Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9356
Peer-review started: May 11, 2016
First decision: July 13, 2016
Revised: August 5, 2016
Accepted: August 19, 2016
Article in press: August 19, 2016
Published online: November 14, 2016
Processing time: 185 Days and 20.2 Hours
To investigate the role of regulatory T cell (Treg) subsets in the balance between Treg and T helper 17 (Th17) cells in various tissues from mice with dextran sulfate sodium-induced colitis.
Treg cells, Treg cell subsets, Th17 cells, and CD4+CD25+FoxP3+IL-17+ cells from the lamina propria of colon (LPC) and other ulcerative colitis (UC) mouse tissues were evaluated by flow cytometry. Forkhead box protein 3 (FoxP3), interleukin 17A (IL-17A), and RORC mRNA levels were assessed by real-time PCR, while interleukin-10 (IL-10) and IL-17A levels were detected with a Cytometric Beads Array.
In peripheral blood monocytes (PBMC), mesenteric lymph node (MLN), lamina propria of jejunum (LPJ) and LPC from UC mice, Treg cell numbers were increased (P < 0.05), and FoxP3 and IL-10 mRNA levels were decreased. Th17 cell numbers were also increased in PBMC and LPC, as were IL-17A levels in PBMC, LPJ, and serum. The number of FrI subset cells (CD4+CD45RA+FoxP3low) was increased in the spleen, MLN, LPJ, and LPC. FrII subset cells (CD4+CD45RA-FoxP3high) were decreased among PBMC, MLN, LPJ, and LPC, but the number of FrIII cells (CD4+CD45RA-FoxP3low) and CD4+CD25+FoxP3+IL-17A+ cells was increased. FoxP3 mRNA levels in CD4+CD45RA-FoxP3low cells decreased in PBMC, MLN, LPJ, and LPC in UC mice, while IL-17A and RORC mRNA increased. In UC mice the distribution of Treg, Th17 cells, CD4+CD45RA-FoxP3high, and CD4+CD45RA-FoxP3low cells was higher in LPC relative to other tissues.
Increased numbers of CD4+CD45RA-FoxP3low cells may cause an imbalance between Treg and Th17 cells that is mainly localized to the LPC rather than secondary lymphoid tissues.
Core tip: The exact etiology and pathology of ulcerative colitis (UC) remains unknown. Here we investigated the role of regulatory T cell (Treg) subsets in the balance between Treg and T helper 17 (Th17) cells in various tissues from mice with dextran sulfate sodium-induced colitis. In this study we found that increased numbers of CD4+CD45RA-FoxP3low cells may cause an imbalance between Treg and Th17 cells, which was mainly localized to the lamina propria of colon rather than secondary lymphoid tissues. Based on our findings, lamina propria-resident Treg cells appear to play important roles in shaping local peripheral tolerance and maintaining intestinal homeostasis, and an imbalance of Treg and Th17 cells in the lamina propria of the colon is critical for UC pathogenesis.
- Citation: Ma YH, Zhang J, Chen X, Xie YF, Pang YH, Liu XJ. Increased CD4+CD45RA-FoxP3low cells alter the balance between Treg and Th17 cells in colitis mice. World J Gastroenterol 2016; 22(42): 9356-9367
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9356.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9356
Ulcerative colitis (UC) is a type of inflammatory bowel disease (IBD) that affects the colon and is confined to the mucosa and superficial submucosa[1]. UC symptoms include diarrhea, abdominal pain, and rectal bleeding, which can all seriously affect quality of life, and the disease is often marked alternating phases of clinical relapse and remission. Although the exact etiology and pathology of UC remains unknown[2], there is increasing evidence that an aberrant immune response is involved in this disease[3].
Acquired immunity plays a vital role in UC pathogenesis, where in T helper cell-type (Th) 1 and Th2 immune responses, as well as alternate subsets of T cells, such as regulatory T cells (Treg) and T helper 17 (Th17) cells, contribute to IBD[4,5].
Regulatory T cells belong to a functionally specialized subset of CD4+ T cells that maintains immune tolerance and homeostasis via cell-cell interactions and secretion of interleukin-10 (IL-10) or other anti-inflammatory cytokines that inhibit activation of effector T cells[6,7]. Notably, Treg cells may play a crucial role in inhibiting intestinal inflammation, maintaining immune tolerance, and providing protection from colitis[8]. A study by Sakaguchi et al[9] demonstrated that Treg cells can be divided into three different functional subsets: Resting Tregs, FrI (rTreg or CD45RA+Foxp3low); activated Tregs, FrII (aTreg or CD45RA-Foxp3high); and non-suppressive Tregs, FrIII (CD45RA-Foxp3low). CD4+CD45RA+Foxp3low cells are resting Treg cells that upon activation become CD4+CD45RA-Foxp3high cells, which are the major suppressive cells that can affect immunologic function when levels of this subtype decrease. Meanwhile, CD4+CD45RA-Foxp3low cells secrete interleukin-17 (IL-17) and have the potential to become Th17 cells, a newly discovered CD4+ T cell subset that lacks immunosuppressive function and is characterized by interleukin 17A (IL-17A), IL-17F, IL-22, IL-21 secretion[10].
Th17 cells show pleiotropic activities and functions that promote immune responses via the adaptive and innate immune systems. Like sentinel cells, Th17 cells help maintain epithelial barrier function in healthy intestines. However, in the presence of chronic intestinal inflammation, Th17 cells present IL-23 and show full pathogenic and antibacterial functions[11]. Aberrant numbers of Th17 cells have been reported to occur in colonic LP of the ileum and colon in conventionally raised mice, and these cells are highly infiltrated in inflamed areas in colitic mice[12].
Furthermore, other studies reported that CD4+ T cells can demonstrate enhanced “plasticity” between T-cell subsets, such as the IL-17 and Foxp3 double-expressing (DE) CD4+ T cell population, which is a crossover transition between Treg and Th17 cells[13]. In IBD patients, the population of circulating IL-17 and Foxp3 DE CD4+ T cells is increased. Furthermore, the finding that IL-17 and Foxp3 DE CD4+ T-cell populations co-express related orphan receptor-γt (RORγt) and Foxp3 suggests that Treg cells can convert to Th17 cells that have decreased suppressive function that is characteristic of CD4+Foxp3+ T lymphocytes[14]. Indeed, in our earlier study of scleroderma patients, we identified a CD4+CD45RA-Foxp3low cell subset that had no suppressive function and co-expressed RORγt and Foxp3[15].
Here we examined Treg, Treg subsets, and Th17 cells in tissues from UC mice. We found abnormal proportions of these cells and a cell population that co-expressed FoxP3 and RORC mRNA, which may represent a crossover transition of Th17 and Treg cells that is related to an imbalance of Treg cells and Th17 cells in dextran sulfate sodium (DSS)-induced colitis.
Male C57BL/6 mice (aged 6-8 wk; 20-22 g) were obtained from the Center for Animal Resource and Development (Weitonglihua Company, Beijing, China). All mice were maintained on a 12 h/12 h light/dark cycle under specific pathogen-free conditions. All animal procedures and stress protocols were approved by the Institutional Animal Care and Committee of Beijing Chaoyang Hospital, Capital Medical University.
The healthy control (HC) mice drank distilled water for 14 d, while the UC mice drank distilled water supplemented with 2.5% w/v (DSS, MW = 40000-50000, MP Biomedical, United States) for 7 d followed by 7 d of drinking water alone. The mice were sacrificed on the 14th d. DSS-induced colitis was characterized by higher disease activity index that included changes in body weight, stool consistency, and the presence of blood in the stool[16].
Histopathological evaluation was performed as described previously[17]. Mouse colons were extracted immediately after sacrifice and examined for macroscopic damage. The resected colons were fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin-eosin for evaluation.
Anti-mouse rat antibodies (conjugated with FITC, PE, PE-Cyanine7, PE-CF594, PerCP, APC, Alexa Fluor® 488, and BV510 as indicated) used in this study were: CD4-PerCP (L3T4, BD Pharmingen™, United States), CD25-APC (IL-2 Receptor α chain, BD Pharmingen™, United States), FoxP3-PE-Cyanine7 (JM2, eBioscience, United States), CD45RA-PE (BD Pharmingen™, United States), IL-17-Alexa Fluor® 488 (BD Pharmingen™, United States), CD3e-PE-CF594 (CD3ε chain, BD Horizon™, United States), CD8a-BV510 (Ly-B, BD Horizon™, United States). In all experiments, a control antibody of the respective IgG isotype was included. Leukocyte Activation Cocktail (BD Pharmingen™, United States), RNeasy Micro Kit (QIAGEN, Germany), FastQuant RT Kit (TIANGEN, China), SuperReal PreMix Plus (TIANGEN, China), and the Mouse Th1/Th2/Th17 Cytokine kit (BD Biosciences, United States) were used according to the manufacturer’s instructions.
Peripheral blood (PBMC), spleen, mesenteric lymph node (MLN), lamina propria of jejunum (LPJ), and lamina propria of colon (LPC) from UC and HC mice were collected and mononuclear cells were prepared. The cells were then incubated with CD4, CD25, and CD45RA antibodies in the dark at room temperature for 15 min. Intracellular FoxP3 staining was subsequently performed according to the manufacturer’s instructions. Intracellular cytokine production was detected after stimulation of 1 × 106 cells with 2 μL Leukocyte Activation Cocktail (BD Pharmingen™, United States) for 4.5 h. Cells were then incubated with CD4, CD25, and CD45RA and stained with antibodies against FoxP3 and IL-17 after fixation and permeabilization (eBioscience, United States). Images of stained cells were acquired using a Gallios flow cytometer (Beckman Coulter, United States) and analyzed with Kaluza v1.20 software.
Mononuclear cells extracted from PBMC, spleen, MLN, and jejunum and colon LP were stained with CD4, CD25, and CD45RA antibodies before sorting with a FACS AriaII flow cytometer (BD Biosciences, United States).
Total RNA was extracted from 2-10 × 103 sorted Treg cells and CD4+CD45RA-Foxp3low cells from UC and HC mice using an RNeasy Micro Kit (QIAGEN). The RNA was then reverse-transcribed to obtain cDNA (FastQuant RT Kit, TIANGEN). Real-time PCR was performed with a SYBR green assay (SuperReal PreMix Plus, TIANGEN) using the ABI 7500 system (Applied Biosystems) with a GAPDH-Primer (PMM04) and other primers listed in Table 1.
| Primers | Forward (5'-3') | Reverse (5'-3') |
| GAPDH mRNA | GGTTGTCTCCTGCGACTTCA | TGGTCCAGGGTTTCTTACTCC |
| FoxP3 mRNA | CTGCCTTGGTACATTCGTGA | CAGATGTTGTGGGTGAGTGC |
| IL-17 mRNA | CTGTGTCAATGCGGAGGGAA | CGACCCTGAAAGTGAAGGGG |
| RORC mRNA | ACGGCCAACTTACTCTTGGA | AGAAACTGGGAATGCAGTGG |
IL-10 and IL-17A levels secreted by mononuclear cells from PBMC, spleen, MLN, LPJ, and LPC samples were determined using the Mouse Th1/Th2/Th17 Cytokines kit (BD Biosciences) according to the manufacturer’s instructions. Serum IL-17A levels were also assessed using the same method. In addition, IL-10 and IL-17A levels were examined using a BD FACScanto II flow cytometer (BD Biosciences, United States).
Data were analyzed with the SPSS v18.0 statistics package (IBM, United States). Variables were summarized as counts and percentages or as medians and ranges. Independent samples t-test and nonparametric Mann-Whitney U tests were used to compare data between groups. P values less than 0.05 were considered to be statistically significant.
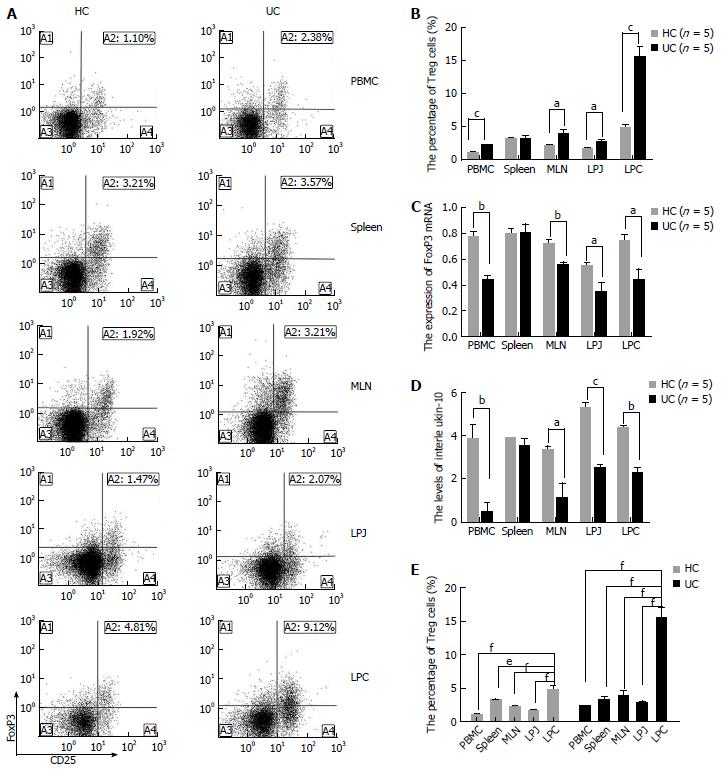
To investigate the roles of Treg cells in DSS colits mice, we compared the levels of Treg, FoxP3 mRNA, and IL-10 from different samples including PBMC, MLN, spleen, LPJ, and LPC isolated from HC and DSS colitis mice. Treg levels increased in PBMC, MLN, LPJ, and LPC samples from DSS colitis mice (P < 0.0001, P = 0.0399, P = 0.0151, P = 0.0001, respectively) (Figure 1A, B). In contrast, FoxP3 mRNA expression (P = 0.0016, P = 0.0062, P = 0.0291, P = 0.0325, respectively) and IL-10 levels (P = 0.0091, P = 0.0353, P = 0.0002, P = 0.0012, respectively) in Treg cells from DSS colitis mice decreased relative to the HC (Figure 1C, D). Meanwhile, spleen tissue from DSS colitis and HC groups showed no difference in the number of Treg cells, FoxP3 mRNA expression, and IL-10 levels (P > 0.05) (Figure 1A-D).
In LPC from DSS colitis mice, Treg cell numbers were increased compared to that of MLN, spleen, LPJ, and PBMC (P = 0.0005, P = 0.0002, P = 0.0002, P = 0.0001, respectively), while MLN, spleen, PBMC, and LPJ showed no difference in Treg numbers (P > 0.05) (Figure 1E). Similarly, in HC mice Treg cell numbers in LPC were increased compared to those in spleen, MLN, PBMC, and LPJ (P = 0.0105, P = 0.0006, P < 0.0001, P = 0.0002, respectively), with the latter two samples showing similar numbers with no significant differences (P > 0.05) (Figure 1E).
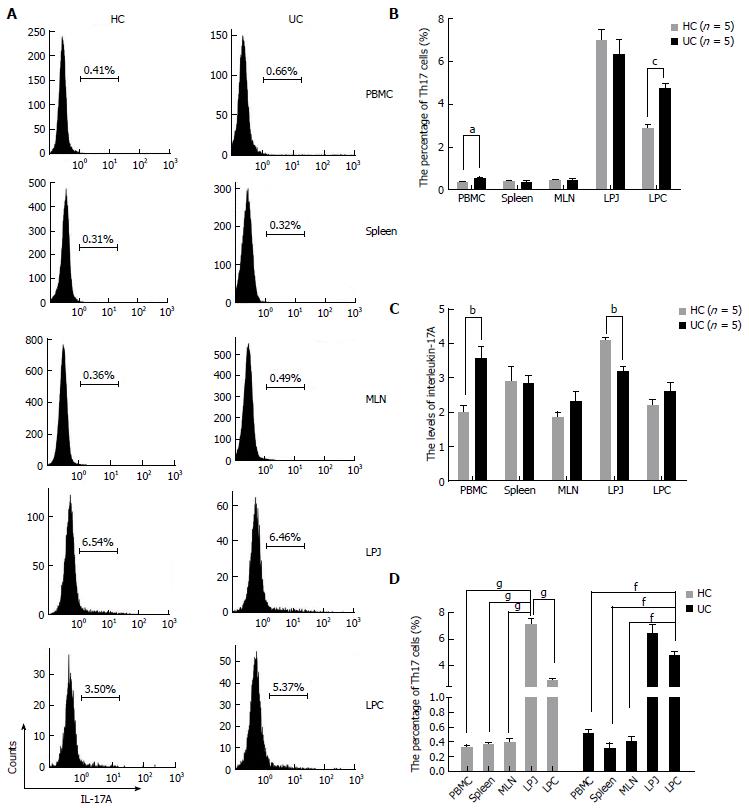
Given the contribution of Th17 cells to inflammatory responses, we next assessed Th17 numbers in DSS colitis mice. There were increased numbers of Th17 cells in PBMC and colon compared to HC (P = 0.0338, P = 0.0004), although spleen, MLN, and showed no differences in Th17 cell numbers between HC and DSS colitis mice (P > 0.05) (Figure 2A, B). In culture supernatants of PBMC, LPJ, and serum, IL-17A levels were increased compared with HC (P = 0.0063, P = 0.0064, P = 0.0016, respectively), while those of spleen, MLN, and LPC were similar (P > 0.05) (Figure 2C).
In DSS colitis mice, Th17 cell numbers in LPC were also increased compared to MLN, spleen, and PBMC (P < 0.0001, respectively), and LPC and LPJ had no differences (P > 0.05) (Figure 2D). Meanwhile, in HC mice Th17 cell numbers in both LPJ were increased compared with LPC, MLN, spleen, and PBMC (P < 0.0001), as were Th17 cell numbers in LPC relative to those for MLN, spleen, and PBMC (P < 0.0001), but MLN, spleen, and PBMC showed no differences among samples (P > 0.05) (Figure 2D).
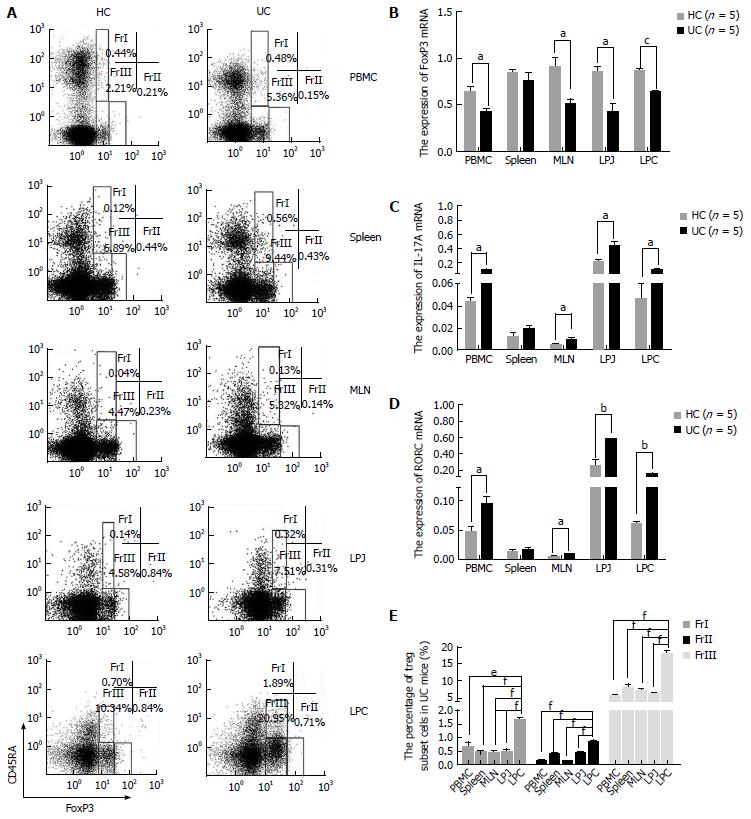
In samples from DSS colitis mice the number of CD4+CD45RA+FoxP3low was increased in spleen, MLN, LPJ, and LPC (P = 0.0020, P = 0.0050, P = 0.0010, P < 0.0010, respectively), but not in PBMC (P = 0.1170) (Figure 3A). Meanwhile, CD4+CD45RA+FoxP3low cell numbers in colon LP were also obviously increased compared to MLN, spleen, PBMC, and LPJ (P < 0.0001, P < 0.0001, P = 0.0013, P < 0.0001, respectively), which all showed similar levels (P > 0.05) (Figure 3E). In HC mice, CD4+CD45RA+FoxP3low cell numbers in LPC were obviously increased compared to MLN, spleen, PBMC, LPJ (P = 0.0033, P = 0.0081, P = 0.0010, P < 0.0001, respectively), with spleen and LPJ showing similar levels (P > 0.05).
In DSS colitis mice, the number of CD4+CD45RA-FoxP3high in PBMC, MLN, LPJ, and LPC samples was lower than that for HC mice (P = 0.0060, P = 0.0000, P = 0.0000, P = 0.0250, respectively), but spleen tissues were similar (P = 0.3980) (Figure 3A). CD4+CD45RA-FoxP3high cell numbers in LPC from UC mice were also increased compared to those for spleen, LPJ, MLN, and PBMC (P < 0.0001, P = 0.0003, P < 0.0001, P < 0.0001, respectively), with the latter two samples showing similar values (P > 0.05) (Figure 3E). In HC mice, CD4+CD45RA-FoxP3high cell numbers in LPC were increased relative to spleen, LPJ, MLN, and PBMC (P = 0.0026, P = 0.0322, P = 0.0002, P = 0.0002, respectively), with MLN and PBMC again having similar numbers (P > 0.05).
CD4+CD45RA-FoxP3lowcell numbers were increased among multiple PBMC, MLN, LPJ, and LPC samples from DSS colitis mice (P = 0.0010, P = 0.0330, P = 0.0420, P < 0.0010, respectively), but not in spleen (P = 0.248) (Figure 3A). In both DSS colitis and HC mice, CD4+CD45RA-FoxP3low cell numbers in LPC were increased relative to those for MLN, spleen, LPJ, and PBMC (P < 0.0001, P = 0.0005, P < 0.0001, P < 0.0001, respectively in DSS colitis; P = 0.0002, P = 0.0175, P = 0.0003, P < 0.0001, respectively in HC), and MLN, spleen, and LPJ showed no difference between DSS colitis and HC mice (P > 0.05) (Figure 3E).
We also evaluated levels of FoxP3, IL-17A, and RORC mRNA in CD4+CD45RA-FoxP3low cells isolated from HC and DSS colitis mice. Compared to HC mice, in DSS colitis mice FoxP3 mRNA expression was decreased among PBMC, MLN, LPJ, and LPC samples (P = 0.0286, P = 0.0284, P = 0.0121, P = 0.0002) (Figure 3B). IL-17A and RORC mRNA from DSS colitis mice was increased among the above samples compared to HC mice (IL-17 mRNA: P = 0.0150, P = 0.0278, P = 0.0247, P = 0.0357; RORC mRNA: P = 0.0369, P = 0.0128, P = 0.0101, P = 0.0016, respectively), but we saw no difference in spleen values (P > 0.05) (Figure 3C and D).
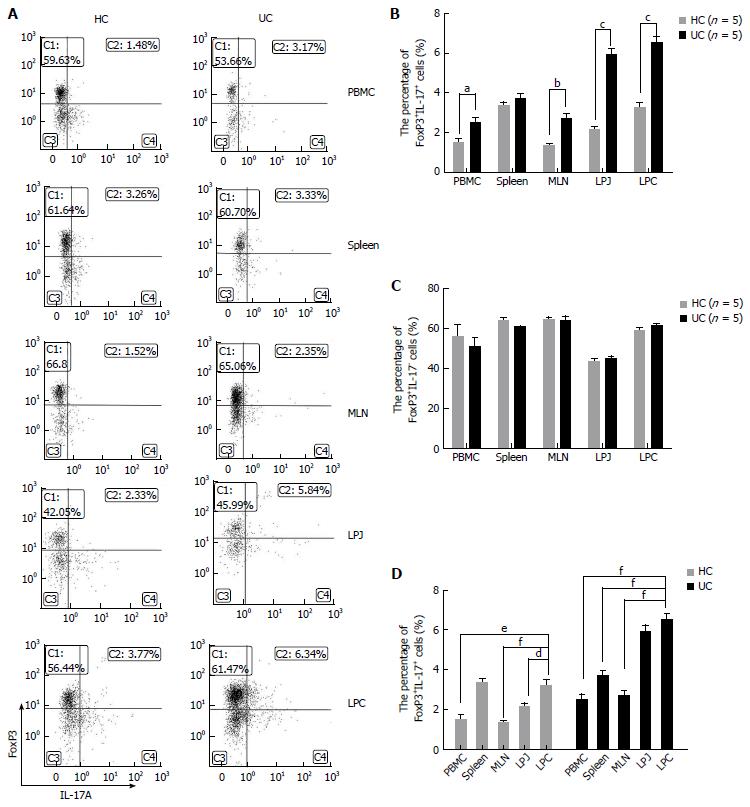
The number of CD4+FoxP3+IL-17A+ cells was higher in DSS colitis mice among multiple samples containing PBMC, MLN, LPJ, and LPC (P = 0.0161, P = 0.0037, P < 0.0001, P = 0.0001, respectively), but not in spleen (P > 0.05) (Figure 4A and B). In DSS colitis mice LPC, the levels of CD4+CD25+FoxP3+IL-17A+ cells were obviously increased compared with spleen, MLN, and PBMC (P = 0.0004, P < 0.0001, P < 0.0001, respectively), while there were no differences between MLN and PBMC (P > 0.05), or LPJ and LPC (P > 0.05). In HC mice, CD4+CD25+FoxP3+IL-17A+ cell numbers were increased in LPC compared to those for LPJ, MLN, and PBMC (P = 0.0193, P = 0.0008, P = 0.0014, respectively), but the numbers in spleen and LPC were similar (P > 0.05), and there were no differences between MLN and PBMC (P > 0.05) (Figure 4D).
When we excluded CD4+CD25+FoxP3+IL-17A+ cells from the total number of Treg cells, our results showed that CD4+FoxP3+IL-17A- cell numbers were similar between multiple PBMC, spleen, MLN, LPJ, and LPC samples from HC and DSS colitis mice (P > 0.05) (Figure 4A and C).
Treg cells are anti-inflammatory cells that secrete the anti-inflammatory cytokine IL-10 and inhibit effector T cell proliferation through cell-cell interactions[18,19]. Upon Treg-specific IL-10 ablation, mice spontaneously develop signs of colitis, illustrating the key role of Treg-derived IL-10 in maintaining intestinal health[20]. Meanwhile, high levels of the transcription regulation factor Forkhead box protein 3 (FoxP3) that is encoded by FoxP3 mRNA[21] enhance the immunosuppressive function of Treg cells[22]. In our study, we established the 2.5% DDS-induced colitis, among the common models of mice colitis: 2,4,6-trinitro benzene sulfonic acid, oxazolone and dextran sodium sulfate (DSS) colitis. Of which the DSS-induced colitis colitis was sample and repetitive[16]. Moreover it resembles the clinical course of human UC occurs frequently in the chronic phase of DSS-induced colitis[23]. Here we observed an increase in Treg cell numbers in UC mice, but this increase likely does not inhibit inflammatory responses given results from previous studies showing the presence of excessive inflammation in the intestines of UC patients[24,25]. Instead, the decreased expression of IL-10 and FoxP3 mRNA in Treg cells from UC mice suggested that Treg cells in these animals may have functional defects that contribute to UC morbidity.
In response to various cytokines such as IL-6 and TGF-β, CD4+ cells differentiate to Treg or Th17 cells, which have opposite functions and thus a balance between these populations is essential. Similar to Treg cells in the UC mice, Th17 cell numbers increased in both PBMC and LPC samples from UC mice, and IL-17A cytokine levels in PBMC and serum were concurrently increased. These results suggest the presence of a hyperactive inflammatory response in the colon mucosa of UC animals[26-28], and also that impaired Treg cell function as well as an imbalance between Treg and Th17 cell populations could be involved in inappropriate immune responses in UC.
Aberrant immune responses that can result from Treg and Th17 imbalances are a feature of autoimmune diseases[29,30], although the underlying mechanisms that promote these imbalances are unclear. On the one hand, there is heterogeneity in among the Treg cell population. Thus, we explored whether Treg subsets contribute to immune imbalance seen in UC mice. In our study, the number of CD4+CD45RA-FoxP3high cells, which are activated Treg cells that display immunosuppressive capacity, was decreased in UC. CD4+CD45RA-FoxP3high cells were previously shown to have higher levels of IL-10 transcription[31], and together with the decreased expression of FoxP3 mRNA and IL-10 in Treg cells observed here, supports that the low levels of CD4+CD45RA-FoxP3high cells contribute to immunosuppressive function of Treg cells in UC.
CD4+CD45RA+FoxP3low cells are resting Treg cells that act as as a reserve of cells that can be activated and differentiate into CD4+CD45RA-FoxP3high cells. There is a tight balance between the continuous development of CD4+CD45RA-FoxP3high cells from activated and proliferating CD4+CD45RA+FoxP3low cells and CD4+CD45RA-FoxP3high cell death after exerting suppressive effects[9,15]. Here we observed an increase in CD4+CD45RA+FoxP3low cells alongside the decrease in CD4+CD45RA-FoxP3high cells. From these results we inferred that UC mice have defects in CD4+CD45RA+FoxP3low to CD4+CD45RA-FoxP3high cell conversion that could affect CD4+CD45RA-FoxP3high cell replenishment.
On the other hand, we observed a concurrent increase in CD4+CD45RA-FoxP3low cells and decrease in CD4+CD45RA-FoxP3high cells. Earlier studies suggested that FoxP3 expression determines Treg lineage and is required for the suppressive function of these cells[32]. FoxP3 expression downregulates expression of the retinoic acid receptor-RORγt, which is the lineage-defining transcription factor for Th17 cells, and in turn contributes to the inhibition of Th17 differentiation[33,34]. However, a more recent study by Voo et al[35] documented FOXP3/RORC double-positive cells in peripheral blood and in lymphoid organs from healthy human volunteers. Moreover, FoxP3+IL-17A+ double-positive cells appear to be the crossover cells that can differentiate into Th17 cells in response to lower amounts of TGF-β together with the presence of IL-6 or IL-21[36,37]. These FoxP3+IL-17A+ double-positive cells also exist in several autoimmune diseases, including allergic rhinitis, psoriasis, and IBD[38], and several studies demonstrated that FOXP3+IL-17+cells can indeed differentiate into Th17 cells in the periphery[35,39]. Meanwhile, FrIII cells can differentiate into Th17 cells that have no immunosuppressive function[9]. Our results also showed that, relative to HC mice, UC mice have increased numbers of both CD4+CD45RA-FoxP3low cells and FoxP3+IL-17A+ double-positive cells, especially in LPC and LPJ samples. Furthermore, we showed that these CD4+CD45RA-FoxP3low cells co-expressed FoxP3 and RORC mRNA, which, together with the increased expression levels of IL-17A mRNA, confer on these cells the characteristics of FOXP3+IL-17+ double-positive cells. Thus, we inferred that CD4+CD45RA-FoxP3low cells acquired the ability to express RORC in the periphery, and have the potential to differentiate into Th17 cells that lack suppressive activity.
CD4+ Treg cells are composed of two major populations: Thymus-derived Treg cells, or natural Tregs (nTregs), and peripherally derived Treg cells, or induced Tregs, which are generated in the periphery[40,41]. To extend the study of functional Treg subsets into non-lymphoid tissues, unique tissue-resident Treg populations have been identified and characterized, such as Treg in visceral adipose tissu[42], in skin[43], in skeletal muscle[44], in lung[45], liver[46], and pancreas[47]. Recent evidence suggests that environmental signals found in peripheral non-lymphoid tissues promote the development of tissue-specific Treg cell subsets. Although the proportion of tissue-specific Treg cell subsets within tissues is difficult to determine due to differences between inflammatory and steady-state conditions[48] accumulating evidence suggests that Treg cells are highly responsive to their local environment. The gut contains a large reservoir of both secondary lymphoid tissue-resident Treg cells and non-lymphoid tissue-resident Treg cells[49]. The development of lamina propria-resident Treg cells differs from that for secondary lymphoid tissue Treg cells[50,51], in that lamina propria Treg cells respond to unique environmental signals that are generated in response to products of commensal bacteria[52,53]. As such, we further evaluated differences in Th17, Treg, and Treg cells subtypes in LPC and LPJ, spleen, MLN, and PBMC in UC and HC mice. In this study, there were more Treg in the colon relative to other tissues, while the jejunum showed the highest number of Th17 cells, even in UC mice. Round et al[52] also found that in B6 mice, distribution of Th17 cells is duodenum > jejunum > ileum > colon, while Treg cells were most abundant in the colon and scarce in the duodenum. This regional difference is associated with the T cell/APC ratio, especially CD11c(+)CD11b(+)CD103(+) dendritic cells. Our results showed that all Tregs and the three Treg subsets were more frequent in the LPC relative to other secondary lymphoid tissue and PBMC in both UC and HC mice. In addition, the number of Treg cells in spleen tissue from both UC and HC mice was similar, suggesting that lamina propria-resident Treg cells rather than secondary lymphoid tissue Treg cells are involved in UC pathogenesis. Since UC is an organ-specific autoimmune disease, the Treg cells in PBMC likely would not reflect the characteristics of UC. Moreover, relative to HC, the number of CD4+CD45RA-FoxP3high cells in the LPC and LPJ of UC mice was decreased, although the numbers were similar in spleen samples. The increased number of CD4+CD45RA-FoxP3low and CD4+CD25+FoxP3+IL-17+ cells mainly occurred in the LPC in UC mice. As such, we inferred that the abnormal differentiation of active Treg cells occurred in local tissues and not secondary lymphoid tissues.
In summary, decreased numbers of CD4+CD45RA-FoxP3high cells together with a reserve of abnormal of CD4+CD45RA+FoxP3low cells and increased numbers of non-suppressive CD4+CD45RA-FoxP3low cells, could present a potential source of Th17 cells that lack suppressive capacity and are an important characteristic of UC mice. These characteristics may contribute to an imbalance between Treg and Th17 cells and the increased numbers of functional Treg cells. Thus, lamina propria-resident Treg cells appear to play important roles in shaping local peripheral tolerance and maintaining intestinal homeostasis, and an imbalance of Treg and Th17 cells in the lamina propria of the colon is critical for UC pathogenesis.
Ulcerative colitis (UC) is a type of inflammatory bowel disease that affects the colon and is confined to the mucosa and superficial submucosa. UC symptoms include diarrhea, abdominal pain, and rectal bleeding, which can all seriously affect quality of life, and the disease is often marked alternating phases of clinical relapse and remission.
The exact etiology and pathology of UC remains unknown, there is increasing evidence that an aberrant immune response is involved in this disease. Morbidity often involves an imbalance between T helper 17 (Th17) cells and regulatory T cells (Treg).
In this study, the authors investigated the role of Treg cell subsets in the balance between Treg and Th17 cells in various tissues from mice with dextran sulfate sodium (DSS)-induced colitis.
It is believed that this study will be of great interest to scientists and critical pathogenesis for UC, and as well as clinicians studying UC.
Regulatory T cells belong to a functionally specialized subset of CD4+ T cells, which can be divided into three different functional subsets: Resting Tregs, FrI (rTreg or CD45RA+Foxp3low); activated Tregs, FrII (aTreg or CD45RA-Foxp3high); and non-suppressive Tregs, FrIII (CD45RA-Foxp3low). FrI cells are resting Treg cells that upon activation become FrII cells, which are the major suppressive cells. Meanwhile, FrIII cells secrete interleukin-17 (IL-17) and have the potential to become Th17 cells, a newly discovered CD4+ T cell subset that lacks immunosuppressive function and is characterized by interleukin 17A, IL-17F, IL-22, IL-21 secretion.
The authors demonstrated that increased numbers of CD4+CD45RA-FoxP3low cells may cause an imbalance between Treg and Th17 cells that is mainly localized to the lamina propria of colon rather than secondary lymphoid tissues. The present study was well organized and well investigated. To improve the quality of this paper, the authors should revise it according to the following suggestions: (1) the authors used a DSS colitis as a model of UC. Don’t use the “UC” in the result session. It is more suitable to use “DSS colitis” instead of “UC” throughout the result session; and (2) to confirm the role of CD4+CD45RA-FoxP3low cells in the pathogenesis in DSS colitis, the authors should show the time-course changes of these cells.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Naito Y S- Editor: Yu J L- Editor: A E- Editor: Zhang FF
| 1. | Kaplan GG, Jess T. The Changing Landscape of Inflammatory Bowel Disease: East Meets West. Gastroenterology. 2016;150:24-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 2. | de Mattos BR, Garcia MP, Nogueira JB, Paiatto LN, Albuquerque CG, Souza CL, Fernandes LG, Tamashiro WM, Simioni PU. Inflammatory Bowel Disease: An Overview of Immune Mechanisms and Biological Treatments. Mediators Inflamm. 2015;2015:493012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 151] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 3. | Geremia A, Biancheri P, Allan P, Corazza GR, Di Sabatino A. Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 706] [Article Influence: 58.8] [Reference Citation Analysis (1)] |
| 4. | Elkord E. Thymus-Derived, Peripherally Derived, and in vitro-Induced T Regulatory Cells. Front Immunol. 2014;5:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Cătană CS, Berindan Neagoe I, Cozma V, Magdaş C, Tăbăran F, Dumitraşcu DL. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2015;21:5823-5830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 141] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 6. | Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8:523-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2593] [Cited by in RCA: 2470] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 7. | Takahashi T, Sakaguchi S. The role of regulatory T cells in controlling immunologic self-tolerance. Int Rev Cytol. 2003;225:1-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+)CD25(+) T cells. J Autoimmun. 2001;16:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, Parizot C, Taflin C, Heike T, Valeyre D. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1588] [Cited by in RCA: 1811] [Article Influence: 113.2] [Reference Citation Analysis (0)] |
| 10. | Newcomb DC, Cephus JY, Boswell MG, Fahrenholz JM, Langley EW, Feldman AS, Zhou W, Dulek DE, Goleniewska K, Woodward KB. Estrogen and progesterone decrease let-7f microRNA expression and increase IL-23/IL-23 receptor signaling and IL-17A production in patients with severe asthma. J Allergy Clin Immunol. 2015;136:1025-1034.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3529] [Cited by in RCA: 3525] [Article Influence: 220.3] [Reference Citation Analysis (0)] |
| 12. | Gálvez J. Role of Th17 Cells in the Pathogenesis of Human IBD. ISRN Inflamm. 2014;2014:928461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 13. | Furuzawa-Carballeda J, Ortíz-Ávalos M, Lima G, Jurado-Santa Cruz F, Llorente L. Subcutaneous administration of polymerized type I collagen downregulates interleukin (IL)-17A, IL-22 and transforming growth factor-β1 expression, and increases Foxp3-expressing cells in localized scleroderma. Clin Exp Dermatol. 2012;37:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG, Beck PL, Iacucci M, Fort Gasia M, Barkema HW. Increased prevalence of circulating novel IL-17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis. 2013;19:2522-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 15. | Liu X, Gao N, Li M, Xu D, Hou Y, Wang Q, Zhang G, Sun Q, Zhang H, Zeng X. Elevated levels of CD4(+)CD25(+)FoxP3(+) T cells in systemic sclerosis patients contribute to the secretion of IL-17 and immunosuppression dysfunction. PLoS One. 2013;8:e64531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1256] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 17. | Martin JC, Bériou G, Josien R. Dextran Sulfate Sodium (DSS)-Induced Acute Colitis in the Rat. Methods Mol Biol. 2016;1371:197-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 18. | Rêgo MJ, da Silva RR, Pereira MC, da Silva Araújo A, Pitta Ida R, Falcão DA, Bezerra MA, Pitta MG. Evaluation of CD4(+)CD25(+)FoxP3(+) T cell populations, IL-10 production, and their correlation with clinical and biochemical parameters in sickle cell anemia patients with leg ulcers. Cytokine. 2015;75:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Guerin LR, Prins JR, Robertson SA. Regulatory T-cells and immune tolerance in pregnancy: a new target for infertility treatment? Hum Reprod Update. 2009;15:517-535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 321] [Cited by in RCA: 344] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 20. | Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1253] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 21. | Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2432] [Cited by in RCA: 2489] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 22. | Buckner JH. Mechanisms of impaired regulation by CD4(+) CD25(+)FOXP3(+) regulatory T cells in human autoimmune diseases. Nat Rev Immunol. 2010;10:849-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 613] [Cited by in RCA: 606] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 23. | Perše M, Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 595] [Cited by in RCA: 647] [Article Influence: 49.8] [Reference Citation Analysis (1)] |
| 24. | Xu XR, Liu CQ, Feng BS, Liu ZJ. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2014;20:3255-3264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 150] [Cited by in RCA: 177] [Article Influence: 16.1] [Reference Citation Analysis (2)] |
| 25. | Niess JH, Leithäuser F, Adler G, Reimann J. Commensal gut flora drives the expansion of proinflammatory CD4 T cells in the colonic lamina propria under normal and inflammatory conditions. J Immunol. 2008;180:559-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 26. | Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1356] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 27. | Fina D, Sarra M, Fantini MC, Rizzo A, Caruso R, Caprioli F, Stolfi C, Cardolini I, Dottori M, Boirivant M. Regulation of gut inflammation and th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 210] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 28. | Weaver CT, Elson CO, Fouser LA, Kolls JK. The Th17 pathway and inflammatory diseases of the intestines, lungs, and skin. Annu Rev Pathol. 2013;8:477-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 363] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 29. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5130] [Cited by in RCA: 5474] [Article Influence: 288.1] [Reference Citation Analysis (0)] |
| 30. | Liao J, Liu Y, Wu H, Zhao M, Tan Y, Li D, Long H, Dai Y, Yung S, Chan TM. The role of icaritin in regulating Foxp3/IL17a balance in systemic lupus erythematosus and its effects on the treatment of MRL/lpr mice. Clin Immunol. 2016;162:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Janson PC, Winerdal ME, Marits P, Thörn M, Ohlsson R, Winqvist O. FOXP3 promoter demethylation reveals the committed Treg population in humans. PLoS One. 2008;3:e1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Kononova TE, Urazova OI, Novitskiĭ VV, Churina EG, Zakharova PA. [Expression of mRNA transcription factors RORC2 and FoxP3 in lymphocytes in patients with pulmonary tuberculosis]. Tsitologiia. 2015;57:56-61. [PubMed] |
| 33. | Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5576] [Cited by in RCA: 5861] [Article Influence: 266.4] [Reference Citation Analysis (0)] |
| 34. | Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3769] [Cited by in RCA: 4135] [Article Influence: 217.6] [Reference Citation Analysis (0)] |
| 35. | Voo KS, Wang YH, Santori FR, Boggiano C, Wang YH, Arima K, Bover L, Hanabuchi S, Khalili J, Marinova E. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793-4798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 581] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 36. | Roose J, Fagarasan S. Editorial overview: lymphocyte development and activation: lymphocytes, integrators of information. Curr Opin Immunol. 2015;33:v-vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Du R, Zhao H, Yan F, Li H. IL-17+Foxp3+ T cells: an intermediate differentiation stage between Th17 cells and regulatory T cells. J Leukoc Biol. 2014;96:39-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Bovenschen HJ, van de Kerkhof PC, van Erp PE, Woestenenk R, Joosten I, Koenen HJ. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J Invest Dermatol. 2011;131:1853-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 327] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 39. | Schmidl C, Hansmann L, Andreesen R, Edinger M, Hoffmann P, Rehli M. Epigenetic reprogramming of the RORC locus during in vitro expansion is a distinctive feature of human memory but not naïve Treg. Eur J Immunol. 2011;41:1491-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 40. | Liu ZM, Wang KP, Ma J, Guo Zheng S. The role of all-trans retinoic acid in the biology of Foxp3+ regulatory T cells. Cell Mol Immunol. 2015;12:553-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 41. | Piccioni M, Chen Z, Tsun A, Li B. Regulatory T-cell differentiation and their function in immune regulation. Adv Exp Med Biol. 2014;841:67-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Cipolletta D, Cohen P, Spiegelman BM, Benoist C, Mathis D. Appearance and disappearance of the mRNA signature characteristic of Treg cells in visceral adipose tissue: age, diet, and PPARγ effects. Proc Natl Acad Sci USA. 2015;112:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 43. | Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 44. | Villalta SA, Rosenthal W, Martinez L, Kaur A, Sparwasser T, Tidball JG, Margeta M, Spencer MJ, Bluestone JA. Regulatory T cells suppress muscle inflammation and injury in muscular dystrophy. Sci Transl Med. 2014;6:258ra142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 197] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 45. | Pesenacker AM, Broady R, Levings MK. Control of tissue-localized immune responses by human regulatory T cells. Eur J Immunol. 2015;45:333-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 46. | Breous E, Somanathan S, Vandenberghe LH, Wilson JM. Hepatic regulatory T cells and Kupffer cells are crucial mediators of systemic T cell tolerance to antigens targeting murine liver. Hepatology. 2009;50:612-621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 196] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 47. | Baeyens A, Saadoun D, Billiard F, Rouers A, Grégoire S, Zaragoza B, Grinberg-Bleyer Y, Marodon G, Piaggio E, Salomon BL. Effector T cells boost regulatory T cell expansion by IL-2, TNF, OX40, and plasmacytoid dendritic cells depending on the immune context. J Immunol. 2015;194:999-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Yadav M, Louvet C, Davini D, Gardner JM, Martinez-Llordella M, Bailey-Bucktrout S, Anthony BA, Sverdrup FM, Head R, Kuster DJ. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713-1722, S1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 524] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 49. | Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 827] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 50. | Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1750] [Cited by in RCA: 1670] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 51. | Richards DM, Delacher M, Goldfarb Y, Kägebein D, Hofer AC, Abramson J, Feuerer M. Treg Cell Differentiation: From Thymus to Peripheral Tissue. Prog Mol Biol Transl Sci. 2015;136:175-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 52. | Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA. 2010;107:12204-12209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1676] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 53. | Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 656] [Article Influence: 46.9] [Reference Citation Analysis (0)] |