Published online Nov 14, 2016. doi: 10.3748/wjg.v22.i42.9251
Peer-review started: July 1, 2016
First decision: August 8, 2016
Revised: September 9, 2016
Accepted: October 19, 2016
Article in press: October 31, 2016
Published online: November 14, 2016
Processing time: 134 Days and 10.7 Hours
Hepatic encephalopathy (HE) is a neuropsychiatric disorder that commonly complicates the course of patients with liver disease. Despite the fact that the syndrome was probably first recognized hundreds of years ago, the exact pathogenesis still remains unclear. Minimal hepatic encephalopathy (MHE) is the earliest form of HE and is estimated to affect more that 75% of patients with liver cirrhosis. It is characterized by cognitive impairment predominantly attention, reactiveness and integrative function with very subtle clinical manifestations. The development of MHE is associated with worsen in driving skills, daily activities and the increase of overall mortality. Skeletal muscle has the ability to shift from ammonia producer to ammonia detoxifying organ. Due to its large size, becomes the main ammonia detoxifying organ in case of chronic liver failure and muscular glutamine-synthase becomes important due to the failing liver and brain metabolic activity. Gut is the major glutamine consumer and ammonia producer organ in the body. Hepatocellular dysfunction due to liver disease, results in an impaired clearance of ammonium and in its inter-organ trafficking. Intestinal bacteria, can also represent an extra source of ammonia production and in cirrhosis, small intestinal bacterial overgrowth and symbiosis can be observed. In the study of HE, to get close to MHE is to get closer to its big bang; and from here, to travel less transited roads such as skeletal muscle and intestine, is to go even closer. The aim of this editorial is to expose this road for further and deeper work.
Core tip: This work is a contribution to the current knowledge of hepatic encephalopathy (HE) and shows new important data about aspect less studied, as ammonia effect inducing morphological ultrastructural damage in skeletal muscle and gut. These alterations were observed in a Minimal HE model without liver damage, which suggests that the damage caused by ammonia may occur before liver failure.
- Citation: Souto PA, Marcotegui AR, Orbea L, Skerl J, Perazzo JC. Hepatic encephalopathy: Ever closer to its big bang. World J Gastroenterol 2016; 22(42): 9251-9256
- URL: https://www.wjgnet.com/1007-9327/full/v22/i42/9251.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i42.9251
Hepatic encephalopathy (HE) is a neuropsychiatric complex syndrome, ranging from subtle behavioral abnormalities to deep coma and death[1]. HE is potentially reversible and emerges as the major complication of acute or chronic liver failure (ALF, CLF)[2]. RF Butterworth in his recent review[3] stressed that brain edema that accompanies ALF is the primarily cytotoxic phenomena, and moreover, this correlates with alterations in expression of genes coding for key astrocytic proteins[4].
There are clinical conditions with a greater chance of developing cerebral edema, such as deep encephalopathy, hyperacute (ALF-acetaminophen induced) liver failure, severe hyperammonemia, younger age and infection[5]. A controversial area is to establish conclusively whether the percentage of deaths could be attributable to a cerebral edema or to a multiple organ failure. Clemmensen reported in patients with ALF a correlation between arterial ammonemia and brain herniation. Therefore, hyperammonemia in ALF is a key factor for the understanding the pathophysiology of ALF[3].
On the other hand, situation changes drastically in CLF. In the first diagnostic stage, patients studied with conventional clinical assessments could be considered normal from a neurological view, but its evaluation with neuro-psychometric tests, flicker, etc., could possibly staged as a subclinical HE, or “minimal hepatic encephalopathy” (MHE)[6,7].
MHE may be persistent or intermittent (spontaneous or precipitated for different circumstances), but the data indicate that between two clinical episodes, patients do not return to their previous neurological status[8]. It becomes necessary to understand HE pathophysiology in early stages, in its big bang, to establish the mechanism that triggers HE and subsequent neurological consequences.
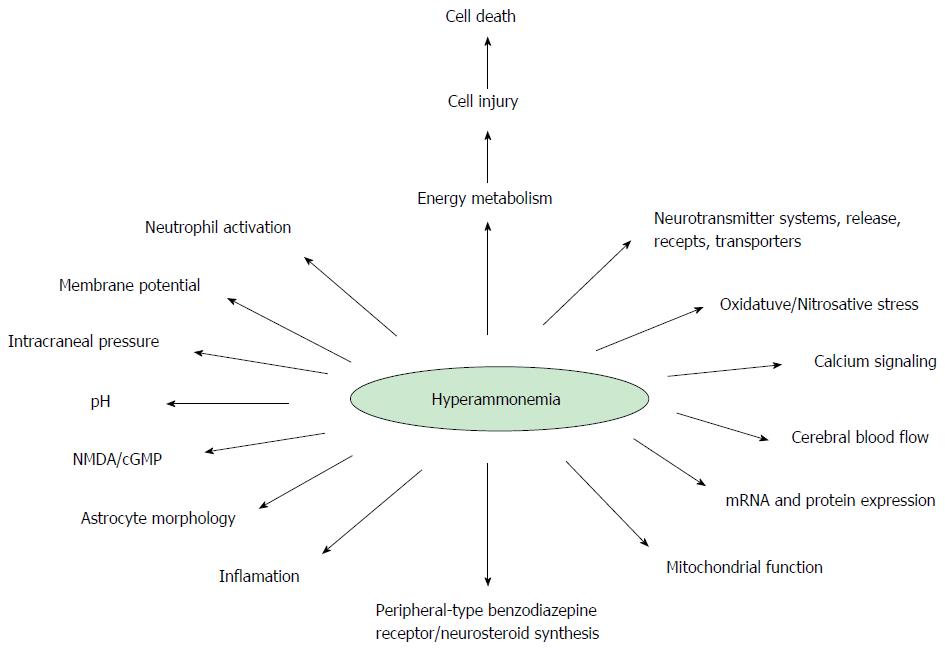
As seen in Figure 1, many paths, molecules, and biological unbalanced equations are involved[1]. The first reference of a patient with chronic liver disease, most likely associated with HE, was documented by G. Morgagni in 1765. Ammonia stands on the scene of HE from the very beginning, and is considered the main culprit. However, no one could give a judgment without risk of injustice. Thus in CLF, the defendant, who is supposed to be under trial in this editorial is ammonia[9]. Although, there are others implicated in HE pathophysiology, such as edema and manganese, it is not possible, to explain HE without the implication of hyperammonemia.
This publication is an approach to MHE, providing in brief some new data that are usually not taken into account, in an attempt to reveal the initial steps of the HE pathophysiology. Also it is suggested that research should be strongly directed to MHE, in order to seek for still unknown mechanisms.
The diagnosis of MHE is still a challenge, and some experimental models, such as the portal vein ligation (PVL), develops two basic characteristics of CLF in its early stages, mild hyperammonemia and MHE[10], thus being a useful tool for the study of MHE pathophysiology and how it finally develops HE without liver disease[11].
Despite the difficulties, it is very important the early diagnose of MHE, because the experimental models of MHE are teaching that there are significant pathological changes, which should be take into account at this stage. We will not discuss here the recognized features such as Alzheimer type II Astrocytes, an important morphological feature of HE[12]. This work will take into account some of the roads less traveled about MHE, skeletal muscle and intestine, which is the major consumer of glutamine (GLN) and the major ammonia producer[13]. Skeletal muscle becomes the main mass of tissue able to metabolize ammonia when liver failure is established and its capacity of ammonia detoxification is reduced drastically. In this way, perhaps we could reconsider that MHE is not so minimal.
Sarcopenia is a common feature of cirrhosis, and the loss of muscle mass and function contributes to its morbidity and mortality. Its prevalence in patients with cirrhosis is estimated to be 40%-70%, well correlated with HE[14,15].
The skeletal muscle damage in cirrhosis is underestimated by physicians although its prevalence is higher than other gastrointestinal complications. Perhaps the poorly understanding of its pathogenesis, the not so well established diagnostic criteria and that none of the proposed treatment options have been well explored in randomized clinical trials, helps to this clinical conduct.
Patients with chronic liver disease (CLD) concomitantly decrease the capability of detoxification of ammonia to urea, shifts ammonia metabolic pathway to the muscular system, to the astrocyte in the central nervous system (CNS) and to the kidney. In the case of liver failure, the first two tissues turn into main organs in detoxifying ammonia. Skeletal muscle tissue, due to its large size, becomes the main ammonia detoxifying organ[16]. In hypoproteic diet which is a very common procedure in treating patients with liver failure, muscular glutamine-synthase becomes an important enzyme in this metabolism, although a normal proteic diet may be metabolically more adequate and can be safely administered to the cirrhotic patient[17]. But hypoproteic diet may decrease the muscular mass and therefore the ammonia detoxifying ability, therefore could be a controversial procedure in patients with liver disease and established hyperammonemia and MHE. On the other side, recently, diet supplementation with branched chain amino acids shown to decrease MHE and to increase muscle mass[18]. This should be balanced because the astrocytic enzyme glutamate synthetase (GS) works at the limit of its possibilities to metabolize ammonia.
Sarcopenia becomes in a very useful tool and in the Model for End-Stage Liver Disease (MELD) should include this new item as a way to assess the nutritional and functional status of cirrhotic patients. Montano-Loza et al[19] used for skeletal muscle evaluation computed tomography and they conclude that sarcopenia is associated with improved prediction of mortality in patients with cirrhosis, primarily in patients with low MELD scores. In hospitalized cirrhotic patients there is a correlation between protein malnutrition and sepsis[20].
Montano-Loza et al[19] sustain that the muscle mass and malnutrition is not taken into account in MELD and Child-Pugh scores. So, there is no possibilities to reflect these important features in prognosis of mortality parameters, risks associated with low muscle mass.
So it could be regarded that sarcopenia and cirrhosis has a very related pathogenesis so that simple dietary interventions are insufficient. Efforts should focus on improved understanding of the multiple mechanisms triggered in chronic liver disease, the overlap of the different pathogenesis involved, to arrive to the development of more effective therapies. Sarcopenia is present in almost one third of patients with HCC[21], and constitutes a strong and independent risk factor for mortality. Our results highlight the importance of body composition assessment in clinical practice.
Myostatin is a potent autocrine growth inhibitor produced by myocytes that inhibits skeletal muscle growth and reduces muscle mass in cirrhosis. Qiu et al[22] showed that exposure of mouse skeletal muscle myotubes in culture to ammonium acetate caused a time and concentration-dependent increase in myostatin mRNA and protein expression. They found that hyperammonemia-activated transcription factor p65 NF-κB who binds to the myostatin promoter with transcriptional up regulation. They also found that myotube diameter was significantly greater in the NF-κB knockdown cells compared with the control cells, further supporting their proposal that NF-κB regulates myostatin expression during hyperammonemia. Their observations show that hyperammonemia induces myostatin expression in myotubes via an NF-κB-dependent pathway[16,22,23].
In our laboratory some new data was registered. PVL rats showed skeletal muscle structural/ultrastructural and functional changes (unpublished data). It has been suggested that ammonia uptake is increased in CLD and that the subsequent increase in GS capacity is a major alternative pathway for ammonia detoxification[24]. Desjardins et al[25] demonstrated in a porto-cava anastomosis model that GS activity was significantly increased as a result of a post-translational modification of the enzyme.
Our results in both, basic biochemical parameters in liver and skeletal muscle conventional microscopy observation, showed no differences when compared with the control group.
Despite these disappointing results, the fact is that the loss of skeletal muscle is nearly universal in cirrhosis and that adversely affects survival, inducing the development of other complications, and that negatively affects outcome after liver transplantation, decreasing quality of life. However, a reduction in skeletal muscle protein synthesis alone is not sufficient to account for continued reduction in muscle mass in cirrhosis, and an increase in proteolysis is necessary. On the other side cirrhosis is a state of accelerated starvation and the enhanced muscle metabolism may serve as a source of essential amino acids for critical cellular function.
There is not a complete answer, but to date the loss of skeletal muscle is associated with advanced stages cirrhotic.
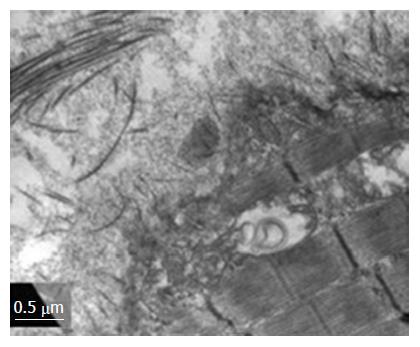
The high-resolution optical microscopy (HROM) and transmission electron microscopy in the MHE showed surprising results. The skeletal muscle Triad showed in the dark cell, extensive convergent structure resembling the streets on a map. These membrane systems increased by altering the fibrillar surface structure generating a serious focal damage (Figure 2). Immunohistochemistry (PCNA, TUNEL, GS), ROS and mitochondrial respiration measure, supports the HROM and MET findings.
These results disaggregate the fact that it may not be absolutely necessary a damaged liver, perhaps hyperammonemia alone could trigger the damage in skeletal muscle, somehow independent of liver damage (Figure 2).
Gut is the major GLN consumer and ammonia producer organ in the body. Ammonia generation derives from the consumption of GLN and glutamate as the most important oxidative fuel for enterocytes and colonocytes[26]. In Gut epithelial cells, these aminoacids have several roles, i.e., represents substrates for another amino acids synthesis[27], oxidative fuel[28], nucleotide and nucleic acid synthesis[29], glutathione production[30], amino sugars, NAD+, N-acetylglucosamine and N-acetylgalactosamine synthesis, etc[31]. GLN administration in patients with different diseases, improved gut function and reduce bacterial translocation, reestablishing normal epithelial permeability[32,33].
Hepatocellular dysfunction due to liver disease, results in an impaired clearance of ammonium and in its inter-organ trafficking[34,35]. Besides acute or chronic liver failure, specific genetic disorders are also characterized by hyperammonemia accompanied by liver and brain disorders with different degrees of severity. Intestinal bacteria, also can represent and extra source of ammonia production. The most relevant bacteria are those with urease enzyme such as enterobacteriaceae. In cirrhosis, small intestinal bacterial overgrowth (SIBO)[36] and dysbiosis[37,38] can be observed. This modification in gut microbiota is produced for various circumstances. First, gut motility is reduced, and acid gastric secretion in stomach is reduced as a consequence of gastric enteropathy, both phenomena influence SIBO[39]. Moreover, an impairment in bile acids production and secretion by the damaged liver was demonstrated[37]. The amount and the profile modification of bile acids are due a reduction in secondary fecal bile acids, a bacteriostatic compound. Another factor involve on SIBO development in cirrhosis, is the alteration in local immunological system, mediated by a low cellular and humoral components of gut immune system[39]. SIBO generates an increase in ammonia gut production, generating an active role of gut in the predisposition in HE development.
In addition of ammonia generation, luminal bacteria can generate other substances such as phenols, mercaptans, benzodiazepine-like compounds and short and medium chain fatty acids, also implicated in pathogenesis of HE[40] exerting a synergic effect on CNS alteration, by modulating the synaptic processes. For example, the neurosteroid allopregnenolone is elevated in patients with HE. This steroid can enhance the effects of GABA on its specific receptors (mainly in GABA-A), increasing it neurodepressive function[41]. On the other hand, endogenous benzodiazepines produced by intestinal bacteria, can augment the opening time of GABA-A receptor after its interaction. The levels of this benzodiazepine like compounds are also elevated in blood of HE patients, exerting a potentiation effect with neurosteroids named before[42-44].
When gut was evaluated in the PVL distal ileum showed impaired contractile response to acetylcholine and potassium chloride, and reduced expression of proteins markers of occlusive and adherents junctions, such as Zonulin 1 and B catenine, respectively. Also, a reduction in number of cells related with mucosal immune system was observed and altered L-Citrulline was recorded (data not published). All these findings suggest an active role of gut in the development of MHE in a PVL model.
This review tries to approach the early stages of the MHE, where even though there are no clinical manifestations, there is evidence of morphological ultrastructural alterations that are present even in the absence of hepatic failure. From this it can be hypothesized that it might not be necessary to have a clinically assessed liver damage to trigger skeletal muscle pathology and maybe it can be started by hyperammonemic condition by itself.
Regarding the gastrointestinal tract, an increase in gut epithelial permeability, alteration in motility and immunological local status were described, generating a predisposition for future complications. Concomitantly, an inespecific inflammatory infiltration is considered guilty of many of the gut impairments of this pathology, followed by mucosal atrophy, edema of lamina propia, fibromuscular proliferation and thickened muscularis mucosa.
In the study of HE, to get close to MHE is to get closer to its big bang; and from here, to travel less transited roads such as skeletal muscle and intestine, is to go even closer. The aim of this review is to expose these roads for further and deeper work.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Argentina
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Zarrinpar A S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Bosoi CR, Rose CF. Identifying the direct effects of ammonia on the brain. Metab Brain Dis. 2009;24:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 180] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 2. | Quero JC, Hartmann IJ, Meulstee J, Hop WC, Schalm SW. The diagnosis of subclinical hepatic encephalopathy in patients with cirrhosis using neuropsychological tests and automated electroencephalogram analysis. Hepatology. 1996;24:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Butterworth RF. Pathogenesis of hepatic encephalopathy and brain edema in acute liver failure. J Clin Exp Hepatol. 2015;5:S96-S103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Thumburu KK, Dhiman RK, Vasishta RK, Chakraborti A, Butterworth RF, Beauchesne E, Desjardins P, Goyal S, Sharma N, Duseja A. Expression of astrocytic genes coding for proteins implicated in neural excitation and brain edema is altered after acute liver failure. J Neurochem. 2014;128:617-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Chastre A, Bélanger M, Nguyen BN, Butterworth RF. Lipopolysaccharide precipitates hepatic encephalopathy and increases blood-brain barrier permeability in mice with acute liver failure. Liver Int. 2014;34:353-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 6. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1594] [Cited by in RCA: 1402] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 7. | Dharel N, Bajaj JS. Definition and nomenclature of hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S37-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 68] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 8. | Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1583] [Cited by in RCA: 1409] [Article Influence: 128.1] [Reference Citation Analysis (1)] |
| 9. | Amodio P. The liver, the brain and nitrogen metabolism. Metab Brain Dis. 2009;24:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Chojkier M, Groszmann RJ. Measurement of portal-systemic shunting in the rat by using gamma-labeled microspheres. Am J Physiol. 1981;240:G371-G375. [PubMed] |
| 11. | Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT. Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int. 2009;29:783-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 12. | Norenberg MD. A light and electron microscopic study of experimental portal-systemic (ammonia) encephalopathy. Progression and reversal of the disorder. Lab Invest. 1977;36:618-627. [PubMed] |
| 13. | Acosta GB, Fernández MA, Roselló DM, Tomaro ML, Balestrasse K, Lemberg A. Glutamine synthetase activity and glutamate uptake in hippocampus and frontal cortex in portal hypertensive rats. World J Gastroenterol. 2009;15:2893-2899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, Beaumont C, Tandon P, Esfandiari N, Sawyer MB. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 15. | Hanai T, Shiraki M, Nishimura K, Ohnishi S, Imai K, Suetsugu A, Takai K, Shimizu M, Moriwaki H. Sarcopenia impairs prognosis of patients with liver cirrhosis. Nutrition. 2015;31:193-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 288] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 16. | Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, Sawyer MB. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166-173, 173.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 609] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 17. | He Y, Hakvoort TB, Köhler SE, Vermeulen JL, de Waart DR, de Theije C, ten Have GA, van Eijk HM, Kunne C, Labruyere WT. Glutamine synthetase in muscle is required for glutamine production during fasting and extrahepatic ammonia detoxification. J Biol Chem. 2010;285:9516-9524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Córdoba J, López-Hellín J, Planas M, Sabín P, Sanpedro F, Castro F, Esteban R, Guardia J. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 260] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 19. | Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, Beaumont C, Esfandiari N, Myers RP. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 263] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 20. | Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, Ridola L, Attili AF, Venditti M. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 21. | Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 22. | Qiu J, Thapaliya S, Runkana A, Yang Y, Tsien C, Mohan ML, Narayanan A, Eghtesad B, Mozdziak PE, McDonald C. Hyperammonemia in cirrhosis induces transcriptional regulation of myostatin by an NF-κB-mediated mechanism. Proc Natl Acad Sci USA. 2013;110:18162-18167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 216] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 23. | Qiu J, Tsien C, Thapalaya S, Narayanan A, Weihl CC, Ching JK, Eghtesad B, Singh K, Fu X, Dubyak G. Hyperammonemia-mediated autophagy in skeletal muscle contributes to sarcopenia of cirrhosis. Am J Physiol Endocrinol Metab. 2012;303:E983-E993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Tallis S, Caltana LR, Souto PA, Delfante AE, Lago NR, Brusco A, Perazzo JC. Changes in CNS cells in hyperammonemic portal hypertensive rats. J Neurochem. 2014;128:431-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Desjardins P, Rao KV, Michalak A, Rose C, Butterworth RF. Effect of portacaval anastomosis on glutamine synthetase protein and gene expression in brain, liver and skeletal muscle. Metab Brain Dis. 1999;14:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Newsholme P, Lima MM, Procopio J, Pithon-Curi TC, Doi SQ, Bazotte RB, Curi R. Glutamine and glutamate as vital metabolites. Braz J Med Biol Res. 2003;36:153-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 253] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Blachier F, Boutry C, Bos C, Tomé D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr. 2009;90:814S-821S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 231] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 29. | Newsholme EA, Carrié AL. Quantitative aspects of glucose and glutamine metabolism by intestinal cells. Gut. 1994;35:S13-S17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Reeds PJ, Burrin DG, Stoll B, Jahoor F, Wykes L, Henry J, Frazer ME. Enteral glutamate is the preferential source for mucosal glutathione synthesis in fed piglets. Am J Physiol. 1997;273:E408-E415. [PubMed] |
| 31. | Reeds PJ, Burrin DG. Glutamine and the bowel. J Nutr. 2001;131:2505S-2508S; discussion 2523S-2524S. [PubMed] |
| 32. | Panigrahi P, Gewolb IH, Bamford P, Horvath K. Role of glutamine in bacterial transcytosis and epithelial cell injury. JPEN J Parenter Enteral Nutr. 1997;21:75-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Neu J, DeMarco V, Li N. Glutamine: clinical applications and mechanisms of action. Curr Opin Clin Nutr Metab Care. 2002;5:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Wright G, Noiret L, Olde Damink SW, Jalan R. Interorgan ammonia metabolism in liver failure: the basis of current and future therapies. Liver Int. 2011;31:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 35. | Olde Damink SW, Jalan R, Dejong CH. Interorgan ammonia trafficking in liver disease. Metab Brain Dis. 2009;24:169-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 36. | Steffen EK, Berg RD. Relationship between cecal population levels of indigenous bacteria and translocation to the mesenteric lymph nodes. Infect Immun. 1983;39:1252-1259. [PubMed] |
| 37. | Kakiyama G, Pandak WM, Gillevet PM, Hylemon PB, Heuman DM, Daita K, Takei H, Muto A, Nittono H, Ridlon JM. Modulation of the fecal bile acid profile by gut microbiota in cirrhosis. J Hepatol. 2013;58:949-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 620] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 38. | Rogers GB, van der Gast CJ, Bruce KD, Marsh P, Collins JE, Sutton J, Wright M. Ascitic microbiota composition is correlated with clinical severity in cirrhosis with portal hypertension. PLoS One. 2013;8:e74884. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 39. | Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int. 2013;33:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 184] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 40. | Zieve FJ, Zieve L, Doizaki WM, Gilsdorf RB. Synergism between ammonia and fatty acids in the production of coma: implications for hepatic coma. J Pharmacol Exp Ther. 1974;191:10-16. [PubMed] |
| 41. | Williams R. Review article: bacterial flora and pathogenesis in hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 42. | Norenberg MD, Itzhak Y, Bender AS. The peripheral benzodiazepine receptor and neurosteroids in hepatic encephalopathy. Adv Exp Med Biol. 1997;420:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Zeneroli ML, Iuliano E, Racagni G, Baraldi M. Metabolism and brain uptake of gamma-aminobutyric acid in galactosamine-induced hepatic encephalopathy in rats. J Neurochem. 1982;38:1219-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Baraldi M, Zeneroli ZL. Experimental hepatic encephalopathy: changes in the binding of gamma-aminobutyric acid. Science. 1982;216:427-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 107] [Article Influence: 2.5] [Reference Citation Analysis (0)] |