Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9613
Peer-review started: August 17, 2016
First decision: September 5, 2016
Revised: September 13, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: November 21, 2016
Processing time: 93 Days and 16.7 Hours
To investigate peg-interferon (peg-IFN) and ribavirin (RBV) therapy in Myanmar and to predict sustained virologic response (SVR).
This single-center, open-label, study was conducted in Myanmar between 2009 and 2014. A total of 288 patients infected with HCV genotypes 1, 2, 3 and 6 were treated with peg-IFN alpha-2a (180 μg/wk) or alpha-2b (50 to 100 μg as a weight-based dose) and RBV as a weight-based dose (15 mg/kg/d). Treatment duration was 48 wk for genotypes 1 and 6, 24 wk for genotype 2, and 24 or 48 wk for genotype 3 based on rapid virologic response (RVR). Those co-infected with hepatitis B received 48 wk of therapy.
Overall, SVR was achieved for 82% of patients and the therapy was well tolerated. All patients achieved SVR at equivalent rates regardless of HCV genotype (P = 0.314). Low fibrosis scores (P < 0.001), high baseline albumin levels (P = 0.028) and low baseline viral loads (P = 0.029) all independently predicted SVR. On the other hand, IL-28B TT and CC genotypes were not found to significantly predict SVR (P = 0.634; P = 0.618). Among those who completed treatment, the occurrence of RVR showed a > 96% positive predictive value for achieving SVR. Treatment duration did not significantly impact the likelihood of achieving SVR for patients infected with genotype 3 HCV (P = 0.371). The most common adverse events were fatigue (71%) and poor appetite (60%). Among patients with genotype 3 HCV, more patients in the 48-wk treatment group required erythropoietin than in the 24-wk treatment group (61.1% vs 49.2%).
SVR rates were high with peg-IFN and RBV therapy in Myanmar. Fibrosis scores, baseline albumin, HCV RNA levels and RVR independently predicted SVR.
Core tip: Peg-interferon (peg-IFN) and ribavirin (RBV) therapy was a viable treatment option for hepatitis C virus (HCV)-infected patients in Myanmar due to its availability and affordability. Currently, direct-acting antiviral agents are not widely accessible to the Myanmar population. Therefore, HCV treatment is still being conducted with peg-IFN and RBV therapy. This treatment regimen was shown to be reasonably efficacious with minimal adverse events. Fibrosis scores, baseline albumin, HCV RNA levels and rapid virologic response all independently predicted sustained virologic response.
- Citation: Hlaing NKT, Banerjee D, Mitrani R, Arker SH, Win KS, Tun NL, Thant Z, Win KM, Reddy KR. Hepatitis C virus therapy with peg-interferon and ribavirin in Myanmar: A resource-constrained country. World J Gastroenterol 2016; 22(43): 9613-9622
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9613.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9613
Recent advances in treatment for hepatitis C virus (HCV) infection lend hope to curing the almost 170 million people worldwide who are chronically infected with HCV[1]. Treating HCV remains a top priority to prevent progression to cirrhosis and to lower the risks of developing hepatocellular carcinoma. Direct-acting antiviral (DAA) therapy has high cure rates and a substantially reduced side effects profile compared to dual therapy with pegylated-interferon (peg-IFN) and ribavirin (RBV), and has revolutionized HCV treatment paradigms in many countries[2]. However, DAA treatment is only just beginning to enter the market in Myanmar, leaving peg-IFN and RBV therapy as a viable option[3,4].
The burden of HCV is enormous in low- and middle-income countries comprising South Asia, East Asia, North Africa, the Middle East and Southeast Asia, which altogether amounts to more than 80% of the global HCV burden[5,6]. Until novel DAA therapy is made more economically accessible to these HCV-endemic regions, clinicians must try to optimize available IFN-based therapies to heterogeneous patient populations seeking treatment.
About half a million or 1%-1.6% of Myanmar’s population is infected by HCV[7,8]. Among people who inject drugs, the prevalence of HCV in Myanmar is much higher, around 48.1%[9]. If HCV remains untreated, then its prevalence could rise through increasing numbers of people who inject drugs. In Myanmar, genotype 3 HCV is the most frequently encountered infection (> 50%), followed by genotypes 6, 1 and 2[10]. As of now, there is a dearth of data on outcomes of treatment in Myanmar. Thus, expectations following HCV therapy are not known for that part of the world. To that end, in an open label trial, we investigated the efficacy and safety of peg-IFN alpha-2a or -2b and RBV in patients from Myanmar infected with HCV genotype 1, 2, 3 and 6, with a particular emphasis on identifying predictors of cure and determining whether 24 wk of treatment was as effective as 48 wk.
This open-label experience was conducted in Myanmar at two centers, namely the Mandalay General Hospital and Yangon GI and Liver Centre. Patients aged between 9-years-old and 70-years-old and who were not previously treated with peg-IFN or RBV were eligible for HCV therapy. Patients included in this analysis were treated between January 2009 and August 2014. Eligible patients had detectable HCV RNA levels ≥ 15 IU/mL and had abstained from alcohol use for at least 3 mo prior to screening. Serum alanine aminotransferase (ALT) levels, liver fibrosis score and coinfection with hepatitis B virus or human immunodeficiency virus were evaluated. Patients with indeterminate HCV genotype, high serum total bilirubin > 3 times the upper limit of the normal range, leukocyte count < 2000 cells/mm3, platelet count < 30000 cells/mm3, hemoglobin levels < 9 g/dL, history of drug abuse, significant medical comorbidities, known psychiatric or autoimmune disease, pregnancy or lactation, or lack of socioeconomic support were excluded from the study.
Patients of any genotype were eligible for enrollment, and HCV genotyping was completed by line probe assay (LiPA) technology (VERSANTTM HCV Genotype Assay (LiPA) Bayer Healthcare, manufactured by Innogenetics, Ghent, Belgium). On-treatment virologic assessments were done using Roche COBAS AmpliPrep/COBAS TaqMan HCV Test, version 2.0, with the lower limit of detection being 15 IU/mL.
Patients received a combination of peg-IFN and RBV treatment. Patients were given one weekly subcutaneous injection of peg-IFN alpha-2 (either peg-IFN alpha-2a at 180 μg, Hoffman-La Roche, Basel, Switzerland; or peg-IFN alpha-2b at 50-100 μg as a weight-based dose, Merck Sharp & Dohme). RBV (Copegus, Roche, or Rebetol, Merck Sharp & Dohme) was administered orally as a weight-based dose (15 mg/kg/d).
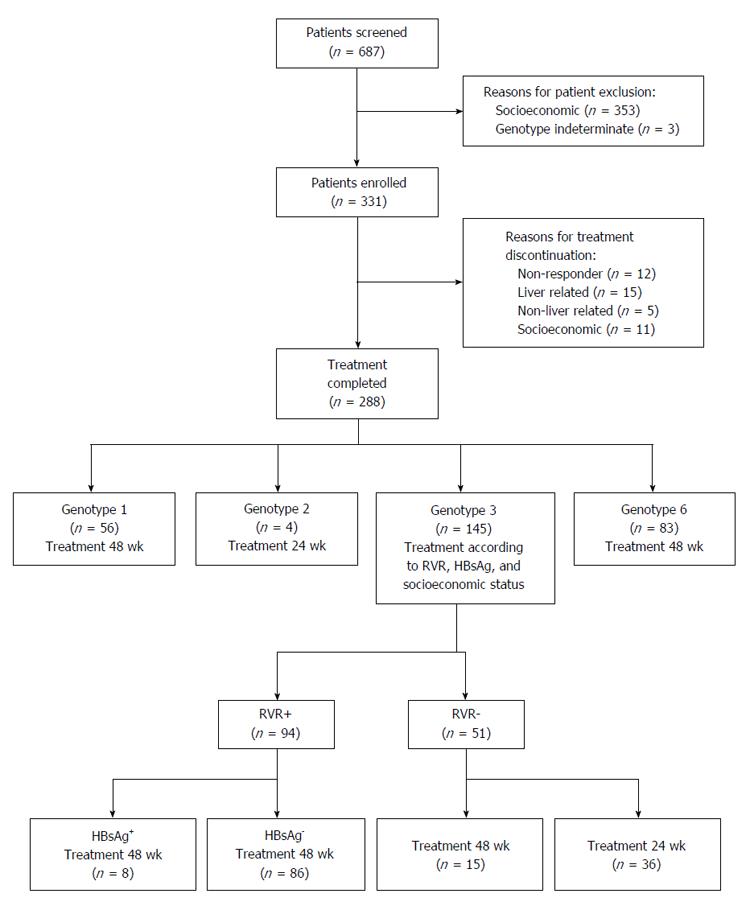
Patients with genotype 1 or 6 HCV infection received treatment for 48 wk, while those with genotype 2 HCV infection received treatment for 24 wk. Treatment for patients with genotype 3 HCV infection varied according to hepatitis B surface antigen (HBsAg), rapid virologic response (RVR) and socioeconomic factors. The patients who achieved RVR at week 4 and did not present with HBsAg were consolidated into the 24-wk treatment group (n = 122). Either the failure to achieve RVR or the presence of HBsAg resulted in placement into the 48-wk treatment group (n = 24).
All patients provided verbal informed consent for collecting data and presenting it. This investigator-initiated study was conducted without financial support from the industry, as patients paid for the medication and study assessments. No limitations on publications were imposed. All authors approved the final version of the manuscript.
All results are presented on the basis of those who completed treatment. The primary efficacy endpoint for this study was sustained virologic response (SVR), defined as undetectable serum HCV RNA 24 wk after treatment was discontinued.
Secondary endpoints included RVR, early virologic response (EVR), end of treatment response (ETR) and relapse. RVR, EVR and ETR were defined as undetectable serum HCV RNA at week 4, week 12 and at the end of treatment, respectively. Virologic relapse was defined as detectable HCV RNA within 24 wk of cessation of therapy after having achieved ETR. Patients who had detectable HCV RNA at 24 wk of treatment were considered non-responders, and treatment was discontinued for these patients.
Safety was assessed by laboratory testing, physical examination and spontaneous/patient-initiated reporting of adverse events. Laboratory tests were carried out at local laboratories. Adverse events were graded as mild, moderate, and severe or life-threatening according to guidelines published by the World Health Organization, while depression was assessed according to the Montgomery-Asber Rating Scale (MADRS). Laboratory assessments and outpatient visits to evaluate the onset of clinical adverse events were scheduled every 2 wk while patients were on treatment and every month after the end of treatment.
Dose modifications of peg-IFN, RBV, or both were permitted if patients were experiencing clinical adverse events or laboratory abnormalities that were not life-threatening, and if the total leukocyte count was < 1500 cells/mm3 or if the absolute neutrophil count was < 750 cells/mm3. Reductions were done in a step-wise fashion: to 135 μg then to 90 μg in patients initially receiving peg-IFN alpha-2a at 180 μg, and reductions according to body weight for patients receiving peg-IFN alpha-2b. Anemia was attributed to treatment when hemoglobin levels were 1.5 g/dL lower than the baseline value, while severe anemia was assessed as when hemoglobin levels dropped below 7 g/dL. RBV dose was reduced upon the onset of severe anemia and was carried out in one or two 200 mg-reductions as needed for anemia to resolve. Treatment was permanently discontinued if the absolute neutrophil count was < 500 cells/mm3.
Blood transfusions were given when hemoglobin levels were < 7 g/dL and if the patient agreed to receive a transfusion. Erythropoietin stimulating agent was administered when hemoglobin levels were < 9 g/dL.
The efficacy analysis was based on 288 enrolled patients, while relapse analysis included 283 patients. t-test was used to determine the P-values for means (standard deviation), while Wilcoxon test was used to determine the P-values for medians (interquartile range) for continuous variables. For categorical variables, χ2 test was used to determine P-values, and frequencies were summarized as n (percent). These methods were used for summarizing baseline characteristics and for completing preliminary efficacy and safety analyses.
Multi-variable logistic regression analysis was used to evaluate the relationships between treatment duration and SVR, baseline factors and SVR, and baseline factors and relapse. This analysis was used to compute P-values and odds ratios (ORs), their 95% confidence intervals (CIs), and P-values. The model for treatment duration was adjusted for several prognostic factors identified by preliminary efficacy analysis including age, sex and liver stiffness. This model evaluated genotype 3 only, as this was the only genotype where treatment duration varied. Models exploring outcomes and baseline factors excluded genotype 2 cases, as broad conclusions from pooled analysis may not be represented in the small sample size of patients with this genotype (n = 4). As treatment duration was not found to be a significant factor for SVR, it was excluded from subsequent models. The final outcome models were adjusted for age, sex, liver stiffness, genotype, platelet levels ≥ 150000/mm3, albumin levels ≥ 3.5 g/dL and pre-treatment HCV RNA ≥ 800000 IU/mL. All analyses were conducted using SAS v9.4 (Cary, NC, United States).
Between January 2009 and August 2014, 687 patients were screened and 331 were ultimately enrolled (Figure 1). A total of 43 patients discontinued treatment and 5 patients were lost during follow-up. Patients received treatment of varying duration according to genotype. Efficacy analysis includes all 288 patients who completed peg-IFN and RBV treatment. Patients with genotype 1 (n = 56) received treatment for 48 wk, patients with genotype 2 (n = 4) received treatment for 24 wk, patients with genotype 6 (n = 83) received treatment for 48 wk, and patients with genotype 3 (n = 145) received treatment for either 24 wk (n = 23) or 48 wk (n = 122) based on RVR. The baseline characteristics of the patient population have been summarized in Table 1.
| Achieved SVR | P value | ||
| Yes (n = 237) | No (n = 51) | ||
| Demographics | |||
| Sex | |||
| Male | 101 (87.1) | 15 (12.9) | 0.081 |
| Female | 136 (79.1) | 36 (20.9) | |
| Age | |||
| Mean ± SD | 45.9 ± 10.4 | 50.1 ± 8.5 | 0.007 |
| Median (range) | 48.00 (11.0-68.0) | 49.00 (34.0-66.0) | 0.016 |
| ≤ 20 | 5 (100) | 0 (0) | 0.017 |
| 21-40 | 57 (87.7) | 8 (12.3) | |
| 41-60 | 164 (82.4) | 35 (17.6) | |
| > 60 | 11 (57.9) | 8 (42.1) | |
| Pretreatment conditions/comorbidities | |||
| HCV/HIV coinfection | 4 (100) | 0 (0) | 0.350 |
| Diabetes mellitus | 21 (91.3) | 2 (8.7) | 0.238 |
| Previous alcohol consumption | 45 (81.8) | 10 (18.2) | 0.919 |
| High pretreatment ALT (> 40) | 166 (81.8) | 37 (18.2) | 0.722 |
| High pretreatment Hgb (≥ 12 g/dL) | 182 (81.3) | 42 (18.8) | 0.386 |
| High pretreatment WBC (≥ 4000 mm3) | 225 (82.7) | 47 (17.3) | 0.432 |
| High pretreatment platelet (≥ 150000/mm3) | 189 (84.8) | 34 (15.2) | 0.043 |
| High pretreatment albumin (≥ 3.5 g/dL) | 212 (84.5) | 39 (15.5) | 0.012 |
| High pretreatment HCV RNA (≥ 800000 IU/mL) | 144 (79.1) | 38 (20.9) | 0.065 |
| Liver stiffness | |||
| F ≤ 2 | 133 (92.4) | 11 (7.6) | < 0.001 |
| F3-4 | 104 (72.2) | 40 (27.8) | |
| IL-28B polymorphism | |||
| rs8099917TT | 204 (82.3) | 44 (17.7) | 0.634 |
| rs8099917Non-TT | 18 (78.3) | 5 (21.7) | |
| Unknown | 15 (88.2) | 2 (11.8) | |
| rs12979860CC | 196 (82.4) | 42 (17.6) | 0.618 |
A total of 237 patients (82%) in this cohort completed treatment and achieved SVR with peg-IFN and RBV therapy (Table 2). All 4 patients with genotype 2 HCV achieved SVR, whereas patients with genotype 1, 3 and 6 HCV achieved SVR at a rates of 75%-85%. Therapy was equally effective across all genotypes (P = 0.31). Several baseline characteristics varied between those who did and did not achieve SVR (Table 1). Patients with an undetectable viral load at 6 mo after treatment cessation were generally younger, had high pretreatment platelet levels (≥ 150000/mm3), and had high pretreatment albumin (≥ 3.5 g/dL) compared to those who did not achieve SVR. Men achieved SVR at slightly higher rates than women. Patients who received dose reductions were not noted to have a lower likelihood of achieving SVR.
| Achieved SVR | P value | ||
| Yes(n = 237) | No(n = 51) | ||
| Dose reduction | |||
| Any dose reduction | 43 (79.6) | 11 (20.4) | 0.570 |
| Peg-IFN only | 9 (100) | 0 (0) | 0.157 |
| Ribavirin only | 12 (70.6) | 5 (29.4) | 0.193 |
| Both peg-IFN and ribavirin | 22 (78.6) | 6 (21.4) | 0.587 |
| Genotype | |||
| 1 | 42 (75.0) | 14 (25.0) | 0.314 |
| 2 | 4 (100) | 0 (0) | |
| 3 | 123 (84.8) | 22 (15.2) | |
| 6 | 68 (81.9) | 15 (18.1) | |
Those with high-grade fibrosis (F3-4) were substantially less likely to clear the virus (P < 0.001) (Table 1). Higher rates of SVR were observed in patients with lower grade fibrosis (F ≤ 2) for genotypes 1, 3 and 6 HCV (P = 0.009, P = 0.008, P = 0.002) (Table 3).
| Total patients | F ≤2 (n = 144) | F 3-4 (n = 144) | P value | |||
| SVR+ | SVR- | SVR+ | SVR- | |||
| All genotypes | 288 | 133 (46) | 11 (4) | 104 (36) | 40 (14) | 0.034 |
| Genotype 1 | 56 | 26 (46) | 3 (5) | 16 (29) | 11 (20) | 0.009 |
| Genotype 2 | 4 | 0 (0) | 0 (0) | 4 (100) | 0 (0) | |
| Genotype 3 | 145 | 60 (41) | 4 (3) | 63 (43) | 18 (12) | 0.008 |
| Genotype 6 | 83 | 47 (57) | 4 (5) | 21 (25) | 11 (13) | 0.002 |
Relapse analysis was completed based on data from 283 patients (Tables 4 and 5). Overall, 49 patients (17%) experienced relapse. Of those who completed 24 wk of treatment relapsed, 15% relapsed compared to 19% of patients who relapsed after completing 48 wk of treatment. Older patients (P = 0.007), those with low pretreatment platelet levels, (P = 0.032) low albumin levels (P = 0.009) and advanced fibrosis (P < 0.001) were likely to have a relapse of HCV infection (Table 4). The proportion of patients who relapsed did not vary substantially by genotype (P = 0.279) (Table 5).
| Relapse (n = 49) | No relapse (n = 234) | P value | |
| Relapse | 49 (17.3) | 234 (82.7) | |
| Demographics | |||
| Sex | |||
| Male | 14 (12.4) | 99 (87.6) | 0.074 |
| Female | 35 (20.6) | 135 (79.4) | |
| Age | |||
| mean ± SD | 50.4 ± 8.5 | 46.0 ± 10.2 | 0.006 |
| Median (range) | 50.00 (34.0-66.0) | 48.00 (11.0-68.0) | 0.011 |
| ≤ 20 | 0 (0) | 4 (100) | 0.013 |
| 21-40 | 7 (10.9) | 57 (89.1) | |
| 41-60 | 34 (17.3) | 162 (82.7) | |
| > 60 | 8 (42.1) | 11 (57.9) | |
| Pre-treatment/comorbidities | |||
| HCV/HIV coinfection | 0 (0) | 3 (100) | 0.426 |
| Diabetes mellitus | 2 (8.7) | 21 (91.3) | 0.254 |
| Previous alcohol consumption | 9 (17.0) | 44 (83.0) | 0.943 |
| High pretreatment ALT (> 40) | 36 (17.9) | 165 (82.1) | 0.678 |
| High pretreatment Hgb (≥ 12g/dL) | 40 (18.3) | 179 (81.7) | 0.434 |
| High pretreatment WBC (≥ 4000 mm3) | 45 (16.9) | 222 (83.1) | 0.403 |
| High pretreatment platelet (≥ 150000/mm3) | 32 (14.7) | 186 (85.3) | 0.032 |
| High pretreatment albumin (≥ 3.5 g/dL) | 37 (15.0) | 209 (85.0) | 0.009 |
| High pretreatment HCV RNA (≥ 800000 IU/mL) | 36 (20.3) | 141 (79.7) | 0.082 |
| Liver stiffness | |||
| F ≤ 2 | 9 (6.5) | 130 (93.5) | < 0.001 |
| F3-4 | 40 (27.8) | 104 (72.2) | |
| IL28B polymorphism | |||
| rs8099917TT | 42 (17.3) | 201 (82.7) | 0.592 |
| rs8099917Non-TT | 5 (21.7) | 18 (78.3) | |
| Unknown | 2 (11.8) | 15 (88.2) | 0.918 |
| rs12979860CC | 40 (17.2) | 193 (82.8) | 0.569 |
| Relapse Yes | Relapse No | P value | |
| (n = 49) | (n = 234) | ||
| Dose reduction | |||
| Any dose reduction | 10 (19.2) | 42 (80.8) | 0.686 |
| Peg-IFN only | 0 (0) | 9 (100) | 0.163 |
| Ribavirin only | 4 (26.7) | 11 (73.3) | 0.325 |
| Both peg-IFN and ribavirin | 6 (21.4) | 22 (78.6) | 0.544 |
| Genotype | |||
| 1 | 14 (25.0) | 42 (75.0) | 0.279 |
| 2 | 0 (0) | 4 (100) | |
| 3 | 21 (14.7) | 122 (85.3) | |
| 6 | 14 (17.5) | 66 (82.5) |
Logistic regression analysis showed that treatment duration did not have a significant impact on likelihood of achieving SVR (P = 0.371), suggesting that treatment lasting 24 wk was just as effective as 48 wk of treatment for curing genotype 3 HCV infection (Table 6). As treatment duration was not found to significantly impact SVR outcome, it was excluded from subsequent models. Bivariate analysis identified age, sex, high HCV RNA at baseline, high pretreatment platelet levels, high pretreatment albumin levels and lower grade fibrosis as potential predictors of response. After stepwise multivariable logistic regression analysis, low pretreatment HCV RNA, high pretreatment albumin level and low-grade fibrosis among patients with genotype 1, 3 and 6 HCV infection remained significant independent predictors of SVR (Table 7). Those with high baseline viral load were less likely to achieve SVR (OR = 0.42, CI: 0.19-0.92, P = 0.029), while those with high baseline albumin levels were substantially more likely to clear the virus (OR = 2.63, CI: 1.11-6.22, P = 0.028). Finally, those with more severe liver fibrosis (F3-4) were predisposed to fail peg-IFN and RBV treatment (OR = 0.19, CI: 0.08-0.48, P < 0.001).
| Comparison or stratum | OR (95%CI) | P value |
| Age | 1.02 (0.97-1.08) | 0.432 |
| Liver fibrosis (F3-4 vs F ≤ 2) | 0.18 (0.05-0.67) | 0.010 |
| Sex (female vs male) | 0.41 (0.14-1.16) | 0.093 |
| Treatment duration (48 wk vs 24 wk) | 2.05 (0.42-9.92) | 0.371 |
| Comparison or stratum | OR (95%CI) | P value |
| Age | 1.00 (0.96-1.04) | 0.904 |
| Genotype (1 vs 6) | 0.74 (0.30-1.84) | 0.433 |
| Genotype (3 vs 6) | 1.33 (0.58-3.05) | 0.433 |
| High pretreatment HCV RNA (≥ 800000 IU/mL) | 0.42 (0.19-0.92) | 0.029 |
| High pretreatment albumin (≥ 3.5 g/dL) | 2.63 (1.11-6.22) | 0.028 |
| High pretreatment platelet (≥ 150000/mm3) | 1.00 (0.45-2.25) | 0.992 |
| Liver fibrosis (F3-4 vs F ≤ 2) | 0.19 (0.08-0.48) | < 0.001 |
| Sex (female vs male) | 0.57 (0.28-1.16) | 0.120 |
| Treatment duration (48 wk vs 24 wk)1 | 2.05 (0.42-9.92) | 0.371 |
Undetectable serum HCV RNA after 4 wk of therapy (RVR) was a substantial/important predictor of SVR for patients with genotype 1 (P < 0.001) and 6 (P < 0.001) who received treatment for 48 wk. For patients with genotype 1, 2 and 6 HCV infection, 100% of those who achieved RVR achieved SVR 6 mo after treatment had been discontinued. RVR showed a similar positive predictive value (PPV) in patients with genotype 3 HCV infection regardless of treatment duration. A total of 96% of patients who achieved RVR demonstrated undetectable HCV RNA levels 6 mo after treatment cessation.
In this study, IL-28B genotype did not demonstrate any type of predictive value for achieving SVR (Table 1). Patients with the rs8099917TT polymorphism exhibited an SVR rate of 82.3% (P = 0.634) whereas patients with the rs12979860CC polymorphism exhibited an SVR rate of 82.4% (P = 0.618). Similar SVR rates were achieved in patients of rs809997Non-TT and unknown polymorphisms.
Adverse events and treatment dose modifications are summarized in Table 8. The proportion of patients requiring dose reductions were comparable for both treatment groups. In the 24-wk treatment group, 15.9% of patients required dose reductions (n = 20/126), while in the 48-wk treatment group, 21% of patients required dose reductions (n = 24/162). Adverse events occurred in roughly similar proportions between the 24-wk and 48-wk treatment groups except for erythropoietin usage. More patients in the 48-wk treatment group required erythropoietin (61.1% vs 49.2%), even though moderate anemia (hemoglobin reduction of 1.5 g/dL) occurred at equivalent rates for both groups. Severe anemia (hemoglobin reduction of 7 g/dL) also occurred at comparable rates in both groups. Treatment was discontinued for a total of 43 patients due to liver-/non-liver, non-responder and socioeconomic reasons. The most common adverse events were fatigue (71%), poor appetite (60%), insomnia (39%), dizziness (23%), hair loss (18%), joint pain (11%), itchiness (9%) and muscle ache (6%).
| 48 wk treatment (n = 162) | 24 wk treatment (n = 126) | |
| Dose reduction | ||
| Any dose reduction | 34 (21.0) | 20 (15.9) |
| Peg-IFN only | 3 (1.9) | 6 (4.8) |
| Ribavirin only | 13 (8.0) | 4 (3.2) |
| Both peg-IFN and ribavirin | 18 (11.1) | 10 (7.9) |
| Hematological adverse events | ||
| Hemoglobin reduction = 1.5 g/dL from pretreatment value | 121 (74.7) | 85 (67.5) |
| Erythropoietin use | 99 (61.1) | 62 (49.2) |
| Severe anemia (Hgb < 7 g/dL) | 4 (2.5) | 3 (2.4) |
| WBC < 4000 cells/mm3 | 110 (67.9) | 86 (68.3) |
| Platelet reduction < 150000 cells/mm3 | 75 (46.3) | 72 (57.1) |
| General adverse events | ||
| Fatigue | 115 (71) | 89 (70.6) |
| Poor appetite | 92 (56.8) | 82 (65.1) |
| Itchiness | 16 (9.9) | 11 (8.7) |
| Skin rash | 5 (3.1) | 0 (0) |
| Joint pain | 20 (12.3) | 13 (10.3) |
| Myalgia/muscle aches | 9 (5.6) | 8 (6.3) |
| Hair loss | 34 (21) | 17 (13.5) |
| Dizziness | 36 (22.2) | 31 (24.6) |
| Insomnia | 63 (38.9) | 49 (38.9) |
| Irritability | 5 (3.1) | 2 (1.6) |
| Depression | 13 (8.0) | 5 (4.0) |
| Thyroid dysfunction | 7 (4.3) | 1 (0.8) |
This study is one of few that investigated the safety and efficacy of traditional HCV treatment in patients from Myanmar, an area of the world where cost and access remains a significant barrier to next-in-line HCV therapies. Study results confirm the efficacy and safety of peg-IFN and RBV combination therapy in Myanmar patients with chronic HCV infection of genotypes 1, 2, 3 and 6. A total of 237 patients (82%) achieved SVR in this study. By HCV genotype, 75% of patients achieved SVR for genotype 1 (n = 42/56), 100% of patients achieved SVR for genotype 2 (n = 4/4), 84.8% of patients achieved SVR for genotype 3 (n = 123/145) and 81.9% of patients achieved SVR for genotype 6 (n = 68/83). These rates are comparable to historical experiences with SVR rates of 60%-90% in Asian countries for all HCV genotypes[11,12]. Genotype 1b HCV infection is more prevalent across Myanmar compared to genotype 1a (6.9% vs 4.1%)[13] and genotype 1b has been shown to be more amenable to combination therapy[14]. These findings could help explain the higher SVR rate observed for genotype 1 in this study compared to those in Western countries[13].
Treatment for 24 wk in patients infected with genotype 3 HCV infection proved to be equally efficacious compared to the traditional 48 wk of treatment. SVR was achieved in 84.4% and 87.5% of cases for patients in the 24-wk and 48-wk treatment groups, respectively. In an American multicenter, open-label, investigator-initiated study, treatment duration was also not found to affect SVR rates for HCV genotypes 2/3[15]. The same combination therapy was used in both studies, yet a greater percentage of genotype 3 HCV-infected patients achieved SVR in this experience compared to the American study (84.8% vs 61.8%). As a general trend, SVR rates for all genotypes have been reported to be higher in Asia compared to Western countries[12].
In this experience, patients infected by genotype 6 HCV were treated for a duration of 48 wk and 81.9% of patients achieved SVR. HCV genotype 6 is endemic to Southeast Asia, and SVR rates for genotype 6 have been reported to be around 80%[16]. Patients infected by genotype 6 HCV were significantly more likely to achieve SVR if their fibrosis scores were equal to 2 or less. Among patients infected by genotype 6, those who present with low fibrosis scores and achieve RVR could potentially be candidates to undergo a shorter treatment duration of 24 wk compared to 48 wk. In a Vietnamese study, Thu Thuy et al[17] reported SVR rates of 60% and 71% for 24 wk and 48 wk of treatment, respectively. Treatment duration was not found to significantly impact SVR rates for patients infected with genotype 6 HCV; however, a larger sample size is needed to confirm these results.
Low-grade fibrosis, low baseline viral loads, and high baseline albumin levels were found to be significant independent predictors of SVR in this study. These factors should be considered in conjunction with age, body mass index and HCV genotype to appropriately determine treatment duration and likelihood of achieving SVR. Patients infected by all genotypes were significantly more likely to achieve SVR if they had RVR. In this study, the PPV of RVR was shown to be > 96%. In a Chinese study, RVR was reported to have a similarly high PPV of 86.7% for HCV genotypes 1-3; however, RVR occurred less frequently in patients infected by genotype 1 HCV[18].
The majority of patients in this study contained either the rs8099917TT and/or the rs12979860CC IL-28B polymorphism. Previous studies have indicated both polymorphisms as independent predictors of virologic responses for genotype 1 HCV, regardless of ethnicity[19]. Moreover, the rs8099917TT polymorphism has been indicated as an independent predictor of achieving SVR for genotypes 2/3 in Asians[18]. In this study, no correlation was found between IL-28B profile and the efficacy of antiviral therapy. Perhaps a larger sample size is needed to assess the relationship between IL-28B profile and the efficacy of antiviral therapy for Asian patients infected with different HCV genotypes, and particularly when there are such high response rates.
Comparable amounts of patients given 24-wk and 48-wk treatments required RBV and/or peg-IFN dose reductions. Meanwhile, a higher proportion of patients in the 48-wk group required erythropoietin compared to patients in the 24-wk group (61.1% vs 49.2%). When possible, 24 wk of combination therapy is preferred in order to reduce the moderate anemia rate and the need to take erythropoietin. Patients in this experience reported higher rates of poor appetite compared to participants in other studies (60% vs 20%-30%)[20]. Poor appetite often coincides with fatigue, which was seen in 71% of patients in this experience. In other studies, fatigue has been noted to occur in 60%-90% of patients[19]. Psychiatric side effects such as depression and irritability were observed in much lower proportions compared to studies in Western countries[21]. Cultural and socioeconomic differences could explain the lower incidences of depression reported in this experience[17].
In conclusion, this study analyzed SVR rates and independent predictors of SVR in Myanmar’s population. Patients infected with HCV genotypes 1 and 6 were treated for a duration of 48 wk and achieved SVR at 75% and 81.9%, respectively. These rates were equivalent or higher compared to historical observations. Patients infected with genotype 2 HCV were treated for a duration of 24 wk and all 4 patients achieved SVR. Depending on RVR and the presence of HBsAg, patients infected by genotype 3 HCV were treated for 24 wk or 48 wk. SVR rates were attained at equivalent rates for patients in both groups. This study found that low fibrosis scores, high baseline albumin levels and low baseline viral loads significantly predict SVR. Patients who achieved RVR were very likely to achieve SVR. This study assessed the safety profiles and efficacy of peg-IFN and RBV combination therapy to better optimize treatment in Myanmar. Most side effects were observed at similar rates compared to historical controls. Peg-IFN and RBV remain the top choice for treating HCV in Myanmar due to higher availability and lower costs compared to DAA agents.
Naomi Khaing Than Hlaing was a Research Fellow at the University of Pennsylvania in 2015-2016, and her Fellowship was funded through the generous support of Eldridge L. Eliason (Professor Shaked A) and Donald Guthrie (Professor Olthoff K) - Endowed Chairs in the Department of Surgery. Debolina Banerjee and Robert Mitrani are Research Assistants in the Division of Gastroenterology and Hepatology and are funded through the generous support of Ruimy Endowed Chair (Professor Reddy R).
Approximately half a million people are chronically infected with hepatitis C virus (HCV) in Myanmar. Treating HCV remains a top priority to prevent progression to cirrhosis and to lower the risks of developing hepatocellular carcinoma. Direct-acting antiviral (DAA) therapy has high cure rates and a substantially reduced side effects profile compared to dual therapy with pegylated-interferon (Peg-IFN) and ribavirin (RBV), and has revolutionized HCV treatment paradigms in many countries. However, DAA treatment is only just beginning to enter the market in Myanmar, leaving peg-IFN and RBV therapy as a viable option.
This open-label experience investigated the efficacy and safety of peg-IFN alpha-2a or -2b and RBV in patients from Myanmar with HCV genotypes 1, 2, 3 and 6, with a particular emphasis on identifying predictors of cure and determining whether 24 wk of treatment was as effective as 48 wk.
There has been a sporadic study that analyzed HCV treatment in Myanmar. In this experience, several baseline clinical and biochemical characteristics were assessed to find predictors of sustained virologic response (SVR). Low fibrosis scores, high baseline albumin levels and low baseline viral loads significantly predicted SVR. Additionally, patients who achieved RVR had a high likelihood of achieving SVR. Patients with genotype 3 HCV were treated for 24 wk or 48 wk, and both groups of patients achieved SVR at equivalent rates. Finally, side effects of peg-IFN and RBV were comparable to historical controls.
Generic DAAs from the chronic hepatitis C treatment expansion by Gilead Sciences are just becoming available in Myanmar. A single tablet regimen of ledipasvir/sofosbuvir will become available to the Myanmar population, but the medication has yet to be approved by the Myanmar Federal Drug Administration. For the next couple of years, peg-IFN plus RBV will be the mainstay of treatment in Myanmar.
Several countries worldwide use peg-IFN and RBV to treat HCV due to extremely high costs of DAA agents. This study reinforces the high SVR observed with such treatment in Asia compared to Western countries. It also reinforces the use of peg-IFN and RBV as the most competitive choice for treatment of HCV in Myanmar due to its high SVR rates and safety profiles. It also shows that some previously described predictors of SVR apply well to the Myanmar population, allowing its use in patients enrolled for treatment. The manuscript is well written, concise and objective.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Keppeke GD S- Editor: Gong ZM L- Editor: Filipodia E- Editor: Wang CH
| 1. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1764] [Cited by in RCA: 1841] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 2. | Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut. 2012;61 Suppl 1:i36-i46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 3. | Yu ML, Chuang WL. New treatments for HCV: Perspective from Asia. Clin Liver Dis. 2015;5:17-21. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Naing C, Sitt T, Aung AT, Aung K. Sustained Virologic Response to a Dual Peginterferon alfa-2a and Ribavirin in Treating Chronic hepatitis C Infection: A Retrospective Cohort Study. Medicine (Baltimore). 2015;94:e1234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1846] [Article Influence: 153.8] [Reference Citation Analysis (3)] |
| 6. | Jayasekera CR, Barry M, Roberts LR, Nguyen MH. Treating hepatitis C in lower-income countries. N Engl J Med. 2014;370:1869-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 945] [Article Influence: 67.5] [Reference Citation Analysis (2)] |
| 8. | Akkarathamrongsin S, Praianantathavorn K, Hacharoen N, Theamboonlers A, Tangkijvanich P, Poovorawan Y. Seroprevalence and genotype of hepatitis C virus among immigrant workers from Cambodia and Myanmar in Thailand. Intervirology. 2011;54:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Luhmann N, Champagnat J, Golovin S, Maistat L, Agustian E, Inaridze I, Myint WM, Butsashvili M, Bouscaillou J. Access to hepatitis C treatment for people who inject drugs in low and middle income settings: Evidence from 5 countries in Eastern Europe and Asia. Int J Drug Policy. 2015;26:1081-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, Barnes E. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61:77-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1077] [Cited by in RCA: 1142] [Article Influence: 114.2] [Reference Citation Analysis (0)] |
| 11. | Tsang OT, Zee JS, Chan JM, Li RS, Kan YM, Li FT, Lo FH, Chow DA, Cheung KW, Chan KH. Chronic hepatitis C genotype 6 responds better to pegylated interferon and ribavirin combination therapy than genotype 1. J Gastroenterol Hepatol. 2010;25:766-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Yu ML, Chuang WL. Treatment of chronic hepatitis C in Asia: when East meets West. J Gastroenterol Hepatol. 2009;24:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 13. | Lwin AA, Shinji T, Khin M, Win N, Obika M, Okada S, Koide N. Hepatitis C virus genotype distribution in Myanmar: Predominance of genotype 6 and existence of new genotype 6 subtype. Hepatol Res. 2007;37:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Jensen DM, Morgan TR, Marcellin P, Pockros PJ, Reddy KR, Hadziyannis SJ, Ferenci P, Ackrill AM, Willems B. Early identification of HCV genotype 1 patients responding to 24 weeks peginterferon alpha-2a (40 kd)/ribavirin therapy. Hepatology. 2006;43:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 367] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Jacobson IM, Brown RS, Freilich B, Afdhal N, Kwo PY, Santoro J, Becker S, Wakil AE, Pound D, Godofsky E. Peginterferon alfa-2b and weight-based or flat-dose ribavirin in chronic hepatitis C patients: a randomized trial. Hepatology. 2007;46:971-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Fung J, Lai CL, Hung I, Young J, Cheng C, Wong D, Yuen MF. Chronic hepatitis C virus genotype 6 infection: response to pegylated interferon and ribavirin. J Infect Dis. 2008;198:808-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Thu Thuy PT, Bunchorntavakul C, Tan Dat H, Rajender Reddy K. A randomized trial of 48 versus 24 weeks of combination pegylated interferon and ribavirin therapy in genotype 6 chronic hepatitis C. J Hepatol. 2012;56:1012-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Yu JW, Wang GQ, Sun LJ, Li XG, Li SC. Predictive value of rapid virological response and early virological response on sustained virological response in HCV patients treated with pegylated interferon alpha-2a and ribavirin. J Gastroenterol Hepatol. 2007;22:832-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 19. | Chen Y, Xu HX, Wang LJ, Liu XX, Mahato RI, Zhao YR. Meta-analysis: IL28B polymorphisms predict sustained viral response in HCV patients treated with pegylated interferon-α and ribavirin. Aliment Pharmacol Ther. 2012;36:91-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Sulkowski MS, Cooper C, Hunyady B, Jia J, Ogurtsov P, Peck-Radosavljevic M, Shiffman ML, Yurdaydin C, Dalgard O. Management of adverse effects of Peg-IFN and ribavirin therapy for hepatitis C. Nat Rev Gastroenterol Hepatol. 2011;8:212-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 21. | Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 220] [Article Influence: 9.6] [Reference Citation Analysis (0)] |