Published online Nov 21, 2016. doi: 10.3748/wjg.v22.i43.9604
Peer-review started: July 13, 2016
First decision: August 19, 2016
Revised: August 25, 2016
Accepted: October 10, 2016
Article in press: October 10, 2016
Published online: November 21, 2016
Processing time: 129 Days and 21.5 Hours
To develop a fast, low-cost diagnostic strategy to identify single point mutations in highly variable genomes such as hepatitis C virus (HCV).
In patients with HCV infection, resistance-associated amino acid substitutions within the viral quasispecies prior to therapy can confer decreased susceptibility to direct-acting antiviral agents and lead to treatment failure and virological relapse. One such naturally occurring mutation is the Q80K substitution in the HCV-NS3 protease gene, which confers resistance to PI inhibitors, particularly simeprevir. Low-cost, highly sensitive techniques enabling routine detection of these single point mutations would be useful to identify patients at a risk of treatment failure. LightCycler methods, based on real-time PCR with sequence-specific probe hybridization, have been implemented in most diagnostic laboratories. However, this technique cannot identify single point mutations in highly variable genetic environments, such as the HCV genome. To circumvent this problem, we developed a new method to homogenize all nucleotides present in a region except the point mutation of interest.
Using nucleotide-specific probes Q, K, and R substitutions at position 80 were clearly identified at a sensitivity of 10% (mutations present at a frequency of at least 10% were detected). The technique was successfully applied to identify the Q80K substitution in 240 HCV G1 serum samples, with performance comparable to that of direct Sanger sequencing, the current standard procedure for this purpose. The new method was then validated in a Catalonian population of 202 HCV G1-infected individuals. Q80K was detected in 14.6% of G1a patients and 0% of G1b in our setting.
A fast, low-cost diagnostic strategy based on real-time PCR and fluorescence resonance energy transfer probe melting curve analysis has been successfully developed to identify single point mutations in highly variable genomes such as hepatitis C virus. This technique can be adapted to detect any single point mutation in highly variable genomes.
Core tip: This report describes a new low-cost, fast and highly sensitive technique based on real-time PCR and specific fluorescence resonance energy transfer probe melting curve analysis to identify single-point mutations in a highly variable background (in this study the Q80K resistance-associated mutation in hepatitis C virus - infected patients) for routine use in clinical laboratories. This technique can be adapted to detect any mutation in a highly variable genome.
- Citation: Chen Q, Belmonte I, Buti M, Nieto L, Garcia-Cehic D, Gregori J, Perales C, Ordeig L, Llorens M, Soria ME, Esteban R, Esteban JI, Rodriguez-Frias F, Quer J. New real-time-PCR method to identify single point mutations in hepatitis C virus. World J Gastroenterol 2016; 22(43): 9604-9612
- URL: https://www.wjgnet.com/1007-9327/full/v22/i43/9604.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i43.9604
Chronic hepatitis C virus (HCV) infection, which affects approximately 160 million individuals worldwide[1] and has an annual incidence of 3 million new infections[2], is one of the leading causes of early-stage liver cirrhosis and hepatocellular carcinoma[3]. HCV is known to have considerable genetic variability.
Direct-acting antivirals (DAAs) are recently-developed drugs based on small molecules that target viral proteins and directly inhibit the viral life cycle. The use of DAAs in triple therapy with pegylated interferon and ribavirin has led to higher sustained virological response (SVR) rates in HCV patients[4]. However, the presence of resistance-associated substitutions conferring decreased susceptibility to DAAs within the viral quasispecies prior to therapy has been found to play a critical role in treatment failure and viral relapse[5,6]. These substitutions can also complicate future therapeutic options using the same class of inhibitors, as resistant mutations may be generated due to the selective pressure exerted by the effect of antiviral treatment.
One well-documented example is the Q80K polymorphism, a simeprevir resistance-associated substitution that is naturally found in more than 30% of naive subtype 1a patients and less than 1% of 1b patients in the United States[7]. Simeprevir, an NS3 protease inhibitor approved in 2014 and currently recommended for HCV G1 and G4 infection in combination with pegylated-interferon and ribavirin, achieved an SVR rate of 85% in naïve patients with chronic HCV G1 infection. However, in patients with the Q80K substitution, SVR dropped to 58%, a value similar to that of patients treated only with pegylated interferon plus ribavirin[8]. In failures to treatment, Q80K will be reinforced and variants with cross-resistance to other NS3 inhibitors, such as R155K, may also be selected[9]. Because of the high prevalence of the Q80K NS3 polymorphism in the HCV G1a-infected population, implementation of an accurate pretreatment screening program is strongly recommended in clinical practice to select the optimal treatment for each individual patient[10].
The current method for detecting these resistance-associated substitutions is direct Sanger sequencing, a technique with a detection limit of 20% to 25%[11]. That is, mutations present in the viral quasispecies in percentages below this value cannot be identified. In addition, it is a costly technique that is not available in many clinical laboratories.
To improve the management of HCV infection, the aim of this study was to develop a low-cost, highly sensitive method based on real-time PCR technology that can be implemented in routine clinical laboratories for Q80K polymorphism screening in HCV G1-infected patients.
Coded serum samples from 442 HCV G1-infected patients were used in the study. These included 240 samples previously tested by first-generation line probe assay (Inno-LiPA v1.0) obtained from the hospital Liver Unit HCV serum bank, which were used to develop the technique, and 202 plasma samples from patients with HCV subtype 1a and 1b infection attending our hospital outpatient clinic, used to validate the method. The prevalence of the Q80K polymorphism in our setting was determined in both groups of samples studied.
HCV RNA was extracted from 650 μL of serum by automatic RNA extraction using a total nucleic acid isolation (TNAI) kit (AmpliPrep system; Roche Diagnostics, West Sussex, United Kingdom) or from 140 μL of serum by manual extraction using the QIAamp viral RNA minikit (Qiagen, Hilden, Germany). The measures to prevent contamination suggested by Kwok and Higuchi were strictly applied[12].
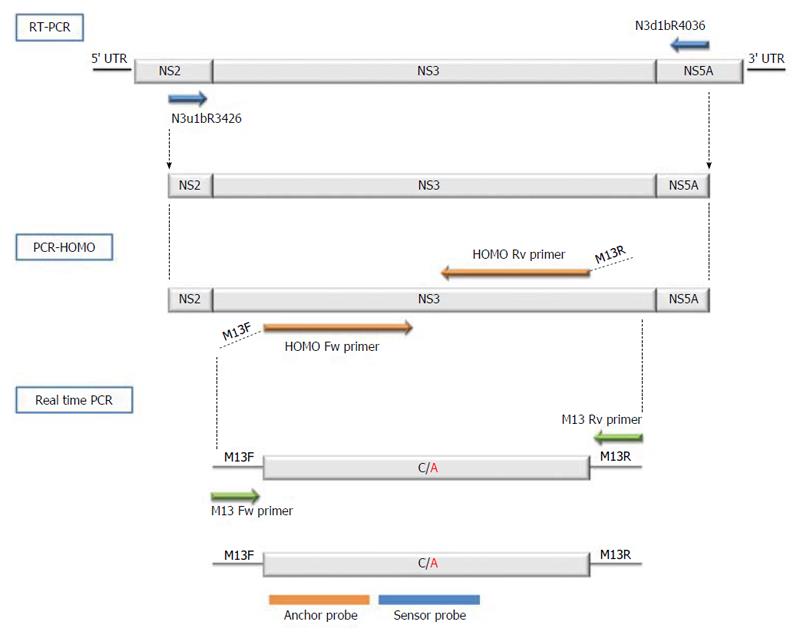
The first step in detection of the Q80K polymorphism was to obtain cDNA from the extracted HCV RNA. The NS3 target for Q80K detection was a 611-nucleotide (nt)-long region spanning nt 3426 to 4036, in accordance with the H77 reference sequence (GenBank NC_004102.1). NS3 target cDNA was obtained using degenerate primers: forward, N3u1bR3426 5’-ATCACGGCYTACKCYCAACARAC-3’ and reverse, N3d1bR4036 5’-GTRGGDGCGTGHARRTGKGCCAC-3’. These primers were able to amplify both HCV subtypes 1a and 1b.
The RT-PCR was performed using the Transcriptor One-Step RT-PCR kit (Roche Applied Science, Basel, Switzerland). The reaction was carried out in a final volume of 25 μL containing 5 μL of 5 × reaction buffer, 1.25 μL of 5% DMSO, 0.6 μmol/L of each primer, (0.75 μL of the two 20-μmol/L forward and reverse primers), 0.5 μL of Transcriptor One-Step enzyme mix, and 5 μL of extracted HCV RNA. The RT-PCR program was run as follows: one cycle at 55 °C for 30 min (reverse transcription) and one cycle at 94 °C for 7 min (reverse enzyme inactivation step), followed by 35 cycles each of 10 s at 94 °C, 30 s at 51 °C and 40 s at 68 °C, followed by 1 final extension cycle at 68 °C for 7 min (amplification), subsequently maintained at 4 °C. A final product of 611 nucleotides was obtained.
A negative control was included in each PCR step to ensure that no contamination had occurred. All the PCR mixes were performed in a DNA-free room and within the laboratory cabin. After each manipulation, the bench surface and cabin were both cleaned with 70% ethanol.
The main problem involved in using specific probes to detect HCV single point mutations is the high variability of the virus. Hence, the aim of the procedure developed was to homogenize the regions flanking Q80K and thereby eliminate the existing variability. This was accomplished by designing two specific long primers [named homogenize the flanking regions (HOMO)-PCR primers] that covered the entire region of interest, except for the single nucleotide base at position 80 causing the C/A polymorphism, which was left uncovered between the primers Thus, the PCR obtained identical sequences throughout except for the nucleotide causing the Q80K mutation (polymorphic nucleotide). The following primers were used for HOMO-PCR: forward primer (5’-GTTGTAAAACGACGGCCAGTCCAAGGGTCCTGTCATCCAGATGTATACCAATGTGGAC-3’) and reverse primer (5’-CACAGGAAACAGCTATGACCCGGGAACCTTGAGGAGCGGGCCAGCCCACAAGGTCTT). The two primers contained a complementary universal M13 primer, which was needed for the next step. A final product of 116 nucleotides was obtained.
Briefly, the PCR reaction mixture consisted of 5 μL of cDNA sample, 2.5 μL of 10 × FastStart reaction buffer, 1.25 μL of 5% DMSO, 0.5 μL of each 0.4-mmol/L dNTP, 1 μL of the two 10-μmmol/L (upstream and downstream) primers, and 0.25 U of FastStart high-fidelity enzyme blend (5 U/μL) (Roche Diagnostics, Mannheim, Germany) in a final volume of 25 μL. After denaturing for 2 min at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at 50 °C, and 10 s at 72 °C were performed, with a final 4-min step at 72 °C, subsequently maintained at 4 °C. The amplification products were analyzed by 2% agarose gel electrophoresis, and negative controls (amplifications in the absence of RNA) were included in parallel to ensure the absence of contamination. All amplicons were purified in agarose gel, using the QIAquick gel extraction kit (Qiagen, Valencia, CA, United States).
Real-time PCR was performed in the LightCycler 2.0 analyzer (Roche Diagnostic, Mannheim, Germany). This thermal cycler detects mutations by analyzing the melting point of one of the two adjacent fluorescent-labeled probes. A schematic representation of the oligonucleotide primers, fluorescence resonance energy transfer (FRET) probes, and sequences used to detect the Q80K variation is shown in Figure 1. To enable sequencing of the fragment of interest, the two HOMO PCR primers (forward and reverse), used to homogenize the regions flanking position 80 were designed with the universal M13 sequencing primer at the 5´end: M13 forward (5’-GTTGTAAAACGACGGCCAGT-3’) and M13 reverse (5’-CACAGGAAACAGCTATGACC-3’). Two FRET probes that hybridize closely together were used to detect the mutation: Anchor (5’-GTCCTGTCATCCAGATGTATAC--FL) spanning nt 3625 to 3646 and labeled with fluorescein at the 3’ end, and Sensor Mut (5’-LC640-ATGTGGACAAAGACCTTGT-PH) spanning nt 3649 to 3666 and labeled with red fluorophore LC640 at the 5’ end. This last probe hybridizes over the mutation position and perfectly matches the mutated sequence while showing a single mismatch with the wild-type variant.
The reaction was conducted at a final volume of 20 μL containing 1.6 mmol/L MgCl2, 0.4 μmol/L of each primer, 0.16 μmol/L of each FRET probe, 2 μL of LightCycler Fast Start DNA master hybridization probe mix (Roche Diagnostics, Mannheim, Germany) and 5 μL of purified HOMO-PCR product. The PCR program was run as follows: an initial denaturation step of 95 °C for 10 min, 15 cycles of denaturation at 95 °C for 10 s, annealing at 43 °C for 10 s, and extension at 72 °C for 15 s. After amplification, melting curves were generated by denaturation at 95 °C for 20 s, maintaining the sample at 40 °C for 20 s and slowly heating the sample at 85 °C. Fluorescent measurements were recorded during each annealing step and during heating in the melting step, with measurements at a wavelength of 640 nm once the run was completed. To analyze the results, the melting curves were converted to melting peaks by LightCycler software, thus enabling the normal and mutated form to be easily differentiated by their characteristic peak patterns based on the differing melting temperatures.
All samples tested using the PCR method developed here underwent Sanger sequencing to verify the reliability of the new method. The PCR product was directly sequenced using the BigDye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA, United States). All sequenced samples were aligned using the BioEdit software and checked for concordance.
To evaluate the sensitivity of the new technique for detecting Q80K variants from HCV G1 samples, one sample containing each variant (Q and K) was selected to provide pure copies of the HCV strands carrying the two variants by molecular cloning.
The nested amplicon with the Q80K polymorphism was 531 nt long and contained a 491-nt-long region (nt 3492 to 3982), in accordance with the H77 reference sequence (GenBank NC_004102.1), as well as the M13 universal primers. The following PCR primers were used: upstream, 13u31b3492 5’- GTTGTAAAACGACGGCCAGTGACARRAACCARGTBGADGGDGA -3’ and downstream, 13d31b3982 5’-CACAGGAAACAGCTATGACCGARTTRTCYGTGAARACCGGRGACC-3’.
The nested reaction mixture contained 5 μL 10 × PfuUltra II reaction buffer, 1.25 μL of each 10mmol/L dNTP, 0.5 μL of each 10-mmol/L forward and reverse primers and 1 μL of PfuUltra II Fusion HS DNA polymerase in a final volume of 50 μL. After denaturing for 2 min at 95 °C, 30 cycles of 20 s at 95 °C, 20 s at 50 °C, and 15 s at 72 °C were performed, with a final 3-min step at 72 °C, subsequently maintained at 4 °C.
Molecular cloning of the two variants (Q and K) was performed using the Zero Blunt Topo PCR cloning kit for sequencing. After treating the colonies with a restriction enzyme and purifying with the Miniprep kit (QIAprep Spin Miniprep Kit), 12 clones of each variant were obtained and identified by direct Sanger sequencing.
To prepare the samples for the next step, 1 sample of each cloned variant (Q and K) was selected and subjected to HOMO-PCR with the following conditions: 1 denaturation cycle for 2 min at 95 °C, 35 cycles of 30 s at 95 °C, 30 s at 50 °C, and 10 s at 72 °C, and a final 4-min step at 72 °C, subsequently maintained at 4 °C.
The 2 amplification products (Q and K) were analyzed by 2% agarose gel electrophoresis including a negative control (amplifications in the absence of RNA) to ensure an absence of contamination. The amplicons were purified using the QIAquick gel extraction kit (Qiagen, Valencia, CA, United States). The purified PCR product was quantified using the PicoGreen assay (Invitrogen, Carlsbad, CA, United States).
Of note, proper serum sample storage and RNA manipulation are essential to succeed in Q80K evaluation. A viral load below 104 copies/mL is an important limitation for this and any other method based on PCR amplification. The protocol presented was designed and validated for Q80K detection because of the high reported prevalence of this mutation, but our aim was to create a procedure that can be adapted for screening resistance mutations occurring in other regions of the HCV genome.
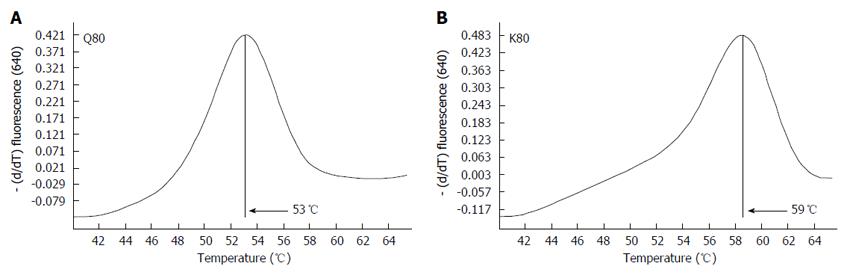
The melting temperature for the K variant was around 59 °C, as the sensor probe was 100% homologous to variant K, whereas the melting temperature for the wild type (Q) was around 53 °C due to the mismatch between the probe and the variant (Figure 2).
The reliability of the new method was verified by testing the same 240 samples using direct Sanger sequencing. The specific nucleotide probes developed for the new real-time technique were able to identify the Q and K variants, and showed complete concordance with direct Sanger sequencing in all the samples compared.
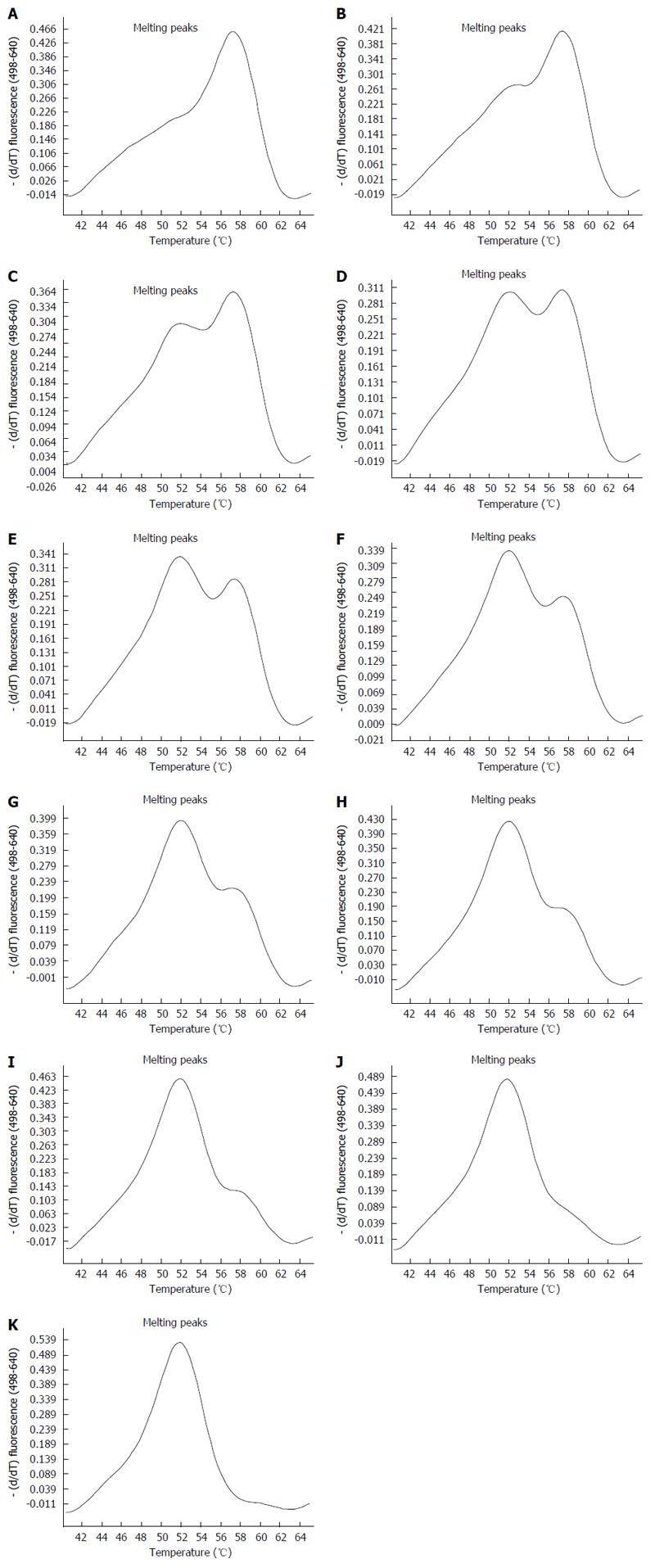
To test the sensitivity of the new real-time technique compared to direct Sanger sequencing, HCV strands carrying the Q and K variants were mixed in different proportions, as described in Table 1. The melting peaks of each mixture obtained by the new real-time method are shown in Figure 3. The method was able to identify the K variant in mixtures containing as little as 10% of the variant. Interestingly, we found that the difference between melting peaks was more prominent in mixtures carrying Q as a minor variant than in those carrying K as a minor variant.
| Mixture samples code | Variant Q | Variant K |
| 1 | 0% | 100% |
| 2 | 10% | 90% |
| 3 | 20% | 80% |
| 4 | 30% | 70% |
| 5 | 40% | 60% |
| 6 | 50% | 50% |
| 7 | 60% | 40% |
| 8 | 70% | 30% |
| 9 | 80% | 20% |
| 10 | 90% | 10% |
| 11 | 100% | 0% |
Once the method had been developed and checked for sensitivity, we determined the prevalence of Q80K in the 240 historical G1 samples from the Liver Unit serum bank collection and the 202 plasma samples from chronically infected Catalonian patients subtyped by a high-resolution HCV method (137 subtype 1a and 65 1b). In total, 442 plasma samples were tested by the new technique. The prevalence of the Q80K polymorphism in the stored samples was 5.4%, whereas in the patient samples, Q80K was detected in 14.6% of subtype 1a infections and none of the subtype 1b infections (Table 2).
| Genotype | Total tested samples | Samples with Q80K variant | Prevalence |
| Genotype 1 | 240 | 13 | 5.4% |
| Genotype 1a | 137 | 20 | 14.6% |
| Genotype 1b | 65 | 0 | 0% |
| Total | 442 | 33 | 7.5% |
In this study, we describe a new strategy to detect single point mutations by real-time PCR using specific FRET probes that can be adapted to the LightCycler platform, a method widely used in clinical laboratories. In the developmental phase reported here, the method was used to detect the Q80K NS3 polymorphism in the HCV G1a and G1b-infected population.
An inherent difficulty with HCV is that it replicates using an RNA-dependent RNA-polymerase that lacks proofreading mechanisms. Because of this characteristic, the virus has an estimated mutation rate of 10-3 to 10-4 substitutions per nucleotide per replication cycle, which means that every time the RNA is copied, the newly generated genome will carry 1 to 10 new mutations in addition to those in the parent viral genome. Because of this high mutation rate, a huge number of variant genomes are continuously generated, which hinders the use of FRET probes to detect a single point mutation in the circulating virion population. The novelty of our method is that we were able to homogenize the surroundings of the particular nucleotide position of interest by using specific primers. This paved the way for application of a FRET probe with melting curve analysis that would enable differentiation of a single point mutation in the highly variable HCV viral population.
The PCR method described was found to be highly reliable when the results were compared with those of Sanger sequencing. G1a samples carrying the Q and K variants clearly showed double peaks with the corresponding melting temperatures. In addition, this method can detect mixed infections, although their relative proportions cannot be quantitatively established. In our G1b samples, however, there were no cases of the Q80K variant. A clinical trial performed by Janssen pharmaceuticals reported less than 1% of the Q80K variant in G1b infections[13]. Despite the low prevalence of Q80K found in these initial studies, the need for Q80K screening in G1b infection should be further evaluated in larger patient populations.
In contrast to other methods used to detect resistance-associated substitutions, which require separate specific primers for G1a and G1b[14-16] our technique can identify a single point mutation using one set of primers for all G1 samples. Simple hybridization is then carried out, in this case with the specific probe that differentiated Q from K at a position 80. We are now working to fully automate the procedure to increase its cost-effectiveness and reduce the risk of contamination.
Of course, mutation detection using next-generation sequencing (NGS) methods would be much more sensitive than our technique for detecting minority mutants (0.5%-1% of the total viral population). However, NGS is an expensive, labor-intensive procedure that is not in extensive use. Our aim was to provide a fast, sensitive, low-cost method for diagnostic applications, particularly keeping in mind the possibility for use in underdeveloped countries where the emergence of HCV infection is of particular concern and routine use of deep sequencing cannot be considered.
Finally, although the technique was designed to detect the Q80K resistance mutation, the protocol can be adapted to detect other mutations in different regions of the HCV genome for use in routine pretreatment clinical screening. We are now working on adapting this method for clinical detection of Y93H, a highly prevalent NS5A mutation that confers resistance to daclatasvir, ledipasvir, and ombitasvir. In addition, Q80K seems to be a natural variant in G5 isolates, which may explain the reduced susceptibility to simeprevir in G5 patients[17]; hence, the technique presented may also have a role in Q80K screening for this population.
In conclusion, this study presents a low-cost, sensitive technique to identify resistance-associated point mutations in samples from HCV-infected patients that is feasible for routine use in clinical laboratories. Application of this method in clinical practice could be a useful aid to providing optimal individualized treatment for patients with HCV infection.
Detection of resistance-associated amino acid substitutions in patients with hepatitis C virus (HCV) infection is clinically relevant information for personalizing therapy. Low-cost, highly sensitive techniques enabling routine detection of any single point mutation would be useful to identify patients at a risk of treatment failure.
Diagnose techniques to detect single point mutations in HCV have been hampered by the huge amount of variation generated by HCV.
A fast, low-cost diagnostic strategy based on real-time PCR and fluorescence resonance energy transfer (FRET) robe melting curve analysis (LightCycler method) has been successfully developed to identify single point mutations in highly variable genomes, in this case for HCV.
This technique can be adapted to detect any single point mutation in highly variable genomes.
With sufficient details presented so that others could repeat and validate this work easily. The logic of what the authors present is well laid out and the figures add to the manuscript. It will likely be used by a limited number of people as SIM pegIFN and Ribavirin are used less frequently than the current second generation direct-acting antivirals (DAAs). However, the authors do make clear that they are doing this for the NS5A mutations, which are much more relevant to the 2nd generation DAAs.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fanning LJ, Urbaczek AC S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1770] [Cited by in RCA: 1846] [Article Influence: 153.8] [Reference Citation Analysis (3)] |
| 2. | Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 933] [Cited by in RCA: 939] [Article Influence: 58.7] [Reference Citation Analysis (1)] |
| 3. | Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2042] [Cited by in RCA: 2025] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 4. | Schinazi RF, Bassit L, Gavegnano C. HCV drug discovery aimed at viral eradication. J Viral Hepat. 2010;17:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Vermehren J, Sarrazin C. The role of resistance in HCV treatment. Best Pract Res Clin Gastroenterol. 2012;26:487-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Pawlotsky JM. Hepatitis: HCV variability, the immune system and resistance to antiviral drugs. Nat Rev Gastroenterol Hepatol. 2009;6:383-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | McCloskey RM, Liang RH, Joy JB, Krajden M, Montaner JS, Harrigan PR, Poon AF. Global origin and transmission of hepatitis C virus nonstructural protein 3 Q80K polymorphism. J Infect Dis. 2015;211:1288-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Forns X, Lawitz E, Zeuzem S, Gane E, Bronowicki JP, Andreone P, Horban A, Brown A, Peeters M, Lenz O. Simeprevir with peginterferon and ribavirin leads to high rates of SVR in patients with HCV genotype 1 who relapsed after previous therapy: a phase 3 trial. Gastroenterology. 2014;146:1669-1679.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 9. | Lindström I, Kjellin M, Palanisamy N, Bondeson K, Wesslén L, Lannergard A, Lennerstrand J. Prevalence of polymorphisms with significant resistance to NS5A inhibitors in treatment-naive patients with hepatitis C virus genotypes 1a and 3a in Sweden. Infect Dis (Lond). 2015;47:555-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Schneider MD, Sarrazin C. Antiviral therapy of hepatitis C in 2014: do we need resistance testing? Antiviral Res. 2014;105:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Leitner T, Halapi E, Scarlatti G, Rossi P, Albert J, Fenyö EM, Uhlén M. Analysis of heterogeneous viral populations by direct DNA sequencing. Biotechniques. 1993;15:120-127. [PubMed] |
| 12. | Kwok S, Higuchi R. Avoiding false positives with PCR. Nature. 1989;339:237-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2583] [Cited by in RCA: 2475] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 13. | Janssen O. Highlights of prescribing information. Titusville, NJ: Janssen 2016; 1-50. |
| 14. | Bae AS, Ku KS, Miller MD, Mo H, Svarovskaia ES. Allele-specific real-time PCR system for detection of subpopulations of genotype 1a and 1b hepatitis C NS5B Y448H mutant viruses in clinical samples. J Clin Microbiol. 2011;49:3168-3174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Fonseca-Coronado S, Escobar-Gutiérrez A, Ruiz-Tovar K, Cruz-Rivera MY, Rivera-Osorio P, Vazquez-Pichardo M, Carpio-Pedroza JC, Ruíz-Pacheco JA, Cazares F, Vaughan G. Specific detection of naturally occurring hepatitis C virus mutants with resistance to telaprevir and boceprevir (protease inhibitors) among treatment-naïve infected individuals. J Clin Microbiol. 2012;50:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Ntziora F, Paraskevis D, Haida C, Manesis E, Papatheodoridis G, Manolakopoulos S, Elefsiniotis I, Karamitros T, Vassilakis A, Hatzakis A. Ultrasensitive amplification refractory mutation system real-time PCR (ARMS RT-PCR) assay for detection of minority hepatitis B virus-resistant strains in the era of personalized medicine. J Clin Microbiol. 2013;51:2893-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lenz O, Vijgen L, Berke JM, Cummings MD, Fevery B, Peeters M, De Smedt G, Moreno C, Picchio G. Virologic response and characterisation of HCV genotype 2-6 in patients receiving TMC435 monotherapy (study TMC435-C202). J Hepatol. 2013;58:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |