Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9642
Peer-review started: July 25, 2016
First decision: September 12, 2016
Revised: September 24, 2016
Accepted: October 19, 2016
Article in press: October 19, 2016
Published online: November 21, 2016
Processing time: 119 Days and 1.8 Hours
To perform meta-analysis of the use of Endocuff during average risk screening colonoscopy.
Scopus, Cochrane databases, MEDLINE/PubMed, and CINAHL were searched in April 2016. Abstracts from Digestive Disease Week, United European Gastroenterology, and the American College of Gastroenterology meeting were also searched from 2004-2015. Studies comparing EC-assisted colonoscopy (EAC) to standard colonoscopy, for any indication, were included in the analysis. The analysis was conducted by using the Mantel-Haenszel or DerSimonian and Laird models with the odds ratio (OR) to assess adenoma detection, cecal intubation rate, and complications performed.
Nine studies (n = 5624 patients) were included in the analysis. Compared to standard colonoscopy, procedures performed with EC had higher frequencies for adenoma (OR = 1.49, 95%CI: 1.23-1.80; P = 0.03), and sessile serrated adenomas detection (OR = 2.34 95%CI: 1.63-3.36; P < 0.001). There was no significant difference in cecal intubation rates between the EAC group and standard colonoscopy (OR = 1.26, 95%CI: 0.70-2.27, I2 = 0%; P = 0.44). EAC was associated with a higher risk of complications, most commonly being superficial mucosal injury without higher frequency for perforation.
The use of an EC on colonoscopy appears to improve pre-cancerous polyp detection without any difference in cecal intubation rates compared to standard colonoscopy.
Core tip: Our meta-analysis of more than 5000 patients demonstrates that when compared to traditional colonoscopy, the use of an Endocuff device improves adenoma detection rates without any adverse effect on procedural efficiency or increased risk of significant adverse events.
- Citation: Chin M, Karnes W, Jamal MM, Lee JG, Lee R, Samarasena J, Bechtold ML, Nguyen DL. Use of the Endocuff during routine colonoscopy examination improves adenoma detection: A meta-analysis. World J Gastroenterol 2016; 22(43): 9642-9649
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9642.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9642
Colorectal cancer is one of the most frequently occurring tumors in the industrialized world[1,2]. One unique benefit of colonoscopy over other noninvasive methods of cancer screening is the potential to not only diagnose precancerous adenomatous polyps, but also to remove them endoscopically and thereby reduce colorectal cancer morbidity and mortality[3]. However, even with excellent colonoscopic technique, some poorly visualized areas of the colon, such as behind folds and at the inner curves of flexures, can be difficult to examine. On tandem colonoscopy studies, adenoma miss rates as high as 27% have been reported[4], and subsequent population-based studies have revealed an interval cancer rate after colonoscopy as high as 3%-8%[5,6]. Investigation has revealed that the adenoma detection rate (ADR), the proportion of one provider’s colonoscopies during which at least one adenoma can be detected, correlates inversely a patient’s risk of developing an interval cancer[7,8]. These findings have led to intense interest in using ADR as a measure of colonoscopy quality.
Another recent area of interest is the observation that the majority of interval cancers are right-sided[9] possibly from missed right-sided sessile serrated adenomas (SSAs)[10]. Through the microsatellite instability pathway, such polyps have been shown to carry a significant risk of progression to malignant change[11]. However, SSAs are known to have traditionally indiscriminate borders and similar endoscopic appearance to other benign colorectal lesions, representing a particular challenge for the endoscopist.
A number of endoscopic devices have been developed in an effort to improve colonoscopic polyp detection and subsequent removal. One such device, the EC (Arc Medical Design Ltd., Leeds, England), is a cap designed to be affixed to the colonoscope head and is comprised of soft projections which remain flattened during insertion and project out, on withdrawal, to spread out the colonic folds. Early experience with the EC suggested that it improved visualization, appeared to have improved polyp detection and facilitated polypectomy[12].
A number of studies have been published comparing the efficacy of EC-assisted colonoscopy (EAC) to that of standard colonoscopy. We performed a meta-analysis of these studies, with a particular interest in adenoma detection, SSA detection, right-sided polyp detection, and other performance characteristics including cecal intubation and complication rates in order to quantitatively summarize the safety and efficacy of EAC.
A three-point systematic and comprehensive literature search was performed on multiple databases. First, Scopus, Cochrane databases, MEDLINE/PubMed, and CINAHL were searched in April 2016. Search terms were “Endocuff”, “Endocuff and colonoscopy”, “Endocuff and adenoma detection”, and “Endocuff and polyp detection”. Second, abstracts from Digestive Disease Week, United European Gastroenterology, and the American College of Gastroenterology meeting were searched from 2004-2015 using the same terms. Third, all the references from the reviewed articles were searched for any other articles that may have been missed. Authors were contacted if the data needed clarification or were not complete.
Studies on adult patients undergoing colonoscopy that compared EAC to standard colonoscopy were included. Two reviewers (Chin M and Nguyen DL) searched the articles and extracted the data independently with any disagreements being settled by a third party (Bechtold ML) or consensus decision.
The quality of studies was assessed using the Effective Public Health Practice Project model. This scale assesses study quality as strong, moderate, or weak based upon criteria ratings for selection bias, study design, confounders, blinding, data collection methods, withdrawal and dropout descriptions, intervention integrity, and analysis. The quality of the study is based upon the number of weak ratings per category (≥ 2 wk ratings = weak, one weak rating = moderate, and no weak rating = strong).
A meta-analysis was performed between EAC compared to the standard colonoscopy. Pooled estimates analyses were conducted for ADR, SSA detection rate, right-sided polyp detection, and cecal intubation. Results were presented as odds ratio (OR) using the Mantel-Haenszel fixed effect model in outcomes with no heterogeneity, and the DerSimonian and Laird, the random effects model in outcomes with significant heterogeneity. The I2 measure of inconsistency (P < 0.10 or I2 > 50% was significant) was utilized to assess heterogeneity. If statistically significant heterogeneity was identified, the results underwent a separate sensitivity analysis. This analysis removed specific studies and re-examined outcome results for continued heterogeneity. RevMan 5.3 (Review Manager, Version 5.3, Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012) was used for statistical analysis. Funnel plots were analyzed for presence of publication bias. A biomedical statistician familiar with the study design performed statistical review.
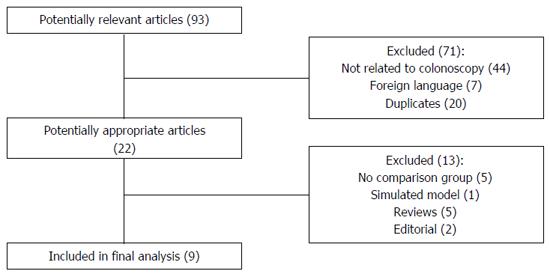
The initial literature search yielded 93 articles and abstracts (Figure 1). Of the 93 articles, 71 were excluded because they were in a foreign language, duplicates, or did not involve colonoscopy examinations. Of the remaining 22 potentially appropriate articles and abstracts, 13, were excluded due to the lack of a comparison group, the use of the device in an anatomic model, or for being reviews or editorial comments. A total of 9 studies comparing EAC with conventional colonoscopy were included in the final analysis.
Of the nine included comparative studies[13-21], summarized in Table 1, four were retrospective cohort studies, four were prospective randomized controlled trials, and one was a prospective observational study. Indications for colonoscopy varied across the index study including average risk screening, high risk screening due to family history, surveillance due to history of previous polyps, follow up for positive stool testing (FOBT or FIT), and diagnostic procedures for concerning symptoms including pain, changes in quality of stools, anemia, or hematochezia. Exclusion criteria also varied across the studies including history of recurrent diverticulitis, colonic stricture, or previous colonic surgery. All studies included procedures performed at academic hospitals with the exception of Shah-Ghassemzadeh et al[18], which indexed patients at VA hospital, and Cattau et al[19], which included procedures performed in a community settings.
| Ref. | Study design | Location | Practice setting | Study period | Procedure Indication | Number patients | Gender | Age (yr) | Primary outcome | ADR EAC standard | EAC Complications | Cecal intubation rate/time EAC standard |
| Marsano et al[13] 2014 | Retrospective chart review | New York | Academic, community | 9/13-11/13 | Screening, surveillance | 318 | NR | NR | ADR | 47% | NR | NR/NR |
| 30% | NR/NR | |||||||||||
| Biecker et al[14] 2015 | Randomized prospective 2-center RCT | Germany | Academic | 2/13-8/13 | Screening, surveillance, diagnostic | 498 | 249 male (50%) | 67 (56-75), | Polyps/procedure | 35% | Mucosal injury (9) | 98%/NR |
| 249 female (50%) | Median (IQR) | 27% | Loss of cuff (6) | 98%/NR | ||||||||
| Floer et al[15] 2014 | Randomized prospective 4-center RCT | Germany | Academic | 2/14-7/14 | Screening, surveillance, diagnostic | 492 | 231 male (47%) | 64 (54-73), | ADR | 35% | Mucosal injury (18) | 96%/NR |
| 261 female (53%) | Median (IQR) | 21% | 94%/NR | |||||||||
| Tsiamoulos et al[16] 2015 | Prospective observational single center | United Kingdom | Academic | 4/13-9/14 | Screening | 399 | NR | NR | ADR | 69% | Elective removal (1) | NR/7.5 min (mean) |
| 58% | Discomfort (1) | NR/9.5 min (mean) | ||||||||||
| van Doorn et al[17] 2015 | Randomized prospective 5-center RCT | Netherlands | Academic | 8/13-10/14 | Surveillance, FIT positive, family history, diagnostic | 1063 | 549 Male (51.6%) | 65, | Adenomas/patient, ADR | 54% | Elective removal (22) | 98%/7 min (median) |
| 514 Female (48.4%) | Median | 53% | Post-polypectomy bleeding (2) | 99%/8.3 min (median) | ||||||||
| Thromboembolic event (1) | ||||||||||||
| Shah et al[18] 2015 | Retrospective chart review | California | Veterans Affair’s Hospital | 1/14-2/15 | Screening, diagnostic | 449 | 417 male (92.9%) | NR | ADR, SSADR | 62% | NR | NR/NR |
| 32 female (7.1%) | 49% | NR/NR | ||||||||||
| Cattau et al[19] 2015 | Prospective randomized multi center RCT | Tennessee | Community | NR | Screening | 658 | 317 male (48.2%) | 58 ± 8, | ADR | 50% | NR | 99%/NR |
| 341 female (51.8%) | mean ± SD | 46% | 98%/NR | |||||||||
| Grewal et al[20] 2015 | Retrospective chart review | California | Academic | 8/14-5/15 | Screening, surveillance, diagnostic | 1237 | 595 male (48.1%) | 61 (54-69) | SSADR | NR | NR | NR/NR |
| 642 female (51.9%) | Median (IQR) | NR/NR | ||||||||||
| Chin et al[21] 2015 | Retrospective chart review | California | Academic | 8/14-5/15 | Screening | 510 | 234 male (45.9%) | 57 (52-61), | ADR | 56% | NR | 99%/12 min (mean) |
| 276 female (54.1%) | Median (IQR) | 45% | 97%/11 min (mean) |
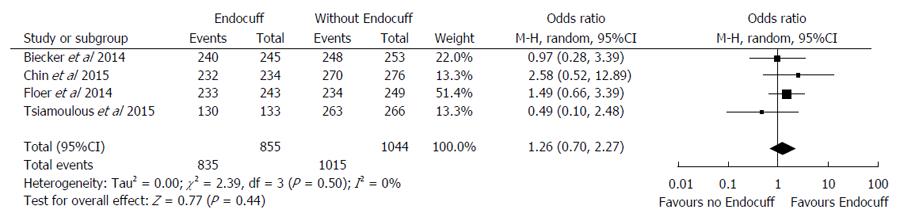
There were three studies that evaluated the cecal intubation time[16,17,21] (Table 1). The reported average cecal intubation times between the EC group were relatively similar to the standard colonoscopy group, with EC group being between 7.0 to 11.7 min compared to 8.3 to 10.7 min in the standard colonoscopy group. In the pooled analysis of four of the nine studies (n = 1899) that evaluated the cecal intubation rate (Figure 2)[14,15,19,21], the results were similar between the EC group compared to the standard colonoscopy group (OR = 1.26 95%CI: 0.70-2.27, I2 = 0%; P = 0.44).
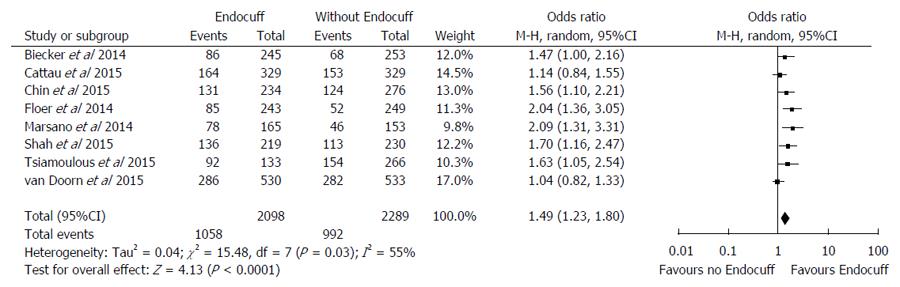
Of the nine studies included in the analysis, eight (n = 4387) reported ADR as an outcome (Figure 3)[13-19,21]. Overall, there was a higher ADR in the EC group of 50.4% compared to the standard colonoscopy group of 43.3% (OR = 1.49 95%CI: 1.23-1.80, I2 = 55%; P = 0.03). The number of procedures needed to be performed with EC in order to yield one additional procedure with an adenoma was 14. Given the significant heterogeneity, we performed a sensitivity analysis through exclusion of the Floer et al[15] study because it reported the lowest adenoma detection amongst the studies. Results remained statistically significant, favoring higher adenoma detection rate in the EC group compared to the standard colonoscopy (OR = 1.42 95%CI: 1.18-1.72, I2 = 50%, P < 0.01). Furthermore, if only indexing randomized control trials in the meta-analysis[14,15,17,19] there remains statistically significant higher adenoma detection rate in the EC compared to the standard colonoscopy group (OR = 1.33 95%CI: 1.01-1.76; I2 = 66%, P = 0.03).
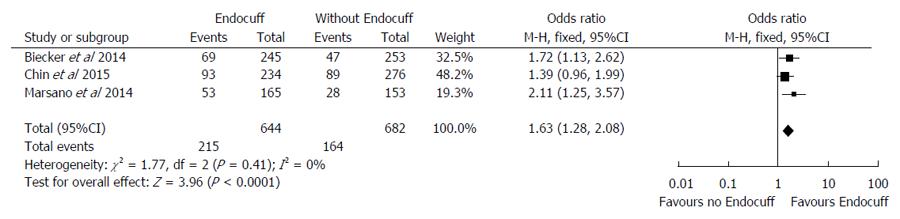
A total of three studies[13,14,20] (n = 1326) evaluated the right-sided polyp detection rate using the EC compared to the standard colonoscopy. Overall, there was an enhanced rate of detection of right-sided colonic polyps in the EC group of 33.4% compared to 24.0% (OR = 1.63, 95%CI: 1.28-2.08, I2 = 0%; P < 0.001) (Figure 4).
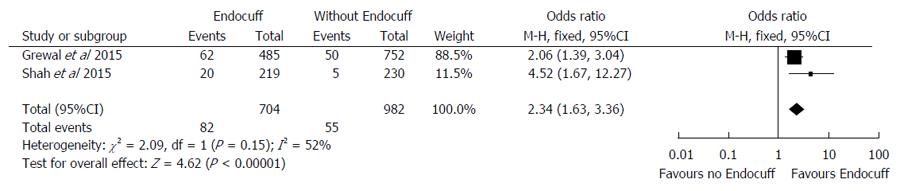
Two studies[18,20] (n = 1686) evaluated SSA detection rates with EC compared to standard colonoscopy. There was a higher SSA detection rate in the EAC group of 11.6% compared to the standard colonoscopy of 5.6% (OR = 2.34, 95%CI: 1.63-3.36; I2 = 52%; P < 0.001) (Figure 5). The number of procedures needed to be performed with EC in order to yield one additional procedure with a sessile serrated adenoma was 17.
Four studies (n = 2452) reported complication rates[14-17]. The complication rate was higher in the EAC group than the standard colonoscopy group (5.47% vs 0.61%, P < 0.001). The most commonly reported complication was clinically insignificant superficial mucosal injury, which was reported in 27 patients in the EC group (2.3%). A total of 23 patients (2.0%) in the EC group required removal of the device due to patient discomfort and the procedure was then successfully completed without the EC. The next most common complication was the loss of the device, and subsequent retrieval, on withdrawal, reported in only one study, occurring in 6 (0.52%) EC patients. No perforations were reported in the EC group.
Colonoscopy with adenoma detection and removal is widely considered the gold standard for the prevention of colorectal cancer, but is limited by an adenoma miss rate as high as 27%[4]. In an effort to increase the effectiveness of colonoscopy, recent interest has evolved around the development of a variety of techniques and devices to improve our ability to examine areas of the colon that are difficult to see including behind folds and turns.
Our meta-analysis of nine comparative studies on one such device, the EC showed benefit over conventional colonoscopy. Strengths of our analysis include variety of clinical settings, including multi-national academic and community settings and, wide variety of patient populations, in which the EC device was shown to improve polyp detection. In particular, EAC was associated with improved adenoma detection. Other benefits were also appreciated in the EAC group when analyzing right-sided adenoma detection and SSA detection.
One concern regarding the use of the EC is that the device may limit cecal intubation in those patients with traditionally difficult anatomy such as angulation, diverticulosis, or previous abdominal surgery, but only 2% of patients required the removal of the EC device due to technical difficulty in studies reporting such events. The procedures were subsequently completed with a standard colonoscope in all patients. The use of an EC did not effect cecal intubation rate or procedure time. Reported complications were minimal and similar to early observational studies of the device and were limited largely to superficial mucosal trauma[12,22]. However, some studies excluded patients with previous abdominal surgery, history of colonic stricture, or recent diverticulitis, i.e., those in whom Endocuff might be more difficult to use in. Furthermore, the retrospective, non-randomized nature of many of the included studies makes it difficult to exclude the possibility that providers may have preferentially performed standard colonoscopy in those with perceived difficult anatomy.
Our study has several limitations and revealed some unanswered questions regarding the potential benefit of EAC over standard colonoscopy. The variability of study designs and outcome measures in this analysis resulted significant heterogeneity. Only English-language studies were included in the analysis, which may introduce publication bias. None of the included studies were performed in tandem fashion, making it difficult to draw conclusions on the incremental benefit of EAC over standard colonoscopy, especially in standard-risk verses high-risk population. While some studies reported whether the EC needed to be removed due to patient discomfort[16,17], no one provided quantified data on patient comfort, or requirement for increased sedation. Our pooled analysis showed the number needed to treat of 14 to achieve one additional adenoma-positive procedure but whether EAC is cost effective or not remains to be determined. The included studies include a heterogeneous list of indications for colonoscopy, which makes conclusions regarding the efficacy of routine EC use on screening colonoscopy somewhat difficult to reach. Finally, bowel prep quality is known to be associated with improved colonoscopy quality[23], yet this important variable was incompletely reported in the included studies, and could confound polyp detection rates, procedural efficiency and safety.
A transparent cap has been used most often prior to the Endocuff to improve polyp detection on colonoscopy. Although it is supposed to improve visualization, the benefit appears to such devices has proven to be modest, with one recent meta-analysis of 16 studies showing a relative risk of 1.08 for polyp detection and a second analysis of 12 studies demonstrating an odds ratio of 1.13[24,25] for cap assisted over standard colonoscopy. The finger-like projections of the Endocuff™ could be better at spreading out the folds to improve detection of polyps explaining the more robust odds ratio for adenoma detection with EAC of 1.49 in our study.
There are several other recently developed accessories that functionally provide the same benefit of spreading folds and affixing the colonoscope in the lumen. These include the EndoRing™ (Endo-Aid, Caesarea, Israel), which is comprised of several clear rings on a cuff designed to be affixed to the colonoscope tip, or the G-Eye™ system (Smart Medical Systems Ltd, Ra-anana, Israel), a novel colonoscope with a balloon integrated at the scope tip that is designed to be inflated upon withdrawal. Several small preliminary studies comparing the EndoRing™ and G-Eye™ system to conventional colonoscopy do suggest some benefit to adenoma detection with such devices[26-28], but clinical data are relatively scant when compared to the Endocuff™. Furthermore, the G-Eye™ system is not yet commercially approved for use in the United States.
In conclusion, EAC improves adenoma detection without any significant adverse effect on procedural efficiency. Further prospective randomized trials or cost-effectiveness studies of an approach of using the Endocuff on routine screening colonoscopy would be warranted.
Polyp detection rates are used as a correlate for colonoscopy quality. The Endocuff is a recently developed device that has been shown is multiple trials to improve the polyp detection.
The development of attachable or integrated colonoscopy devices to improve polyp detection represents one innovation in improving polyp detection and colorectal cancer prevention.
Multiple small studies have demonstrated that the Endocuff can improve polyp detection, however, there have been no previously published efforts to analyze the aggregated data as a whole of such studies.
The results of this study can form the basis for future development of colonoscopy-assist devices and inform a physician’s decision to utilize such a device.
Endocuff: a novel cap affixed to the colonoscope head comprised of soft projections that remain flattened during insertion and project out on withdrawal to spread out the colonic folds
This study is of special interest, as methods to improve adenoma detection during colonoscopy are desired.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Luigiano C, Schramm C, Sieg A, Tan TY S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25535] [Article Influence: 1823.9] [Reference Citation Analysis (7)] |
| 2. | Draft Recommendation Statement: Colorectal Cancer: Screening. U.S. Preventive Services Task Force. October 2015. Available from: http://www.uspreventiveservicestaskforce.org/Page/Document/draft-recommendation-statement38/colorectal-cancer-screening. |
| 3. | Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1390] [Cited by in RCA: 1502] [Article Influence: 100.1] [Reference Citation Analysis (1)] |
| 4. | Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24-28. [PubMed] |
| 5. | Bressler B, Paszat LF, Chen Z, Rothwell DM, Vinden C, Rabeneck L. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 448] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 6. | Singh H, Nugent Z, Demers AA, Bernstein CN. Rate and predictors of early/missed colorectal cancers after colonoscopy in Manitoba: a population-based study. Am J Gastroenterol. 2010;105:2588-2596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 207] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 7. | Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, Zwierko M, Rupinski M, Nowacki MP, Butruk E. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795-1803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1287] [Cited by in RCA: 1462] [Article Influence: 97.5] [Reference Citation Analysis (0)] |
| 8. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1552] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 9. | Richter JM, Campbell EJ, Chung DC. Interval colorectal cancer after colonoscopy. Clin Colorectal Cancer. 2015;14:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Lu FI, van Niekerk de W, Owen D, Tha SP, Turbin DA, Webber DL. Longitudinal outcome study of sessile serrated adenomas of the colorectum: an increased risk for subsequent right-sided colorectal carcinoma. Am J Surg Pathol. 2010;34:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Fujita K, Yamamoto H, Matsumoto T, Hirahashi M, Gushima M, Kishimoto J, Nishiyama K, Taguchi T, Yao T, Oda Y. Sessile serrated adenoma with early neoplastic progression: a clinicopathologic and molecular study. Am J Surg Pathol. 2011;35:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Lenze F, Beyna T, Lenz P, Heinzow HS, Hengst K, Ullerich H. Endocuff-assisted colonoscopy: a new accessory to improve adenoma detection rate? Technical aspects and first clinical experiences. Endoscopy. 2014;46:610-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Marsano J, Tzimas D, Mckinley M, Robbins D, Mammen A, Sun E, Chugh P, Razavi F, Hasan N, Buscaglia J. Endocuff assisted colonoscopy increases adenoma detection rates: a multi-center study. Gastrointest Endosc. 2014;79:AB550. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Biecker E, Floer M, Heinecke A, Ströbel P, Böhme R, Schepke M, Meister T. Novel endocuff-assisted colonoscopy significantly increases the polyp detection rate: a randomized controlled trial. J Clin Gastroenterol. 2015;49:413-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Floer M, Biecker E, Fitzlaff R, Röming H, Ameis D, Heinecke A, Kunsch S, Ellenrieder V, Ströbel P, Schepke M. Higher adenoma detection rates with endocuff-assisted colonoscopy - a randomized controlled multicenter trial. PLoS One. 2014;9:e114267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Tsiamoulos ZP, Mirsa R, Bourikas LA, Rajaratnam R, Patel KP, Thomas-Gibson S, Haycock A, Suzuki N, Beintaris I, Saunders BP. Endocuff-vision: impact on colonoscopist performance during screening. Gastrointest Endosc. 2015;81 Suppl 5: AB209. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | van Doorn SC, van der Vlugt M, Depla A, Wientjes CA, Mallant-Hent RC, Siersema PD, Tytgat K, Tuynman H, Kuiken SD, Houben G. Adenoma detection with Endocuff colonoscopy versus conventional colonoscopy: a multicentre randomised controlled trial. Gut. 2015; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Shah-Ghassemzadeh M, Baek M, Jackson C, Lunn J, Nguyen C, Serrao S, Juma D, Strong R. Endocuff-assisted colonoscopy increases sessile serrated adenoma/polyp detection and adenoma detection rates: a quality improvement study. Am J Gastroenterol. 2015;110:S666. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Cattau EL, Leal RK, Ormseth EJ, Aycock R, Ward JD, Thompson BF, Dragutsky M, Towne C. The effect of Endocuff-assisted colonoscopy on adenoma detection rate: a randomized trial in community ambulatory surgical centers. Am J Gastroenterol. 2015;110:S602. |
| 20. | Grewal J, Chin M, Chen C, Karnes W. Endocuff-assisted colonoscopy improves sessile serrated adenoma detection rate: a single academic center observational study. Am J Gastroenterol. 2015;110:S626. |
| 21. | Chin M, Grewal J, Chen C, Karnes W. Endocuff-assisted colonoscopy: a single-center experience with an average-risk, asymptomatic population. Am J Gastroenterol. 2015;110:S613. |
| 22. | Tsiamoulos ZP, Saunders BP. A new accessory, endoscopic cuff, improves colonoscopic access for complex polyp resection and scar assessment in the sigmoid colon (with video). Gastrointest Endosc. 2012;76:1242-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Nguyen DL, Wieland M. Risk factors predictive of poor quality preparation during average risk colonoscopy screening: the importance of health literacy. J Gastrointestin Liver Dis. 2010;19:369-372. [PubMed] |
| 24. | Ng SC, Tsoi KK, Hirai HW, Lee YT, Wu JC, Sung JJ, Chan FK, Lau JY. The efficacy of cap-assisted colonoscopy in polyp detection and cecal intubation: a meta-analysis of randomized controlled trials. Am J Gastroenterol. 2012;107:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 25. | Westwood DA, Alexakis N, Connor SJ. Transparent cap-assisted colonoscopy versus standard adult colonoscopy: a systematic review and meta-analysis. Dis Colon Rectum. 2012;55:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Dik VK, Gralnek IM, Segol O, Suissa A, Moons LM, Domanov S, Segev M, Rex DK, Siersema PD. Comparing standard colonoscopy with EndoRings” colonoscopy: a randomized, multicenter tandem colonoscopy study interim results of the CLEVER study. Gastroenterology. 2014;146:S160. |
| 27. | Halpern Z, Ishaq S, Neumann H, Dobosz N, Viale E, Hoffman A, Hendel J, Senturk H, Kiesslich R. G-EYE colonoscopy significantly improves adenoma detection rates initial results of a multicenter prospective cohort study. Gastroenterology. 2014;146:S402. |
| 28. | Gross S, Halpern Z, Santo E, Kiesslich R, Hoffman A, Pochapin M, Shpak B. A novel balloon-colonoscope for increased polyp/adenoma detection rate: results of a randomized tandem study. Am J Gastroenterol. 2013;108:S632-S633. [DOI] [Full Text] |