Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9775
Peer-review started: July 6, 2016
First decision: August 29, 2016
Revised: September 11, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: November 28, 2016
Processing time: 144 Days and 22.8 Hours
To explore the protective effects and mechanisms of naringenin (NRG) on hepatic injury induced by isoniazid (INH) and rifampicin (RIF).
Male mice were randomly divided into four groups and treated for 14 d as follows: normal control group was administered intragastrically with normal saline solution alone; model group was administered intragastrically with INH (100 mg/kg) and RIF (100 mg/kg); low- and high-dosage NRG pretreatment groups were administered intragastrically with different doses of NRG (50 or 100 mg/kg) 2 h before INH and RIF challenge. Mice were killed 16 h after the last dose of drug treatment to determine activity of serum transaminases. Oxidative stress was evaluated by measuring hepatic glutathione (GSH) and superoxide dismutase (SOD) and malondialdehyde (MDA) levels. Histopathological changes in hepatic tissue were observed under the optical microscope. Hepatocyte apoptosis was measured by TUNEL assay and caspase-3 activation. Expression of Bcl-2 and Bax in liver was determined by western blot.
Both low- and high-dosage NRG pretreatment obviously alleviated serum levels of alanine aminotransferase and aspartate aminotransferase, liver index, hepatic MDA content, and increased hepatic GSH content and SOD activity compared with the INH and RIF-treated group (44.71 ± 8.15 U/L, 38.22 ± 6.64 U/L vs 58.15 ± 10.54 U/L; 98.36 ± 14.78 U/L, 92.41 ± 13.59 U/L vs 133.05 ± 19.36 U/L; 5.34% ± 0.26%, 4.93% ± 0.25% vs 5.71% ± 0.28%; 2.76 ± 0.67 nmol/mgprot, 2.64 ± 0.64 nmol/mgprot vs 4.49 ± 1.12 nmol/mgprot; 5.91 ± 1.31 mg/gprot, 6.42 ± 1.42 mg/gprot vs 3.11 ± 0.73 mg/gprot; 137.31 ± 24.62 U/mgprot, 148.83 ± 26.75 U/mgprot vs 102.34 ± 19.22 U/mgprot; all P < 0.01 or 0.05). Histopathological evaluation showed obvious necrosis and inflammatory cell infiltration in liver of mice administered INH and RIF; however, mice pretreated with NRG showed minor hepatic injury. In addition, INH and RIF resulted in hepatocyte apoptosis, and NRG pretreatment dramatically suppressed INH- and RIF-induced hepatocytes apoptosis. Furthermore, NRG-mediated anti-apoptotic effects seemed to be in connection with its regulation of Bax and Bcl-2 protein expression in hepatic tissue.
NRG might attenuate INH- and RIF-induced hepatic injury via suppression of oxidative stress and hepatocyte apoptosis.
Core tip: Anti-tuberculosis drugs can cause drug-induced liver injury through a series of complex mechanisms. The main purposes of the study were to explore the effects of naringenin (NRG) on liver injury induced by isoniazid (INH) and rifampicin (RIF). Mice were administered intragastrically with NRG before INH and RIF challenge. The findings revealed that NRG protects against INH- and RIF-induced oxidative stress and hepatocyte apoptosis.
- Citation: Wang C, Fan RQ, Zhang YX, Nie H, Li K. Naringenin protects against isoniazid- and rifampicin-induced apoptosis in hepatic injury. World J Gastroenterol 2016; 22(44): 9775-9783
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9775.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9775
Tuberculosis (TB) is a deadly communicable disease caused by mycobacteria, and it remains a serious global public health issue. In 2014, about 9.6 million people fell ill with TB and 1.5 million died from the disease[1]. The TB treatment recommended by the World Health Organization is a regimen of isoniazid (INH), rifampicin (RIF), pyrazinamide, and ethambutol for 2 mo in the initiation stage, followed by 4 mo of INH and RIF in the continuation stage. Unfortunately, the two most common anti-TB drugs, INH and RIF, can cause drug-induced hepatic injury through a series of complex mechanisms. Peroxidation of endogenous lipids has been shown to be the dominant factor in the hepatotoxic action of INH and RIF[2]. Anti-TB drug-mediated oxidative injury is usually ascribed to formation of highly reactive oxygen species, which act as stimulators of lipid peroxidation and damage the cell membrane[3]. Oxidative stress in the hepatocyte results in altered mitochondrial permeability and induces apoptosis[4]. Therefore, antioxidants could serve as possible drugs against INH- and RIF-induced hepatic toxic injury[5,6].
In recent years, natural plant extracts have been extensively regarded as a rich resource for drug development. Naringenin (NRG) is a type of natural flavonoid compound, which widely exists in grapefruit, bitter orange and other plants. NRG has wide range of biological and pharmacological activities, such as anti-inflammatory[7], anti-mutagenic[8], anti-atherogenic[9], anti-oxidant[10] and anti-cancer[11]. Related studies have shown that NRG attenuates liver injury induced by lead[12], acetaminophen[13], cholesterol overdose[14] and carbon tetrachloride (CCl4) intoxication[15]. It was suggested that NRG can exert hepatoprotective effects and may has therapeutic value for various liver diseases.
Therefore, this study was conducted to investigate the potent protective effects of NRG on INH- and RIF-induced hepatic injury in mice. In addition, we discussed the underlying mechanisms of its therapeutic action.
NRG, INH and RIF were purchased from Sigma Aldrich (St. Louis, MO, United States). Kits to measure aspartate aminotransferase (AST), alanine aminotransferase (ALT), superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA) were purchased from Nanjing Jiancheng Institute of Bioengineering (Nanjing, China). TUNEL detection kit was purchased from Boster Biological Engineering Co., Ltd (Wuhan, China). Rabbit anti-mouse cleaved caspase-3 monoclonal antibody was purchased from Cell Signaling Technology (Danvers, MA, United States). Rabbit anti-mouse Bax, Bcl-2 and β-actin polyclonal antibodies were purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, United States).
Forty male specific pathogen-free BALB/c mice, weighing 20-22 g, were purchased from the Central Animal Facility of Wuhan University (Wuhan, China). All mice were housed in an environmentally controlled room (25 ± 2 °C, 55% ± 5% humidity) with a 12 h light and dark cycle and had free access to laboratory standard food and water. All experiments received approval by the Yangtze University’s institutional laboratory animal care and use committee.
After an acclimatization period of 1 wk, animals were randomly divided into four equal groups, each consisting of 10 mice, and treated for 14 d as follows: normal control group was administered intragastrically with normal saline solution (NSS) alone; model group was administered intragastrically with INH (100 mg/kg) and RIF (100 mg/kg); low-dosage and high-dosage NRG pretreatment groups were administered intragastrically with different doses of NRG (50 or 100 mg/kg) 2 h before INH and RIF challenge. NRG was dissolved in 5% carboxyl methyl cellulose and then diluted with NSS to the required concentration for oral administration. Mice were killed 16 h after the last dose of drug treatment to collect serum and liver tissue samples.
The liver index was calculated according to the formula: liver index (%) = mouse liver weight (g)/mouse weight (g) × 100%. The serum biochemical parameters, such as ALT and AST, were measured by absorbance photometry using commercial automated chemistry analyzers.
GSH level was assayed in the liver tissue according to the method of Griffith[16] and concentration was expressed as mg/g protein. SOD activity was assayed in the liver tissue through nitroblue tetrazolium coloration according to the manufacture’s protocols and was expressed as U/mg protein. Lipid peroxidation was assayed by measuring MDA in the liver tissue according to the method of Wills[17], and MDA content was expressed as nmol/mg protein.
Small pieces of liver were fixed in 10% neutral buffered formalin and then embedded into paraffin. Paraffin-embedded sections at 6 µm and stained with hematoxylin and eosin, and then analyzed under the optical microscope for histopathological evaluation.
The sections were stained for detection of apoptosis in live cells using a commercially available TUNEL kit and following the manufacturer’s instructions. The ratios of apoptosis were analyzed under the optical microscope by counting TUNEL positive cells in six randomly selected areas.
The sections were dewaxed in xylene and rehydrated in descending graded alcohols, followed by incubation in 3% hydrogen peroxide for 15 min to neutralize endogenous peroxidase activity, and then microwave antigen retrieval for 15 min in citric acid buffer. Nonspecific binding was blocked with 10% normal goat serum. Subsequently, the sections were incubated overnight with a rabbit anti-mouse cleaved caspase-3 monoclonal antibody at 4 °C, and then the goat anti-rabbit secondary antibody (Zhongshan Golden Bridge Biotechnology Company, China) was incubated at room temperature for 30 min. Finally, the sections were treated with diaminobenzidine working solution, and then counterstained with hematoxylin and covered with cover slips.
Proteins were extracted from liver tissue using the Bullet Blender homogenizer (Next Advance Inc., Averill Park, NY, United States) in RIPA lysis buffer (Beyotime, Shanghai, China), according to the manufacturer’s protocols. Equal amounts of proteins were subjected to electrophoresis on 10% SDS-polyacrylamide gel electrophoresis, and then the separated protein bands were transferred onto polyvinylidene difluoride membranes (Hybond, Escondido, CA, United States). Next, the membranes were incubated respectively with rabbit anti-mouse β-actin, Bax or Bcl-2 polyclonal antibodies overnight at 4 °C. After incubation with the goat anti-rabbit secondary antibody, proteins were detected by enhanced chemiluminescence detection kit (Pierce, Rockford, IL, United States). The densitometric analysis of the protein bands was carried out with Image J software.
All data were expressed as mean ± SEM. Statistical differences were analyzed by Student’s t test or one-way ANOVA. The analysis was conducted using SPSS 17.0 software. P values below 0.05 were considered statistically significant.
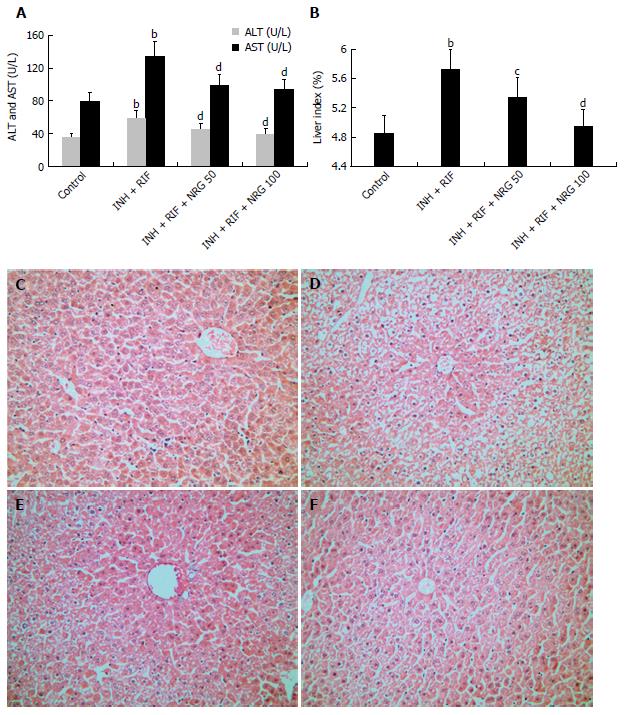
To investigate the protective effects of NRG against INH and RIF, levels of serum transaminases were analyzed. As shown in Figure 1A, administration of INH and RIF led to a significant increase in serum ALT and AST levels, whereas NRG pretreatment obviously alleviated INH- and RIF-induced elevation of ALT and AST (44.71 ± 8.15 U/L, 38.22 ± 6.64 U/L vs 58.15 ± 10.54 U/L; 98.36 ± 14.78 U/L, 92.41 ± 13.59 U/L vs 133.05 ± 19.36 U/L; all P < 0.01). Next, as shown in Figure 1B, administration of INH and RIF led to a significant increase in the liver index, while NRG pretreatment obviously attenuated INH- and RIF-induced elevation of the liver index in a dose-dependent manner (5.34% ± 0.26%, 4.93% ± 0.25% vs 5.71% ± 0.28%, P < 0.01 or 0.05). Furthermore, HE staining was performed to observe the protective effects of NRG against INH and RIF. As shown in Figure 1C-F, histopathological evaluation showed obvious necrosis and inflammatory cell infiltration in liver of mice administered with INH and RIF. Conversely, mice pretreated with NRG showed minor hepatic injury.
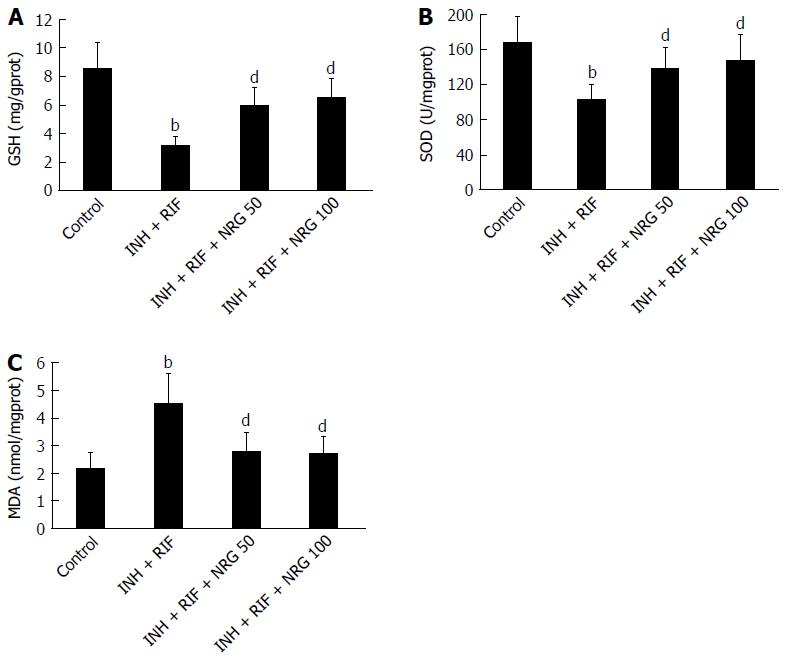
To investigate the protective effects of NRG against INH- and RIF-induced oxidative stress, levels of GSH, SOD and MDA in the liver tissue were evaluated. As shown in Figure 2A and B, hepatic GSH content and SOD activity were markedly decreased after administration of INH and RIF; however, NRG pretreatment obviously increased INH- and RIF-induced reduction of GSH and SOD (5.91 ± 1.31 mg/gprot, 6.42 ± 1.42 mg/gprot vs 3.11 ± 0.73 mg/gprot; 137.31 ± 24.62 U/mgprot, 148.83 ± 26.75 U/mgprot vs 102.34 ± 19.22 U/mgprot; all P < 0.01). In contrast, as shown in Figure 2C, hepatic MDA content, a marker of lipoperoxide, was significantly increased after administration of INH and RIF, but NRG pretreatment obviously attenuated INH- and RIF-induced elevation of MDA (2.76 ± 0.67 nmol/mgprot, 2.64 ± 0.64 nmol/mgprot vs 4.49 ± 1.12 nmol/mgprot, P < 0.01).
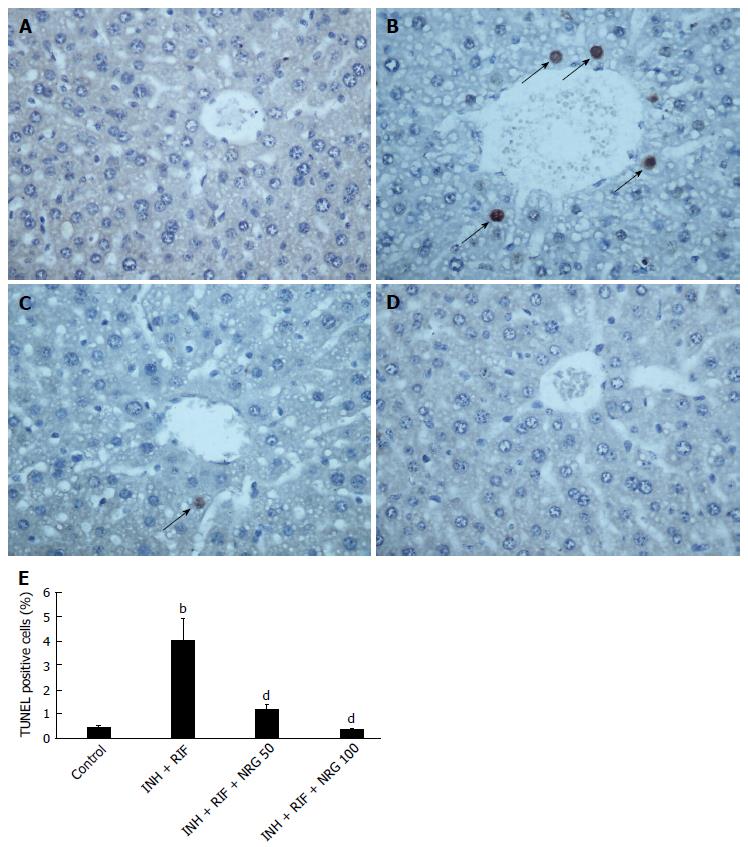
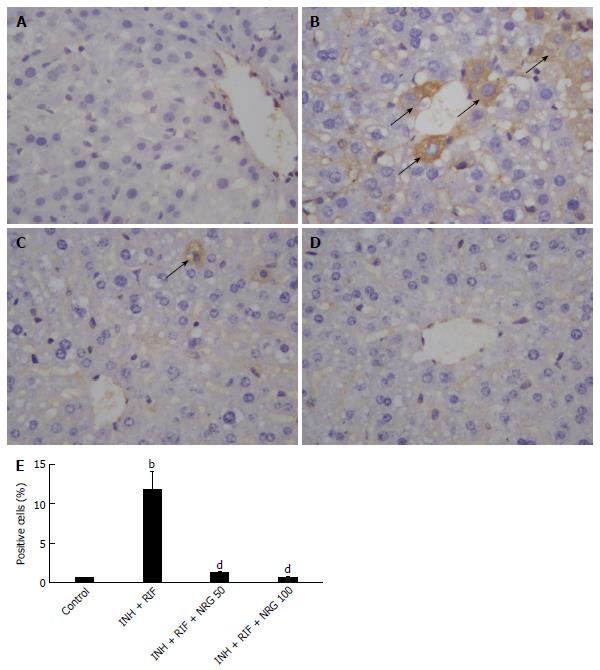
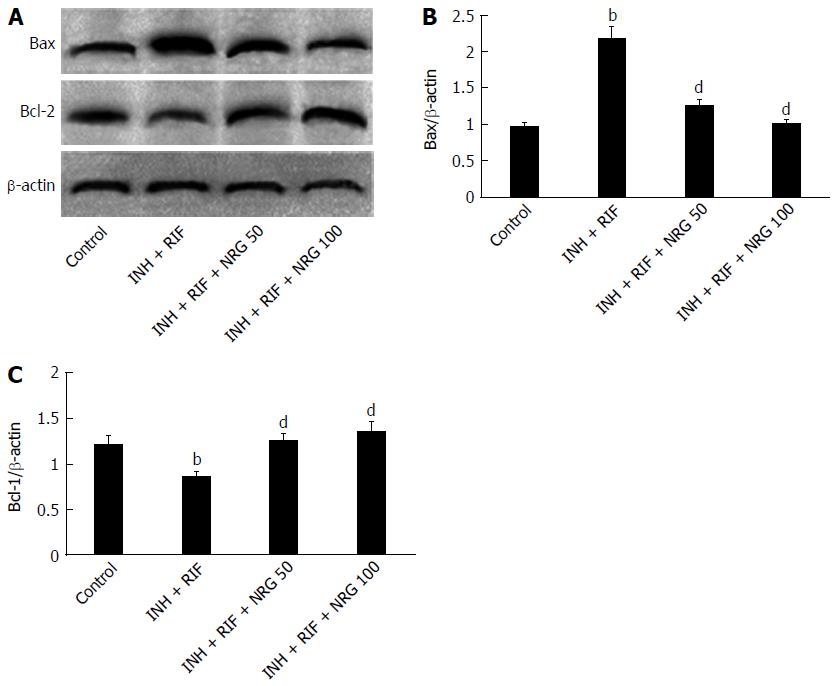
To further investigate the potential mechanisms by which NRG alleviates INH- and RIF-induced liver injury, TUNEL staining was used to estimate the apoptotic changes in hepatocytes. As shown in Figure 3, the number of TUNEL positive cells in liver was obviously increased after INH and RIF administration, while NRG pretreatment dramatically suppressed INH- and RIF-induced hepatocyte apoptosis. In agreement with TUNEL assay, as shown in Figure 4, caspase-3 activity was obviously increased after administration of INH and RIF, and NRG pretreatment markedly prevented caspase-3 activity induced by INH and RIF. In addition, expression of the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 were measured to investigate the effects of NRG against INH and RIF. As shown in Figure 5, the Bax expression level was significantly increased and the Bcl-2 expression level was significantly decreased in mice treated with INH and RIF. Conversely, NRG pretreatment down-regulated Bax expression and up-regulated Bcl-2 expression compared with INH- and RIF-treated mice (1.25 ± 0.09, 0.99 ± 0.07 vs 2.16 ± 0.15; 1.23 ± 0.08, 1.35 ± 0.08 vs 0.85 ± 0.06; all P < 0.01).
Anti-TB drugs are considered as potentially hepatotoxic and can induce hepatic injury. The co-administration of INH and RIF generates metabolic and morphological changes in hepatic tissue because of the fact that the liver is the primary detoxifying organ for the anti-TB drugs[2]. In this study, the liver injury mouse model was developed successfully via intragastric infusion of INH and RIF for 14 d; results show that serum transaminases levels of INH- and RIF-treated mice are significantly increased as compared to those in normal control mice.
NRG is considered to have beneficial effects on human health and has been vigorously researched as a potential candidate to protect against various liver diseases[18,19]. Previous studies have shown that NRG could protect against CCl4-induced chemical liver injury by reducing the oxidative stress reaction[15]. NRG also was shown to attenuate cholesterol-induced hepatitis in rats through inhibition of the NF-κB signaling pathway[14]. In addition, NRG had been shown to inhibit the hepatitis C virus by silencing its infection pathway[20].
In this study, for the first time, we explored the effects of NRG on INH- and RIF-induced liver injury and the possible underlying mechanisms in mice. We found that NRG pretreatment significantly attenuated INH- and RIF-induced elevation of the liver index, and serum ALT and AST. Furthermore, NRG pretreatment obviously attenuated INH- and RIF-induced pathologic injury. These results indicate hepatoprotective effects of NRG against anti-TB drug-induced hepatic injury.
Earlier studies showed that INH- and RIF-induced liver injury were linked to the occurrence of oxidative stress[21,22]. In this study, we observed that hepatic GSH content and SOD activity were markedly decreased after INH and RIF administration. Conversely, hepatic MDA level, a marker of lipoperoxide, was found to be significantly increased after INH and RIF administration. These results suggest that oxidative stress might play a partial role in INH- and RIF-induced hepatic injury.
NRG has exhibited antioxidant activity against oxidative stress damage. Recent studies showed that NRG could attenuate oxidative stress by decreasing the lipid peroxide level in lead-treated liver and kidney[12]. Additionally, NRG was shown to inhibit lipid peroxidation and thus to protect against the damaging effects of free radicals in acetaminophen-induced acute liver injury[13]. Moreover, NRG was shown to help to increase hepatic GSH and SOD levels and to decrease MDA content, lipid peroxidation and DNA damage in cholesterol-induced hepatic inflammation[14].
In the current study, we observed that NRG pretreatment obviously increased INH- and RIF-induced reduction of GSH and SOD. In contrast, NRG pretreatment obviously attenuated INH- and RIF-induced elevation of MDA. The results of the present study are in accordance with previous reports showing that NRG exerts significant hepatoprotective effects via inhibition of oxidative stress.
In this study, we also observed that the percentage of TUNEL positive cells in liver was markedly increased after INH and RIF administration. In agreement with findings from the TUNEL assay, caspase-3 activity was found to be markedly elevated in mice treated with INH and RIF. To further explore the molecular mechanisms of INH- and RIF-induced apoptosis of hepatocytes, the hepatic Bax and Bcl-2 protein expression were examined. Unsurprisingly, the pro-apoptotic protein Bax expression level was significantly increased and anti-apoptotic protein Bcl-2 expression level was significantly decreased after INH and RIF challenge. These data imply that hepatocytes apoptosis might play a partial role in INH- and RIF-induced hepatic injury.
Inhibiting the activation of apoptosis could help prevent liver injury. Earlier studies have suggested that NRG induces apoptosis of human cancer cells, whereas it shows no toxic effect on normal cells at a similar dose[23]. Moreover, recent studies showed that NRG protected human keratinocytes against UVB-induced apoptosis to decrease skin aging and cancer through its anti-apoptotic and anti-lipid peroxidation effects[24]. Additionally, NRG inhibited the activation of the caspase-3 and -9 to prevent hepatocytes from undergoing CCl4-induced apoptosis[25].
In the present study, we found that NRG pretreatment dramatically suppressed INH- and RIF-induced hepatocyte apoptosis. Moreover, NRG pretreatment markedly prevented caspase-3 activity induced by INH and RIF. These results suggest that NRG could protect against INH- and RIF-induced hepatic injury and the mechanism could be related to the anti-apoptotic effect. However, the underlying mechanisms of NRG-mediated anti-apoptotic effect are not well understood.
A recent study has shown that NRG prevents high glucose-induced mitochondria-mediated apoptosis involving apoptosis inducing factor, endonuclease-G and caspases. While preventing mitochondrial depolarization, NRG causes a decline in the release of apoptogenic factors like cytochrome C and endonuclease-G from mitochondria, and an increase in expression of anti-apoptogenic factors like Bcl-2[26]. In the present study, we found that NRG pretreatment down-regulated Bax expression and up-regulated Bcl-2 expression, compared with levels found in the INH- and RIF-treated mice. These results indicate that the protective effect of NRG against INH- and RIF-induced hepatocyte apoptosis involves regulation of the Bax and Bcl-2 balance.
In conclusion, the findings of the present study indicate that NRG alleviates INH- and RIF-induced hepatic injury in mice. The protective effect of NRG could be related with suppression of oxidative stress. Moreover, the beneficial effect is associated with its anti-apoptotic effect.
Isoniazid (INH) and rifampicin (RIF) are the two first-line anti-tuberculosis drugs and are known to be potentially hepatotoxic, with potential for causing drug-induced liver injury. INH is considered to be initiated by cytochrome P450-mediated metabolism of INH to acetylhydrazine and hydrazine, which is hepatotoxic. RIF, which is usually co-administered with INH, is toxic to hepatocytes. Therefore, new and safe preventive measures against INH- and RIF-induced liver injury are urgently needed.
Previous studies have shown that naringenin (NRG) has wide range of biological and pharmacological activities, such as anti-inflammatory, anti-oxidant and anti-cancer. Related studies have shown that NRG attenuates liver injury induced by lead, acetaminophen, cholesterol overdose and carbon tetrachloride (CCl4) intoxication. It was suggested that NRG exerts hepatoprotective effects and may have therapeutic value in various liver diseases.
This study is the first to explore the effects of NRG on INH- and RIF-induced liver injury and the possible mechanisms in mice. The results indicate that NRG protects against INH- and RIF-induced oxidative stress and hepatocyte apoptosis.
Caspase-3 is a member of the cysteine-aspartic acid protease family. It is activated in the apoptotic cell, both by extrinsic (death ligand) and intrinsic (mitochondrial) pathways. It plays an important role in the execution-phase of cell apoptosis.
NRG widely exists in grapefruit, bitter orange and other plants. It can be processed and purified using modern separation technology and has a broad application prospect.
The data show significant protective effect of NRG against anti-tuberculosis drug-induced liver injury through suppression of apoptosis, which is demonstrated by serum transaminases, liver histology, immunohistochemistry and western blotting. Since liver injury induced by INH and RIF is a serious problem for the treatment of tuberculosis (TB), the data are important and may provide a novel clue for management of TB.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: de F Higuera-de la Tijera M, Sherif IO, Shimizu Y, Strom SC S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Zhang FF
| 1. | WHO. Global Tuberculosis Report. World Health Organization Report 2015. . |
| 2. | Santhosh S, Sini TK, Anandan R, Mathew PT. Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur J Pharmacol. 2007;572:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 3. | Tasduq SA, Peerzada K, Koul S, Bhat R, Johri RK. Biochemical manifestations of anti-tuberculosis drugs induced hepatotoxicity and the effect of silymarin. Hepatol Res. 2005;31:132-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Sankar M, Rajkumar J, Devi J. Hepatoprotective activity of hepatoplus on isonaizid and rifampicin induced hepatotoxicity in rats. Pak J Pharm Sci. 2015;28:983-990. [PubMed] |
| 5. | Sano K, Tomioka H, Sato K, Sano C, Kawauchi H, Cai S, Shimizu T. Interaction of antimycobacterial drugs with the anti-Mycobacterium avium complex effects of antimicrobial effectors, reactive oxygen intermediates, reactive nitrogen intermediates, and free fatty acids produced by macrophages. Antimicrob Agents Chemother. 2004;48:2132-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Nicoletti NF, Rodrigues-Junior V, Santos AA, Leite CE, Dias AC, Batista EL, Basso LA, Campos MM, Santos DS, Souto AA. Protective effects of resveratrol on hepatotoxicity induced by isoniazid and rifampicin via SIRT1 modulation. J Nat Prod. 2014;77:2190-2195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Tsai SJ, Huang CS, Mong MC, Kam WY, Huang HY, Yin MC. Anti-inflammatory and antifibrotic effects of naringenin in diabetic mice. J Agric Food Chem. 2012;60:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 8. | Shi Y, Dai J, Liu H, Li RR, Sun PL, Du Q, Pang LL, Chen Z, Yin KS. Naringenin inhibits allergen-induced airway inflammation and airway responsiveness and inhibits NF-kappaB activity in a murine model of asthma. Can J Physiol Pharmacol. 2009;87:729-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Lee S, Lee CH, Moon SS, Kim E, Kim CT, Kim BH, Bok SH, Jeong TS. Naringenin derivatives as anti-atherogenic agents. Bioorg Med Chem Lett. 2003;13:3901-3903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Mershiba SD, Dassprakash MV, Saraswathy SD. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep. 2013;40:3681-3691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | Abaza MS, Orabi KY, Al-Quattan E, Al-Attiyah RJ. Growth inhibitory and chemo-sensitization effects of naringenin, a natural flavanone purified from Thymus vulgaris, on human breast and colorectal cancer. Cancer Cell Int. 2015;15:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 75] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 12. | Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X. Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res. 2012;146:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Lv Y, Zhang B, Xing G, Wang F, Hu Z. Protective effect of naringenin against acetaminophen-induced acute liver injury in metallothionein (MT)-null mice. Food Funct. 2013;4:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Chtourou Y, Fetoui H, Jemai R, Ben Slima A, Makni M, Gdoura R. Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway. Eur J Pharmacol. 2015;746:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 15. | Esmaeili MA, Alilou M. Naringenin attenuates CCl4 -induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin Exp Pharmacol Physiol. 2014;41:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 16. | Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem. 1980;106:207-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3338] [Cited by in RCA: 3096] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 17. | Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 972] [Cited by in RCA: 1072] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 18. | Yen HR, Liu CJ, Yeh CC. Naringenin suppresses TPA-induced tumor invasion by suppressing multiple signal transduction pathways in human hepatocellular carcinoma cells. Chem Biol Interact. 2015;235:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (4)] |
| 19. | Hermenean A, Ardelean A, Stan M, Hadaruga N, Mihali CV, Costache M, Dinischiotu A. Antioxidant and hepatoprotective effects of naringenin and its β-cyclodextrin formulation in mice intoxicated with carbon tetrachloride: a comparative study. J Med Food. 2014;17:670-677. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Nahmias Y, Goldwasser J, Casali M, van Poll D, Wakita T, Chung RT, Yarmush ML. Apolipoprotein B-dependent hepatitis C virus secretion is inhibited by the grapefruit flavonoid naringenin. Hepatology. 2008;47:1437-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 190] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 21. | Bhadauria S, Mishra R, Kanchan R, Tripathi C, Srivastava A, Tiwari A, Sharma S. Isoniazid-induced apoptosis in HepG2 cells: generation of oxidative stress and Bcl-2 down-regulation. Toxicol Mech Methods. 2010;20:242-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Chowdhury A, Santra A, Bhattacharjee K, Ghatak S, Saha DR, Dhali GK. Mitochondrial oxidative stress and permeability transition in isoniazid and rifampicin induced liver injury in mice. J Hepatol. 2006;45:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 119] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 23. | Kanno S, Shouji A, Hirata R, Asou K, Ishikawa M. Effects of naringin on cytosine arabinoside (Ara-C)-induced cytotoxicity and apoptosis in P388 cells. Life Sci. 2004;75:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | El-Mahdy MA, Zhu Q, Wang QE, Wani G, Patnaik S, Zhao Q, Arafa el-S, Barakat B, Mir SN, Wani AA. Naringenin protects HaCaT human keratinocytes against UVB-induced apoptosis and enhances the removal of cyclobutane pyrimidine dimers from the genome. Photochem Photobiol. 2008;84:307-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 25. | Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl(4)-induced acute liver failure. Pharm Res. 2009;26:893-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 26. | Kapoor R, Rizvi F, Kakkar P. Naringenin prevents high glucose-induced mitochondria-mediated apoptosis involving AIF, Endo-G and caspases. Apoptosis. 2013;18:9-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |