Published online Nov 28, 2016. doi: 10.3748/wjg.v22.i44.9765
Peer-review started: July 22, 2016
First decision: September 20, 2016
Revised: October 5, 2016
Accepted: October 27, 2016
Article in press: October 27, 2016
Published online: November 28, 2016
Processing time: 128 Days and 9.2 Hours
To investigate the anticancer mechanisms of the monoterpenoid alcohol linalool in human colon cancer cells.
The cytotoxic effect of linalool on the human colon cancer cell lines and a human fibroblast cell line was examined using the WST-8 assay. The apoptosis-inducing effect of linalool was measured using the terminal deoxynucleotidyl transferase dUTP nick-end labeling assay and flow cytometry with Annexin V. Oxidative stress was investigated by staining for diphenyl-1-pyrenylphosphine, which is a cellular lipid peroxidation marker, and electron spin resonance spectroscopy. Sixteen SCID mice xenografted with human cancer cells were randomized into 3 groups for in vivo analysis: control and low-dose and high-dose linalool groups. The control group was administered tap water orally every 3 d. The linalool treatment groups were administered 100 or 200 μg/kg linalool solution orally for the same period. All mice were sacrificed under anesthesia 21 d after tumor inoculation, and tumors and organs were collected for immunohistochemistry using an anti-4-hydroxynonenal antibody. Tumor weights were measured and compared between groups.
Linalool induced apoptosis of cancer cells in vitro, following the cancer-specific induction of oxidative stress, which was measured based on spontaneous hydroxyl radical production and delayed lipid peroxidation. Mice in the high-dose linalool group exhibited a 55% reduction in mean xenograft tumor weight compared with mice in the control group (P < 0.05). In addition, tumor-specific lipid peroxidation was observed in the in vivo model.
Linalool exhibited an anticancer effect via cancer-specific oxidative stress, and this agent has potential for application in colon cancer therapy.
Core tip: We elucidated the anticancer mechanism of the monoterpenoid alcohol, linalool, which induces apoptosis specifically in cancer cells via lipid peroxidation. Electron spin resonance (ESR) spectroscopy, which enables the real-time visualization of free radicals in live cells, revealed that oxidative stress developed immediately after treatment only in cancer cells. This study demonstrated that the natural compound linalool exerted an anticancer effect without causing serious side effects, and that the further utilization of ESR may support the application of linalool as a new and cost-effective cancer therapy.
- Citation: Iwasaki K, Zheng YW, Murata S, Ito H, Nakayama K, Kurokawa T, Sano N, Nowatari T, Villareal MO, Nagano YN, Isoda H, Matsui H, Ohkohchi N. Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J Gastroenterol 2016; 22(44): 9765-9774
- URL: https://www.wjgnet.com/1007-9327/full/v22/i44/9765.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i44.9765
Colorectal cancer is the fourth most common cause of cancer-related deaths globally, and the number of deaths has increased to approximately 700000 annually[1]. Chemotherapy is an effective treatments for colorectal cancer, but its side effects, such as hair loss, low blood counts, hand-foot syndrome, and neuropathy, may depress the patient’s quality of life[2,3]. In addition, the current anticancer drugs are expensive[4]. Therefore, efforts are underway worldwide to identify new, effective, and inexpensive anticancer compounds with fewer side effects, and several types of natural compounds have recently been recognized as possible sources for anticancer drugs[5-9].
This study examined the anticancer effects of the monoterpenoid alcohol linalool, which is commonly used as a flavoring agent. Linalool is found abundantly in red wine, essential oil of lavender, and coriander fruits[10]. Several studies have reported the anticancer potential of linalool against solid tumor cell lines, such as gastric cancer, lung cancer, skin cancer[11], and hepatic cancer (HepG2)[12], as well as several leukemia cell lines[13]. Some of these studies reported that linalool also exerted an apoptotic effect[11,13], induced oxidative stress[12,14], and exhibited immunomodulation[15]. However, the mechanism by which linalool exerts its cytotoxic effect has not yet been elucidated[14]. We hypothesized that linalool’s anticancer effects are mediated through the cancer-specific generation of hydroxyl radical followed by apoptosis. We investigated the cytotoxic effects of linalool in the human colon cancer cell line HCT 116 by analyzing the cell death mechanisms and measuring oxidative stress.
We focused on the detection of instant reactive oxygen species (ROS) production by using electron spin resonance (ESR) spectroscopy. ESR is a highly sensitive and the most definitive method for the detection of short-lived ROS using the spin-trapping technique, such as the hydroxyl radical, superoxide, and hydroperoxyl radical[16-18]. ESR was developed in the early 1970s, and it is often used in research of ischemia-reperfusion injury[19-21] and oxidative stress after exercise[22]. The method is not commonly used in cancer biology studies, but it has potential for wide application in cancer screening and therapeutic evaluation in the near future, because it is becoming evident that both the ROS levels and redox signaling can affect the phenotypic profile of cancer cells and their responsiveness to therapeutic interventions[23,24].
Linalool (97% pure; Sigma Aldrich, St. Louis, MO, USA), diphenyl-1-pyrenylphosphine(DPPP) (Dojindo, Kumamoto, Japan), 5,5-dimethyl-1-pyrroline-N-oxide(DMPO) (Radical Research Inc., Tokyo, Japan), dimethyl sulfoxide (DMSO) (Wako, Osaka, Japan), and Dulbecco’s modified Eagle’s medium (DMEM) (Wako, Osaka, Japan) were purchased.
Six-week-old male severe combined immune deficiency (SCID) mice (Clea, Tokyo, Japan) were maintained in plastic cages in a temperature-controlled room on a 12-h light/dark cycle with free access to water and a standard pellet diet throughout the experiment. After an acclimation period of 7 d, the solid tumor was developed by the subcutaneous inoculation of 1 × 106 HCT 116 cells on the right flank of each mouse. The mice were divided into the following three groups: control group (n = 5), mice treated with saline; low-dose linalool group (n = 5), mice treated with 100 mg/kg linalool; and high-dose linalool group, mice treated with 200 mg/kg linalool (n = 6). Seven days after tumor injection, saline, low-dose linalool, and high-dose linalool were administered orally via gavage every 3 d in a volume of 25 μL. All the animals were sacrificed 21 d after tumor inoculation. The serum levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were detected. All animal experiments were performed in a humane manner after approval from the Institutional University Experiment Committee of the University of Tsukuba (protocol number: 15-254).
Human colon cancer cell lines, e.g., HCT 116, and a human fibroblast cell line CCD18Co were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA) and subdivided into multiple tubes for stocking in liquid nitrogen. HCT 116: a human colon cancer cell line that is commonly used to study cancer invasion and tumorigenesis (passage 4), WiDr: colon adenocarcinoma cell line established from a 78 year old female (passage 6), RKO: a poorly differentiated colon carcinoma cell line (passage 4), HCT-15: a quasidiploid human colon cancer cell line (passage 6), SW480: established from Dukes’ type B, colorectal adenocarcinoma cells (passage 5), and CCD18Co: a human fibroblast cell line used as a substitute for normal cells (passage 6), were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with inactivated 10% fetal bovine serum and 1% penicillin/streptomycin. All cells were cultured in a 5% CO2 cell culture incubator at 37 °C.
Cell viability was examined using the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan), according to the manufacturer’s instructions. Cancer cells ware seeded in a 96-well dish at 3000 cells/well, incubated overnight, and incubated with different concentrations of linalool for 24 h. The medium was replaced with the medium containing 10% WST-8, and the cells were incubated further for 2 h. The absorbance of each well at 450 nm was measured by a SUNRISE Rainbow microplate reader (TECAN, Groedig, Austria).
The In Situ Cell Death Detection kit(Roche Diagnostics, Basel, Switzerland) was used to detect apoptosis in the cell culture. HCT 116 cells were seeded at 3000 cells/well onto Lab-Tek II Chamber Slides (Nalge Nunc International, Tokyo, Japan) and incubated overnight. Cells were incubated with linalool (0, 100 μmol/L) or 50 nM staurosporine as a positive control for 24 h. Cells were incubated with the terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) reaction mixture according to the manufacturer’s instructions.
A flow cytometer (Muse Cell Analyzer) and Muse Annexin V & Dead Cell Kit (Merck Millipore, Darmstadt, Germany) were used for quantitative analyses of live, early and late apoptosis, and cell death in suspension cell lines. HCT 116 cells were seeded at a density of 1 × 105 cells per dish (diameter, 6 cm) and incubated overnight. Cells were incubated with different concentrations of linalool (0, 10, 100, 1000 μmol/L) and 50 nM staurosporine as the positive control for 24 h. Cells were prepared with Annexin V and Dead Cell reagent according to the manufacturer’s instructions, and the samples were evaluated using the MUSE analyzer with the appropriate thresholds for cell size and apoptotic profile.
HCT 116 cells and CCD18Co cells were seeded at a density of 3000 cells/well each onto Lab-Tek Chambered Borosilicate Coverglass Slides (Nalge Nunc International, Tokyo, Japan) and incubated overnight. CCD18Co cells were used as a normal cell control. diphenyl-1-pyrenylphosphine (DPPP) (5 mmol/L) was dissolved in DMSO, and the solution was diluted using DMEM without Phenol red or FBS. The final concentration was adjusted to 50 μmol/L. A volume of 500 μL of the solution was added to each well and incubated for 10 min. Cells were incubated with different concentrations of linalool for 2 h. The oxidation of DPPP was monitored using KEYENCE BZ-X700 (Keyence, Osaka, Japan). Wavelengths of excitation and emission were set at 352 nm and 380 nm, respectively.
HCT 116 cells were cultured on rectangular cover slides and incubated overnight. Cells were incubated for 15 min with different concentrations of linalool (0, 250, 500, 1000 μmol/L) and were immersed in a culture medium with 5 mmol/L respiratory substrates (succinate, glutamate, and malate), 5 mmol/L nicotinamide adenine dinucleotide (NADH), and 10 mmol/L DMPO. Immersed-slide glasses were placed on the tissue glass and measured using the EPR apparatus for 10 min, 30 min, 1 h, and 2 h after treatment. All spectra were recorded using a JEOL-TE X-band spectrometer (JEOL Ltd., Tokyo, Japan) and analyzed using Win-Rad Radical Analyzer System (Radical Research, Inc., Tokyo, Japan).
Immunohistochemistry for 4-HNE was performed in all samples as follows. Paraffin was removed with xylene, and endogenous peroxidase was blocked in tissues using 3% of H2O2 in methanol. All tissue sections were pretreated in citrate buffer (pH 6.0) for 10 min at 100 °C in a microwave oven. Nonspecific binding sites were blocked using 10% BSA before incubation with the primary antibody. Slides were washed, incubated with biotinylated secondary IgG for 30 min and exposed to a biotin-peroxidase complex.
Statistical analysis was performed using GraphPad Prism 5. Significant probability (P value) was calculated using ANOVA followed by Dunnett’s test. The results are expressed as the mean ± SD, and P < 0.05 was considered statistically significant.
The statistical methods of this study were reviewed by Isao Muraki, MD, PhD, an epidemiologist in the Department of Cardiovascular Disease Prevention, Osaka Center for Cancer and Cardiovascular Disease Prevention.
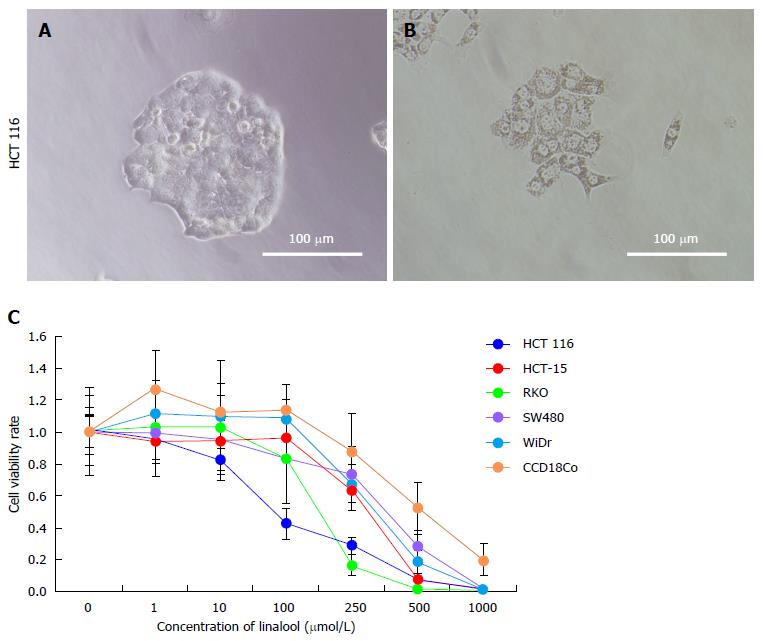
Phase contrast microscopy was used to observe the morphologic changes of HCT 116 cell line, and the WST assay was performed for each cell line to evaluate cytotoxicity after exposure to linalool. Cancer cells exhibited rapid proliferation under control conditions (Figure 1A). In contrast, cancer cells stopped proliferating after treatment with 250 μmol/L linalool and exhibited fragmentation of chromatin, bleb formation on the cell surface, and shrinkage, which are representative of apoptosis (Figure 1B). We observed a dose-dependent increase in the cytotoxic effect of linalool in all cancer cell lines and fibroblast cell lines, but the cell viability of CCD18Co was suggested to be higher than that of HCT 116 at 100-500 μmol/L (Figure 1C). Micrographs of HCT 116 cells treated with different linalool concentrations for 24 h revealed shrinkage of cells and tightly packed organelles at concentrations over 100 μmol/L (data not shown).
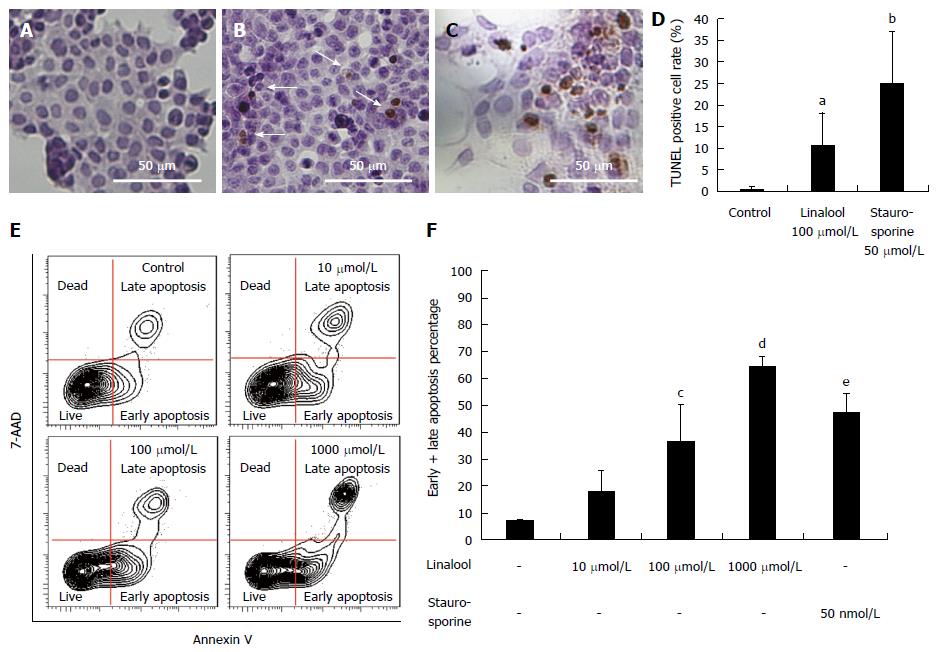
TUNEL staining was performed using HCT 116 cells to clarify the mechanism of linalool cytotoxicity. The cells were divided into the following three groups: control, linalool, and staurosporine groups. The corresponding percentages of TUNEL-positive cells were 0.7% ± 0.7% (Figure 2A), 10.5% ± 7.7% (with 100 μmol/L linalool treatment; Figure 2B), and 25.1% ± 11.2% (with 100 μmol/L staurosporine treatment; Figure 2C). The percentage of apoptotic cells in each group is shown as a column graph with error bars (Figure 2D).
The Annexin V assay was performed for a further quantitative analysis of live cells and cells showing early and late apoptosis, and cell death. Figure 2E and 2F shows the percentage of gated cells in each quadrant, and the data revealed a dose-dependent increase in cells in early and late apoptosis phases.
These results clearly demonstrated that linalool induced apoptosis of human colon cancer cells in a dose-dependent manner in vitro.
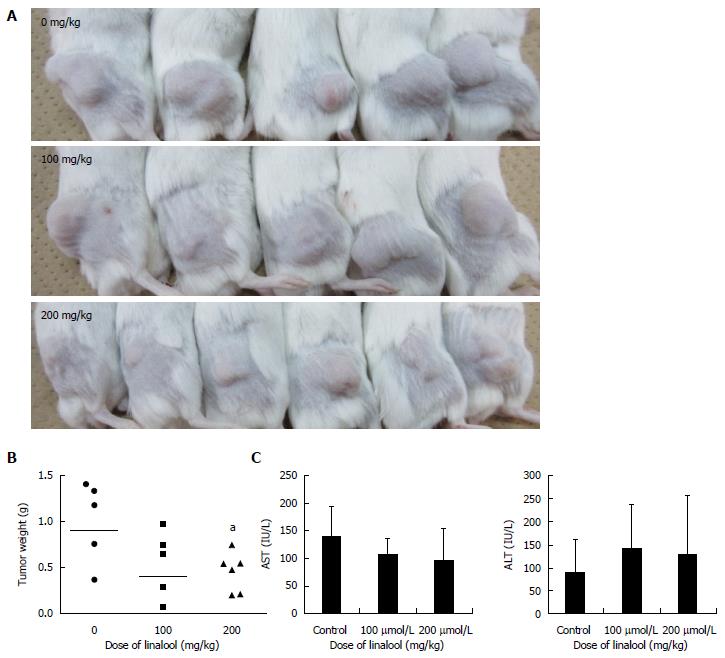
A human cancer xenografted mouse model was established, and linalool was administered orally to investigate whether linalool exhibited an anticancer effect in vivo. Treatment with linalool caused a decrease in the size and weight of subcutaneous tumors (Figure 3A). And the difference in the resected tumor weight between the control group and the high-dose (200 mg/kg) linalool treatment group was significant (Figure 3B). No changes in the mean body weight and serum AST and ALT levels among the three groups were detected (Figure 3C). Skin damage, hair loss, intestinal bleeding, and other abnormal symptoms were not observed in any of the three groups. These results demonstrated that linalool exhibited an anti-proliferation effect on transplanted human cancer cells without significant weight loss or damage to organs.
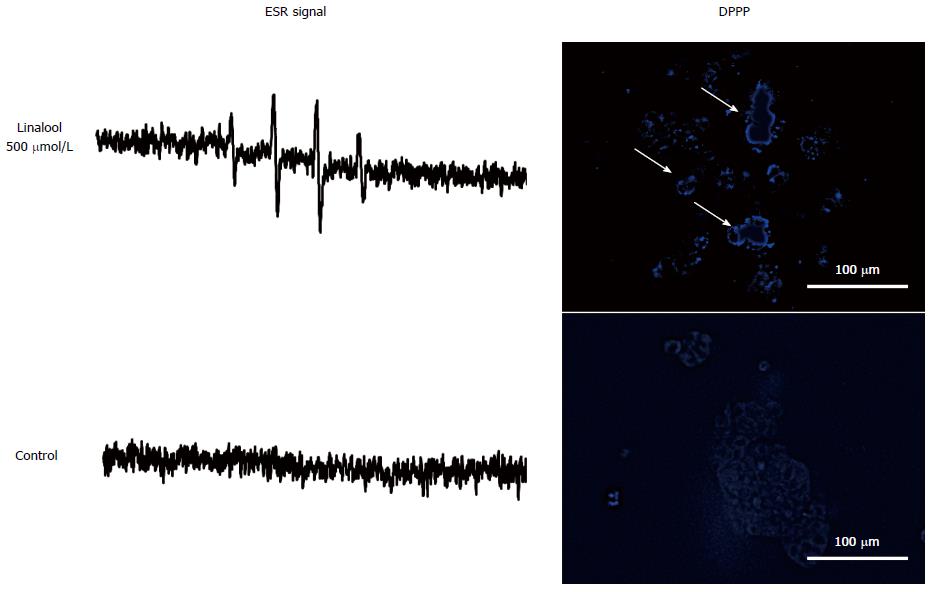
An oxidative stress assay was performed to further confirm the mechanism of the apoptotic effect of linalool. ESR spectroscopy was performed in HCT 116 and CCD18Co cells using DMPO as a spin-trapping reagent. The signal intensity of DMPO reflects linalool-induced hydroxyl radical production. Cancer-specific signal intensity gain was observed only for 500 μmol/L linalool 30 min after treatment (Figure 4A), which was diminished at an earlier time point (10 min) and at later time points (1 and 2 h). The signal intensity of control is shown in (Figure 4B). There was no gain in signal intensity for CCD18Co cells at any time point (data not shown). The DPPP assay was performed to detect lipid peroxidation as green fluorescence on the cell surface. Linalool induced HCT 116 cell membrane lipid peroxidation(Figure 4C), whereas the signal was not observed without linalool(Figure 4D). CCD18Co also showed no lipid peroxidation by linalool (data not shown).
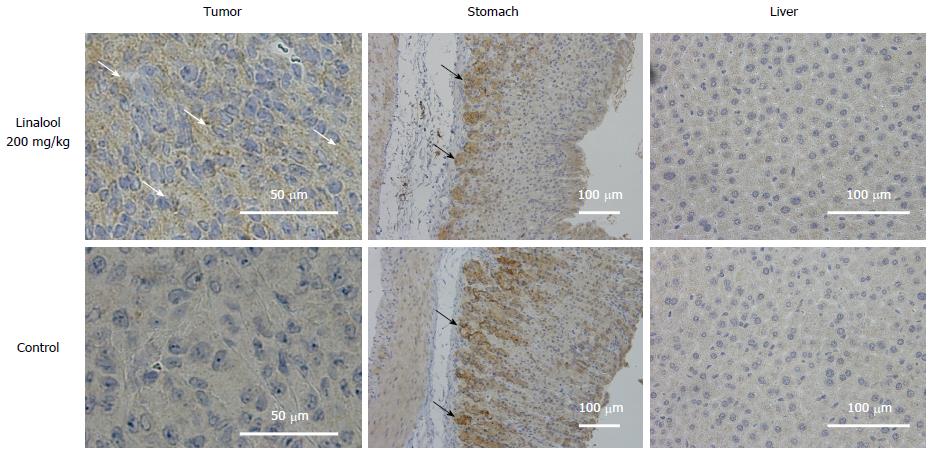
Histological analyses of mouse tumor samples demonstrated a reduction of cancer cells and a relatively large area of necrotizing tissue inside the tumor. Immunohistochemistry for 4-HNE, which is a marker of oxidative stress due to increased lipid peroxidation, revealed an accumulation of 4-HNE in tumors from the high-dose linalool group compared to tumors from the control group. However, no significant difference in 4-HNE accumulation in stomach or liver tissues was observed between any of the groups (Figure 5).
These results revealed that linalool induced cancer-specific oxidative stress that was first induced in the form of hydroxyl radical production, followed by lipid peroxidation, and exhibited a selective anticancer apoptotic effect.
In this study, we have demonstrated for the first time that the rapid induction of ROS by linalool as a trigger of subsequent apoptotic cascade via lipid peroxidation with living-cellular ESR technique. We also revealed that this mechanism was cancer specific and significant weight loss or damage to normal organs were not observed in the human cancer xenografted mice with oral administration of linalool. These results raise the possibility that linalool is applicable as a new resource for cancer therapy.
The anticancer activity of some essential oils from various plants has received substantial attention in recent years because these essential oils are likely to serve as new sources of chemotherapeutic drugs[25]. Existing chemotherapeutic agents exert cytotoxic effect not only on cancer cells but also on normal cells, and various side effects are observed. In contrast, some essential oils exhibit maximum efficacy against cancer cells and cause minimal toxic effects in normal cells[26]. The elucidation of the mechanism by which these essential oils exert the anticancer effect possibly contribute to the improvement in survival rate and quality of life (QOL) of cancer patients. Monoterpenes are non-nutritive dietary component found in the essential oils of citrus fruits, mints and herbs and belong to a group of chemical compounds named “terpenes”, representing a group of natural compounds whose basic structure consists of two linked isoprene units, which are formed by a 5-carbon base each[27]. Linalool is an acyclic monoterpenoid alcohol with a chemical formula of C10H18O and molecular mass of 154.25 g/mol, and has widely been used for its fragrance and odorant qualities in cosmetics, soaps, and perfumes. It is an inexpensive, familiar, and well-known chemical with little toxicity for a human body. According to the aforementioned references, there are three phenomena as anticancer activities of linalool: apoptotic effect[11,13], induction of oxidative stress[12,14], and immunomodulation[15]. However, its underlying mechanism and the reason why linalool exerts cancer selective cytotoxicity have not been confirmed. Therefore, we focused on the detection of instant reactive oxygen species (ROS) production by using ESR spectroscopy.
Free electrons of ROS align with their low energy status (βspin) or high energy status (αspin) in an external magnetic field. A transition between low- and high-energy states may occur when sufficient microwave energy is absorbed[28]. An ESR spectrum is obtained and recorded by observing variations in magnetic field strength at a fixed microwave frequency. ROS are generally present at low concentrations and very reactive, and the use of spin-trapping reagents solves these problems. The ESR spin-trapping technique involves the use of chemical species called spin traps, which react with short-lived free radicals to form relatively stable adducts with a half-life that is adequate to observe the ESR spectrum[29]. This study used DMPO as a spin trap to specifically detect hydroxyl radicals with a signal intensity of 1:2:2:1[29]. Furthermore, more than 90% of intracellular ROS are generated from mitochondria, and these ROS peroxidize lipids at the same location and produce HNE around the mitochondria, which leads to apoptosis[30]. ROS was detected almost immediately and directly after treatment by using an ESR system. The findings of our in vitro experiments following ESR signal detection are consistent with the aforementioned findings, which means that ROS generation, as a sign of apoptosis of cancer cells. The result of our study indicates that detection of ROS by ESR is applicable to the screening and therapeutic evaluation of anticancer agents.
The results obtained from this study showed that linalool has distinct roles in lipid peroxidation in cancer and other tissues such as the stomach and the liver. It has been described that such a cancer selective effect probably depend on the redox state of the cell[31]. The cancer cellular concentrations of ROS used to be higher than that of normal cells because the cancer cellular metabolism is completely different: it is well known as the Warburg effect[32]. Under such condition, cancer cells should have a higher ability for ROS regulation as its survival strategy[33,34]. This is a reason why cancer cells can endure the oxidative stress environments. In addition, one recent study reported an inhibitory effect of linalool on mitochondrial complexes I and II, the electron transport, followed by mitochondrial derangements and cell death in HepG2 cells[12]. Thus, the difference of the redox, metabolic, and mitochondrial states between the cancer and normal organ tissues might be responsible for linalool’s selective oxidative stress induction and selective cytotoxicity to cancer cells. Although we focused on the instant generation of ROS using ESR, which produced complementary findings, further studies are required to establish the strategy to produce a state of selective oxidative stress in cancer cells.
In conclusion, we demonstrated that linalool induced apoptosis of human colon cancer cells via cancer-specific oxidative stress, which was detectable as an ESR signal. The further utilization of ESR can reveal novel uses for natural compounds, such as linalool, as new, cost-effective cancer therapies.
The authors thank Ako Takahashi for providing technical assistance.
Colorectal cancer is a major public health problem, and its mortality is very high globally. Chemotherapy is the most effective treatment for colorectal cancer, but the problems of side effects and high costs remain unresolved.
There is a growing interest in the search for the cancer preventive or therapeutic potential of natural compounds, which are not expensive and exhibit few side effects. Linalool is a monoterpenoid alcohol that is present abundantly in red wine and coriander and exhibits anticancer effects in some types of human cancer. However, the mechanisms of this effect are not clear.
The authors focused on the detection of instant reactive oxygen species (ROS) production using electron spin resonance (ESR) spectroscopy, which is a highly sensitive and definitive method for the detection of short-lived ROS. We revealed that the first sign of apoptosis in cancer cells induced by linalool may be detected almost immediately and directly after treatment by using an ESR system. This result is an important finding for the screening and therapeutic evaluation of new anticancer agents.
This study was designed to evaluate the anticancer mechanisms of linalool on human colon cancer cell lines for the development of new effective anticancer strategies.
Linalool is an acyclic monoterpenoid alcohol with a chemical formula of C10H18O and molecular mass of 154.25 g/mol. Approximately 12000 T of linalool is produced for the industrial use annually, and its natural biosynthesis through plants, primarily herbs, trees and fruits, is higher by dimension. More than 95% of synthetic linalool is used for its fragrance and odorant qualities in cosmetics, soaps, and perfumes, and only approximately 1% is added to food and beverages for aroma and flavoring. Linalool is an inexpensive, familiar, and well-known chemical with great prospects for medical application.
Article “Anticancer activity of linalool via cancer-specific hydroxyl radical generation in human colon cancer”, provides significant and interesting results regarding the anticancer mechanism of linalool by inducing apoptosis specifically in cancer cells via lipid peroxidation.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gopcevic KR, Lakatos PL S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Brody H. Colorectal cancer. Nature. 2015;521:S1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 378] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 2. | Braun MS, Seymour MT. Balancing the efficacy and toxicity of chemotherapy in colorectal cancer. Ther Adv Med Oncol. 2011;3:43-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Teker F, Demirag G, Erdem D, Kemal Y, Yucel I. Quality of life in colorectal cancer patients during chemotherapy in the era of monoclonal antibody therapies. J BUON. 2015;20:443-451. [PubMed] |
| 4. | Hoyle M, Crathorne L, Peters J, Jones-Hughes T, Cooper C, Napier M, Tappenden P, Hyde C. The clinical effectiveness and cost-effectiveness of cetuximab (mono- or combination chemotherapy), bevacizumab (combination with non-oxaliplatin chemotherapy) and panitumumab (monotherapy) for the treatment of metastatic colorectal cancer after first-line chemotherapy (review of technology appraisal No.150 and part review of technology appraisal No. 118): a systematic review and economic model. Health Technol Assess. 2013;17:1-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 5. | Tan BL, Norhaizan ME, Huynh K, Yeap SK, Hazilawati H, Roselina K. Brewers’ rice modulates oxidative stress in azoxymethane-mediated colon carcinogenesis in rats. World J Gastroenterol. 2015;21:8826-8835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Maruyama T, Murata S, Nakayama K, Sano N, Ogawa K, Nowatari T, Tamura T, Nozaki R, Fukunaga K, Ohkohchi N. (-)-Epigallocatechin-3-gallate suppresses liver metastasis of human colorectal cancer. Oncol Rep. 2014;31:625-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Murata S, Shiragami R, Kosugi C, Tezuka T, Yamazaki M, Hirano A, Yoshimura Y, Suzuki M, Shuto K, Ohkohchi N. Antitumor effect of 1, 8-cineole against colon cancer. Oncol Rep. 2013;30:2647-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Samet I, Han J, Jlaiel L, Sayadi S, Isoda H. Olive (Olea europaea) leaf extract induces apoptosis and monocyte/macrophage differentiation in human chronic myelogenous leukemia K562 cells: insight into the underlying mechanism. Oxid Med Cell Longev. 2014;2014:927619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Boulaaba M, Tsolmon S, Ksouri R, Han J, Kawada K, Smaoui A, Abdelly C, Isoda H. Anticancer effect of Tamarix gallica extracts on human colon cancer cells involves Erk1/2 and p38 action on G2/M cell cycle arrest. Cytotechnology. 2013;65:927-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Burdock GA, Carabin IG. Safety assessment of coriander (Coriandrum sativum L.) essential oil as a food ingredient. Food Chem Toxicol. 2009;47:22-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 107] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Cherng JM, Shieh DE, Chiang W, Chang MY, Chiang LC. Chemopreventive effects of minor dietary constituents in common foods on human cancer cells. Biosci Biotechnol Biochem. 2007;71:1500-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Usta J, Kreydiyyeh S, Knio K, Barnabe P, Bou-Moughlabay Y, Dagher S. Linalool decreases HepG2 viability by inhibiting mitochondrial complexes I and II, increasing reactive oxygen species and decreasing ATP and GSH levels. Chem Biol Interact. 2009;180:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Gu Y, Ting Z, Qiu X, Zhang X, Gan X, Fang Y, Xu X, Xu R. Linalool preferentially induces robust apoptosis of a variety of leukemia cells via upregulating p53 and cyclin-dependent kinase inhibitors. Toxicology. 2010;268:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Srithar G, Sudha M, Nalini N. Linalool exerts dose dependent chemopreventive effect against 1 , 2-dimethylhydrazine induced rat colon carcinogenesis. Int J Pharm Biol Arch. 2013;4:758-770. |
| 15. | Chang MY, Shen YL. Linalool exhibits cytotoxic effects by activating antitumor immunity. Molecules. 2014;19:6694-6706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Kominami S, Rokushika S, Hatano H. Studies of short-lived radicals in the gamma-irradiated aqueous solution of uridine-5’-monophosphate by the spin-trapping method and the liquid chromatography. Int J Radiat Biol Relat Stud Phys Chem Med. 1976;30:525-534. [PubMed] |
| 17. | Kohno M, Mizuta Y, Kusai M, Masumizu T, Makino K. Measurements of superoxide anion radical and superoxide anion scavenging activity by electron spin resonance spectroscopy coupled with DMPO spin trapping. Bull Chem Soc Jpn. 1994;67:1085-1090. [RCA] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Noda Y, Anzai K, Mori A, Kohno M, Shinmei M, Packer L. Hydroxyl and superoxide anion radical scavenging activities of natural source antioxidants using the computerized JES-FR30 ESR spectrometer system. Biochem Mol Biol Int. 1997;42:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Li XY, McCay PB, Zughaib M, Jeroudi MO, Triana JF, Bolli R. Demonstration of free radical generation in the “stunned” myocardium in the conscious dog and identification of major differences between conscious and open-chest dogs. J Clin Invest. 1993;92:1025-1041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 92] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Zini I, Tomasi A, Grimaldi R, Vannini V, Agnati LF. Detection of free radicals during brain ischemia and reperfusion by spin trapping and microdialysis. Neurosci Lett. 1992;138:279-282. [PubMed] |
| 21. | Arroyo CM, Kramer JH, Dickens BF, Weglicki WB. Identification of free radicals in myocardial ischemia/reperfusion by spin trapping with nitrone DMPO. FEBS Lett. 1987;221:101-104. [PubMed] |
| 22. | Ashton T, Young IS, Peters JR, Jones E, Jackson SK, Davies B, Rowlands CC. Electron spin resonance spectroscopy, exercise, and oxidative stress: an ascorbic acid intervention study. J Appl Physiol (1985). 1999;87:2032-2036. [PubMed] |
| 23. | Santos J, Alonso C, Guilarte M, Vicario M, Malagelada JR. Targeting mast cells in the treatment of functional gastrointestinal disorders. Curr Opin Pharmacol. 2006;6:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Schumacker PT. Reactive oxygen species in cancer: a dance with the devil. Cancer Cell. 2015;27:156-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 25. | Miyashita M, Sadzuka Y. Effect of linalool as a component of Humulus lupulus on doxorubicin-induced antitumor activity. Food Chem Toxicol. 2013;53:174-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Jana S, Patra K, Sarkar S, Jana J, Mukherjee G, Bhattacharjee S, Mandal DP. Antitumorigenic potential of linalool is accompanied by modulation of oxidative stress: an in vivo study in sarcoma-180 solid tumor model. Nutr Cancer. 2014;66:835-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Almeida JR, Souza GR, Silva JC, Saraiva SR, Júnior RG, Quintans Jde S, Barreto Rde S, Bonjardim LR, Cavalcanti SC, Quintans LJ. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. ScientificWorldJournal. 2013;2013:808460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 28. | He W, Liu Y, Wamer WG, Yin JJ. Electron spin resonance spectroscopy for the study of nanomaterial-mediated generation of reactive oxygen species. J Food Drug Anal. 2014;22:49-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 111] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 29. | Buettner GR. Spin trapping: ESR parameters of spin adducts. Free Radic Biol Med. 1987;3:259-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1360] [Cited by in RCA: 1291] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 30. | Indo HP, Davidson M, Yen HC, Suenaga S, Tomita K, Nishii T, Higuchi M, Koga Y, Ozawa T, Majima HJ. Evidence of ROS generation by mitochondria in cells with impaired electron transport chain and mitochondrial DNA damage. Mitochondrion. 2007;7:106-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 31. | Lee JC, Kim J, Park JK, Chung GH, Jang YS. The antioxidant, rather than prooxidant, activities of quercetin on normal cells: quercetin protects mouse thymocytes from glucose oxidase-mediated apoptosis. Exp Cell Res. 2003;291:386-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794-798. [PubMed] |
| 33. | Warburg O, Wind F, Negelein E. THE METABOLISM OF TUMORS IN THE BODY. J Gen Physiol. 1927;8:519-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 34. | Kroemer G. Mitochondria in cancer. Oncogene. 2006;25:4630-4632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |