001), without lymph node metastasis (P = 0.023) or with early TNM stage disease (P < 0.001). In contrast, MYC amplification was not significantly correlated with clinicopathological features.
| Variables | FGFR1, n (%) | MYC, n (%) | ||||
| No amplification | Amplification | P value | No expression | Expression | P value | |
| Age (yr) | ||||||
| ≤ 60 | 30/44 (68.2) | 14/44 (31.8) | 0.058 | 18/46 (39.1) | 28/46 (60.9) | 0.862 |
| > 60 | 106/129 (82.2) | 23/129 (17.8) | 56/134 (41.8) | 78/134 (58.2) | ||
| Smoking | ||||||
| No | 21/28 (75) | 7/28 (25) | 0.619 | 12/28 (42.9) | 6/28 (57.1) | 0.836 |
| Yes | 115/145 (79.3) | 30/145 (20.7) | 61/151 (40.4) | 90/151 (59.6) | ||
| Histological grade | ||||||
| WD | 29/34 (85.3) | 5/34 (14.7) | 0.350 | 17/35 (48.6) | 18/35 (51.4) | 0.267 |
| MD | 90/117 (76.9) | 27/117 (23.1) | 43/119 (36.1) | 76/119 (63.9) | ||
| PD | 11/16 (68.8) | 5/16 (31.2) | 10/18 (55.6) | 8/18 (44.4) | ||
| Others | 6/6 (100) | 0/6 (0) | 4/8 (50) | 4/8 (50) | ||
| Localization | ||||||
| Upper | 6/7 (85.7) | 1/7 (14.3) | 0.981 | 4/7 (57.1) | 3/7 (42.9) | 0.688 |
| Middle | 34/42 (81) | 8/42 (19) | 20/44 (45.5) | 24/44 (54.5) | ||
| Lower | 86/111 (77.5) | 25/111 (22.5) | 45/116 (38.8) | 71/116 (61.2) | ||
| EGJ | 8/10 (80) | 2/10 (20) | 4/10 (40) | 6/10 (60) | ||
| T | ||||||
| 1 | 66/80 (82.5) | 14/80 (17.5) | 0.602 | 22/81 (27.2) | 59/81 (72.8) | < 0.001 |
| 2 | 12/16 (75) | 4/16 (25) | 6/17 (35.3) | 11/17 (64.7) | ||
| 3 | 55/73 (75.3) | 18/73 (24.7) | 43/78 (55.1) | 35/78 (44.9) | ||
| 4 | 3/4 (75) | 1/4 (25) | 3/4 (75) | 1/4 (24) | ||
| N | ||||||
| 0 | 73/90 (81.1) | 17/90 (18.9) | 0.460 | 30/92 (32.6) | 62/92 (67.4) | 0.023 |
| 1-3 | 63/83 (75.9) | 20/83 (24.1) | 44/88 (50) | 44/88 (50) | ||
| Stage | ||||||
| I | 51/62 (82.3) | 11/62 (17.7) | 0.694 | 18/63 (28.6) | 45/64 (71.4) | < 0.001 |
| II | 40/52 (76.9) | 12/52 (23.1) | 17/53 (32.1) | 36/53 (67.9) | ||
| III | 45/59 (76.3) | 14/59 (23.7) | 39/64 (60.9) | 25/64 (39.1) | ||
FGFR1 amplification was evaluated in matched primary tumors and metastatic lymph nodes of 56 patients, and a significantly positive correlation was found (P < 0.001; Table 3). Briefly, FGFR1 amplification in the primary tumor was observed in 11 of 56 cases, and 7 (63.6%) patients also showed FGFR1 amplification in metastatic tumors of the regional lymph nodes. In contrast, only 3 (6.7%) of the 45 cases who did not have FGFR1 amplification in the primary tumor exhibited FGFR1 amplification in metastatic tumors of the lymph nodes. In contrast, MYC gene copy status was not correlated with the primary tumors or metastatic tumors of the lymph nodes (Table 3).
| Metastatic lymph nodes | Total | P value | |||
| No amplification | Amplification | ||||
| FGFR1 Primary tumor | No amplification | 42 (91.3) | 3 (6.7) | 45 (100) | < 0.001 |
| Amplification | 4 (36.4) | 7 (63.6) | 11 (100) | ||
| Total | 46 (82.1) | 10 (17.9) | 56 (100) | ||
| MYC Primary tumor | No amplification | 17 (63.0) | 10 (37.0) | 27 (100) | 1.000 |
| Amplification | 12 (60.0) | 8 (40.0) | 20 (100) | ||
| Total | 29 (61.7) | 18 (38.3) | 47 (100) | ||
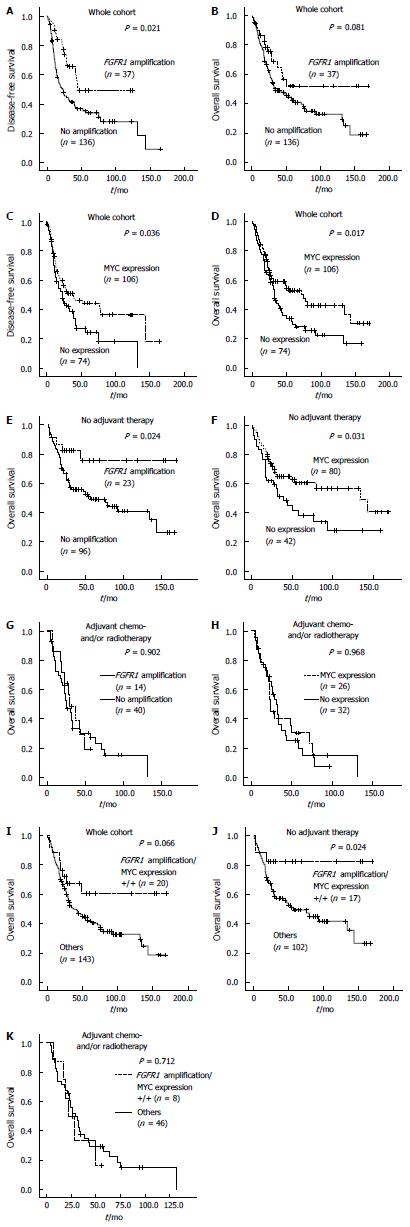
The mean and median follow-up times of 180 patients were 43.2 and 29.8 mo (range, 0.6-169.4 mo). The 5-year DFS and OS rates for all patients were 24% and 26%, respectively, depending on the stage as follows: 40.6% and 44.7% in stage I, 22.9% and 27.6% in stage II and 5.0% and 7.7% in stage III, respectively. Kaplan-Meier analysis revealed that DFS of ESCC patients with FGFR1 amplification was significantly prolonged compared with those without FGFR1 amplification (P = 0.021; Figure 2A). OS also tended to be longer in patients with FGFR1 amplification compared with those without FGFR1 amplification (P = 0.081; Figure 2B). ESCC patients with MYC amplification tended to have a longer DFS and OS than did those without MYC amplification, but statistically insignificant (P = 0.064 and 0.423, respectively; data not shown). However, patients with MYC expression had a significantly longer DFS (P = 0.036) and OS (P = 0.017) compared with those without MYC expression (Figure 2C and D).
Adjuvant chemo- and/or radiotherapy after surgical tumor resection was performed in 58 (32.2%) patients with ESCC. The mean OS of 112 patients who did not receive adjuvant chemo- and/or radiotherapy was 89.5 mo, which was significantly better than the OS of patients who received adjuvant therapy (42.5 mo). The patients who received adjuvant treatment showed a higher pT, nodal metastasis and a higher stage (all P < 0.001). These data suggest that patients given adjuvant therapy have unfavorable clinical features and aggressive biological behavior, leading to adjuvant therapy. Thus, we performed survival analysis in patients with and without adjuvant therapy separately. In patients without adjuvant therapy, FGFR1 amplification and MYC expression were significantly associated with prolonged OS (P = 0.024 and 0.031, respectively; Figure 2E and F), but not in patients who received adjuvant chemo- and/or radiotherapy (Figure 2G and H).
Multivariate Cox analysis for OS incorporating age, T stage, lymph node metastasis, FGFR1 amplification and MYC expression revealed that age, T and N stage were independent poor prognostic factors in all patients as well as in both groups of patients with and without adjuvant therapy (Table 4). In contrast, FGFR1 amplification was found to be an independent favorable prognostic factor in all patients (HR = 0.532 with 95%CI: 0.302-0.937, P = 0.029) and in patients without adjuvant therapy (HR = 0.301 with 95%CI: 0.117-0.774, P = 0.013), but not in patients with adjuvant therapy (Table 4). MYC expression lost its prognostic significance in multivariate Cox analysis (Table 4).
| Variables | Category | Whole cohort | No adjuvant chemo- and/or radiotherapy | Adjuvant chemo- and/or radiotherapy | |||
| HR (95%CI) | P value | HR (95%CI) | P value | HR (95%CI) | P value | ||
| Age (yr) | ≤ 60 vs > 60 | 1.805 (1.102-2.955) | 0.019 | 2.371 (1.088- 5.167) | 0.030 | 1.81 (0.896-3.656) | 0.098 |
| T | 1, 2, 3, 4 | - | 0.005 | - | 0.091 | - | 0.009 |
| 1 vs 2 | 1.204 (0.523-2.773) | 0.663 | 1.589 (0.591-4.269) | 0.358 | 1.260 (0.251-6.324) | 0.779 | |
| 1 vs 3 | 2.373 (1.454-3.872) | 0.001 | 2.115 (1.126-3.973) | 0.020 | 4.136 (1.691-10.119) | 0.002 | |
| 1 vs 4 | 1.902 (0.550-6.575) | 0.310 | 0.632 (0.078-5.130) | 0.667 | 4.256 (0.820-22.092) | 0.085 | |
| N | 0 vs 1-3 | 1.981 (1.275-3.077) | 0.002 | 2.351 (1.338-4.133) | 0.003 | 0.319 (0.125-0.814) | 0.017 |
| FGFR1 amplification | None vs Amplification | 0.532 (0.302-0.937) | 0.029 | 0.301 (0.117-0.774) | 0.013 | 0.830 (0.386-1.783) | 0.633 |
| MYC expression | None vs Expression | 0.993 (0.636-1.550) | 0.975 | 0.873 (0.478-1.595) | 0.659 | 1.566 (0.811-3.024) | 0.181 |
ESCC patients (25/173) with both FGFR1 amplification and MYC expression (hereafter referred to as combined positivity) exhibited prolonged DFS (P = 0.023; data not shown) and OS (P = 0.066) in Kaplan-Meier analysis (Figure 2I). Combined positivity was significantly associated with longer OS (P = 0.024) in patients who did not receive adjuvant therapy, but not in patients who received adjuvant therapy (P = 0.712; Figure 2J and K). Combined positivity was also shown to be an independent favorable prognostic factor among patients who did not receive adjuvant therapy; this was determined when multivariate Cox analysis for OS was performed and incorporated age, T stage, lymph node metastasis, and combined positivity (HR = 0.275 with 95%CI: 0.083-0.97, P = 0.034; data not shown).
In this study, we comprehensively investigated FGFR1 amplification and MYC amplification and expression in ESCC to elucidate the associated clinicopathological characteristics and explore the potential of FGFR1 and MYC as targets for cancer therapy.
Several previous studies reported the prognostic implication of FGFR1 amplification in ESCC, but the results were controversial[11,12]. FGFR1 amplification was associated with poor prognosis or had no prognostic significance in ESCC; however, the FISH criteria for FGFR1 amplification were not identical[11,12]. In the present study, FGFR1 amplification was a favorable prognostic indicator in patients with resected ESCC, which was in conflict with a previous report using the same FISH criteria[11]. In a study using FISH and different criteria, FGFR1 amplification was not associated with clinical outcomes in patients with ESCC[12]. Similarly, in the case of pulmonary SCC, the prognostic implication of FGFR1 amplification was controversial[19-21]. One study demonstrated that FGFR1 amplification was an independent favorable prognostic factor in pulmonary SCC and large cell carcinoma[19], which contrasted with another study showing that FGFR1 amplification was an independent negative prognostic factor in resected pulmonary SCC[20]. Consequently, a recent meta-analysis concluded that FGFR1 amplification had no influence on the survival of patients with pulmonary SCC[22]. Notably, in this study, the association of FGFR1 amplification with clinical outcome of resected ESCC patients was dependent on the status of adjuvant therapy; i.e., FGFR1 amplification was a favorable prognostic factor in patients with ESCC who did not receive adjuvant therapy. Adjuvant therapy after surgery for patients with stage III-IV or lymph node metastasis prolonged survival compared with surgery alone in ESCC[23]. Therefore, adjuvant chemotherapy with or without radiotherapy is increasingly used for the treatment of advanced ESCC, although no definite criteria or regimen for adjuvant therapy has been established in ESCC. In this study, patients with adjuvant chemo- and/or radiotherapy tended to be in the advanced stage compared with those with no adjuvant therapy. Thus, FGFR1 might play variable biological roles during the progression of cancer and thereby have different prognostic significance depending on the stage and subsequent adjuvant therapy status of patients. Otherwise, it could be possible that ESCC with FGFR1 amplification represents a biologically less aggressive group among ESCCs having variable genetic alterations. This could result in the prolonged survival of patients receiving no adjuvant therapy. FGFR1 could affect the efficacy of chemo- or radiotherapy in patients with ESCC, and thus be differently associated with the prognosis in those receiving adjuvant therapy.
To the best of our knowledge, this is the largest study to evaluate MYC status using IHC and FISH in ESCC. Kaplan-Meier analysis demonstrated that MYC expression, but not amplification, was associated with prolonged survival. This result might be contradictory to the role of MYC as an oncogene. In this study, MYC expression was more common in ESCC patients of younger age and in the early TNM stage, and it was not an independent prognostic factor. Thus, the favorable prognosis of patients with ESCC who showed combined FGFR1 amplification and MYC expression in the group without adjuvant therapy might be due to the association of FGFR1 amplification with prognosis. Based on this study, MYC status might have little, if any, prognostic implication in patients with ESCC. However, more studies using a large cohort of patients are needed to validate the prognostic significance of MYC in ESCC.
However, this study had some limitations. First, it was a retrospective study and, as such, the specific regimen of adjuvant therapy may not have been well-controlled. Second, we used a TMA of 2 mm diameter, which may not reflect the intratumoral heterogeneity of FGFR1 and MYC status. However, comparative analysis showed that FGFR1 amplification status was not significantly different between primary and nodal metastatic tumors. In contrast, MYC amplification status was significantly different between these two groups. Third, small groups were compared as a result of subgroup analysis according to the different treatment modalities. Thus, another study using large prospective cohorts is required to validate the prognostic role of FGFR1 amplification in ESCC according to adjuvant therapy status.
Although ESCC is an aggressive cancer with poor clinical outcomes, treatment approaches remain limited, requiring the development of novel strategies including targeted molecular therapy. This study demonstrated that FGFR1 was amplified in approximately 20% of ESCCs, and moreover, FGFR1 amplification status was maintained during lymph node metastasis; hence, this group may benefit from therapeutic inhibition of FGFR1. FGFR1 amplification is considered an adequate factor to predict sensitivity to FGFR inhibitors[24]. However, FGFR inhibitors resulted in insufficient clinical responses in patients with FGFR1-amplified lung cancer[25,26]. A recent study showed that MYC expression might modulate the sensitivity of FGFR1-amplified pulmonary SCC to FGFR1 inhibitors[13]. In that study, 40% of FGFR1-amplified pulmonary SCCs expressed high levels of MYC[13], which was similar to our results in that 54.1% (20/37) of FGFR1-amplified ESCCs expressed MYC. Among the all patients with resected ESCC, 12.3% (20/163) exhibited both FGFR1 amplification and MYC expression. Based on the pulmonary SCC study, this population could be a potential candidate for FGFR inhibitor therapy in ESCC patients. The role of therapy targeting FGFR1 or MYC in ESCC remains to be explored by further in vitro and clinical studies.
In conclusion, FGFR1 amplifications were observed in 21.4% of patients and combined FGFR1 amplification and MYC expression was observed in 12.3% of patients with resected ESCC. FGFR1 amplification had prognostic implications in patients with resected ESCC with respect to adjuvant therapy. The role of targeted therapy against FGFR1 or MYC in ESCC remains to be explored.
It has been demonstrated that fibroblast growth factor receptor 1 (FGFR1) and MYC are frequently co-amplified and play a role in neoplastic transformation in pulmonary squamous cell carcinoma (SCC). Moreover, a potential role of MYC as a predictor of the sensitivity to FGFR inhibitors in pulmonary SCC has been reported. Although FGFR1 and MYC alterations have been reported by genomic studies for esophageal squamous cell carcinoma (ESCC), the prevalence of FGFR1 and MYC alterations and their relationship remains to be clarified in patients with ESCC. Thus, we investigated FGFR1 amplification and MYC amplification and expression in patients with ESCC and analyzed their clinicopathological features and prognostic significance.
ESCC is one of the leading causes of cancer-related mortality worldwide and novel treatment strategies other than surgery and conventional chemo- and radio-therapy are required to improve clinical outcome. However, molecular targeted therapy for ESCC remains to be established. The results of this study contribute to clarifying the biological role of FGFR1 and MYC, and therapeutic potential of FGFR targeted therapy in patients with ESCC.
In this study, FGFR1 amplifications were observed in 21.4% of patients and combined FGFR1 amplification and MYC expression was observed in 12.3% of patients with resected ESCC. FGFR1 amplification had prognostic implications in patients with resected ESCC with respect to adjuvant therapy. The role of FGFR1-targeted therapy in ESCC remains to be explored.
This study suggests that patients with ESCC harboring combined FGFR1 amplification and MYC expression might benefit from therapies targeting FGFR1 and/or MYC, especially those with advanced disease requiring adjuvant therapies.
FGFR1 is a receptor tyrosine kinase playing an oncogenic role in many cancers and can be targeted for molecular therapy. MYC is an oncogene and contributes to sensitivity to FGFR inhibitor in pulmonary squamous cell carcinoma (SCC). Fluorescence in situ hybridization (FISH) is a tool useful to evaluate the gene amplification using tumor tissues from patients with solid tumor.
It is a very interesting article presenting novel data on role of FGFR1 and MYC status in ESCC. All parts of the manuscript were composed correctly and they contain suitable information. Tables and figures were constructed appropriately. Statistical analysis of data was performed correctly with using the appropriate tests. All references are actual and relevant to the text of article.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Alshehabi Z, Chen XL, Diakowska D, Ishiguro H S- Editor: Gong ZM L- Editor: A E- Editor: Liu WX
| 1. | Higuchi K, Koizumi W, Tanabe S, Sasaki T, Katada C, Azuma M, Nakatani K, Ishido K, Naruke A, Ryu T. Current management of esophageal squamous-cell carcinoma in Japan and other countries. Gastrointest Cancer Res. 2009;3:153-161. [PubMed] |
| 2. | Lin DC, Hao JJ, Nagata Y, Xu L, Shang L, Meng X, Sato Y, Okuno Y, Varela AM, Ding LW. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 504] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 3. | Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 855] [Article Influence: 77.7] [Reference Citation Analysis (0)] |
| 4. | Kang X, Chen K, Li Y, Li J, D’Amico TA, Chen X. Personalized targeted therapy for esophageal squamous cell carcinoma. World J Gastroenterol. 2015;21:7648-7658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Dienstmann R, Rodon J, Prat A, Perez-Garcia J, Adamo B, Felip E, Cortes J, Iafrate AJ, Nuciforo P, Tabernero J. Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Ann Oncol. 2014;25:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 6. | Jang M, Kim E, Choi Y, Lee H, Kim Y, Kim J, Kang E, Kim SW, Kim I, Park S. FGFR1 is amplified during the progression of in situ to invasive breast carcinoma. Breast Cancer Res. 2012;14:R115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Turner N, Grose R. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer. 2010;10:116-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1812] [Cited by in RCA: 2041] [Article Influence: 136.1] [Reference Citation Analysis (1)] |
| 8. | Lin DC, Wang MR, Koeffler HP. Targeting genetic lesions in esophageal cancer. Cell Cycle. 2014;13:2013-2014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Sugiura K, Ozawa S, Kitagawa Y, Ueda M, Kitajima M. Co-expression of aFGF and FGFR-1 is predictive of a poor prognosis in patients with esophageal squamous cell carcinoma. Oncol Rep. 2007;17:557-564. [PubMed] |
| 10. | Göke F, Franzen A, Hinz TK, Marek LA, Yoon P, Sharma R, Bode M, von Maessenhausen A, Lankat-Buttgereit B, Göke A. FGFR1 Expression Levels Predict BGJ398 Sensitivity of FGFR1-Dependent Head and Neck Squamous Cell Cancers. Clin Cancer Res. 2015;21:4356-4364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 11. | Kim HS, Lee SE, Bae YS, Kim DJ, Lee CG, Hur J, Chung H, Park JC, Jung DH, Shin SK. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival in patients with resected esophageal squamous cell carcinoma. Oncotarget. 2015;6:2562-2572. [PubMed] |
| 12. | von Loga K, Kohlhaussen J, Burkhardt L, Simon R, Steurer S, Burdak-Rothkamm S, Jacobsen F, Sauter G, Krech T. FGFR1 Amplification Is Often Homogeneous and Strongly Linked to the Squamous Cell Carcinoma Subtype in Esophageal Carcinoma. PLoS One. 2015;10:e0141867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Malchers F, Dietlein F, Schöttle J, Lu X, Nogova L, Albus K, Fernandez-Cuesta L, Heuckmann JM, Gautschi O, Diebold J. Cell-autonomous and non-cell-autonomous mechanisms of transformation by amplified FGFR1 in lung cancer. Cancer Discov. 2014;4:246-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 14. | Lockwood W, Politi K. MYCxing it up with FGFR1 in squamous cell lung cancer. Cancer Discov. 2014;4:152-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Li B, Simon MC. Molecular Pathways: Targeting MYC-induced metabolic reprogramming and oncogenic stress in cancer. Clin Cancer Res. 2013;19:5835-5841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 16. | Robanus-Maandag EC, Bosch CA, Kristel PM, Hart AA, Faneyte IF, Nederlof PM, Peterse JL, van de Vijver MJ. Association of C-MYC amplification with progression from the in situ to the invasive stage in C-MYC-amplified breast carcinomas. J Pathol. 2003;201:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Kim MY, Koh J, Kim S, Go H, Jeon YK, Chung DH. Clinicopathological analysis of PD-L1 and PD-L2 expression in pulmonary squamous cell carcinoma: Comparison with tumor-infiltrating T cells and the status of oncogenic drivers. Lung Cancer. 2015;88:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 18. | Schildhaus HU, Heukamp LC, Merkelbach-Bruse S, Riesner K, Schmitz K, Binot E, Paggen E, Albus K, Schulte W, Ko YD. Definition of a fluorescence in-situ hybridization score identifies high- and low-level FGFR1 amplification types in squamous cell lung cancer. Mod Pathol. 2012;25:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 19. | Tran TN, Selinger CI, Kohonen-Corish MR, McCaughan BC, Kennedy CW, O’Toole SA, Cooper WA. Fibroblast growth factor receptor 1 (FGFR1) copy number is an independent prognostic factor in non-small cell lung cancer. Lung Cancer. 2013;81:462-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Kim HR, Kim DJ, Kang DR, Lee JG, Lim SM, Lee CY, Rha SY, Bae MK, Lee YJ, Kim SH. Fibroblast growth factor receptor 1 gene amplification is associated with poor survival and cigarette smoking dosage in patients with resected squamous cell lung cancer. J Clin Oncol. 2013;31:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Russell PA, Yu Y, Young RJ, Conron M, Wainer Z, Alam N, Solomon B, Wright GM. Prevalence, morphology, and natural history of FGFR1-amplified lung cancer, including squamous cell carcinoma, detected by FISH and SISH. Mod Pathol. 2014;27:1621-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Jiang T, Gao G, Fan G, Li M, Zhou C. FGFR1 amplification in lung squamous cell carcinoma: a systematic review with meta-analysis. Lung Cancer. 2015;87:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Zhang SS, Yang H, Xie X, Luo KJ, Wen J, Bella AE, Hu Y, Yang F, Fu JH. Adjuvant chemotherapy versus surgery alone for esophageal squamous cell carcinoma: a meta-analysis of randomized controlled trials and nonrandomized studies. Dis Esophagus. 2014;27:574-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Guagnano V, Kauffmann A, Wöhrle S, Stamm C, Ito M, Barys L, Pornon A, Yao Y, Li F, Zhang Y. FGFR genetic alterations predict for sensitivity to NVP-BGJ398, a selective pan-FGFR inhibitor. Cancer Discov. 2012;2:1118-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 272] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 25. | Sos ML, Dietlein F, Peifer M, Schöttle J, Balke-Want H, Müller C, Koker M, Richters A, Heynck S, Malchers F. A framework for identification of actionable cancer genome dependencies in small cell lung cancer. Proc Natl Acad Sci USA. 2012;109:17034-17039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 152] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 26. | Venugopal B, Baird R, Kristeleit RS, Plummer R, Cowan R, Stewart A, Fourneau N, Hellemans P, Elsayed Y, McClue S. A phase I study of quisinostat (JNJ-26481585), an oral hydroxamate histone deacetylase inhibitor with evidence of target modulation and antitumor activity, in patients with advanced solid tumors. Clin Cancer Res. 2013;19:4262-4272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |