Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9127
Peer-review started: July 2, 2016
First decision: August 8, 2016
Revised: August 28, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: November 7, 2016
Processing time: 127 Days and 22.8 Hours
To cure typically life-threatening esophagogastric anastomosis in rats, lacking anastomosis healing and sphincter function rescue, in particular.
Because we assume esophagogastric fistulas represent a particular NO-system disability, we attempt to identify the benefits of anti-ulcer stable gastric pentadecapeptide BPC 157, which was in trials for ulcerative colitis and currently for multiple sclerosis, in rats with esophagocutaneous fistulas. Previously, BPC 157 therapies have promoted the healing of intestinal anastomosis and fistulas, and esophagitis and gastric lesions, along with rescued sphincter function. Additionally, BPC 157 particularly interacts with the NO-system. In the 4 d after esophagogastric anastomosis creation, rats received medication (/kg intraperitoneally once daily: BPC 157 (10 μg, 10 ng), L-NAME (5 mg), or L-arginine (100 mg) alone and/or combined or BPC 157 (10 μg, 10 ng) in drinking water). For rats underwent esophagogastric anastomosis, daily assessment included progressive stomach damage (sum of the longest diameters, mm), esophagitis (scored 0-5), weak anastomosis (mL H2O before leak), low pressure in esophagus at anastomosis and in the pyloric sphincter (cm H2O), progressive weight loss (g) and mortality. Immediate effect assessed blood vessels disappearance (scored 0-5) at the stomach surface immediately after anastomosis creation.
BPC 157 (all regimens) fully counteracted the perilous disease course from the very beginning (i.e., with the BPC 157 bath, blood vessels remained present at the gastric surface after anastomosis creation) and eliminated mortality. Additionally, BPC 157 treatment in combination with L-NAME nullified any effect of L-NAME that otherwise intensified the regular course. Consistently, with worsening (with L-NAME administration) and amelioration (with L-arginine), either L-arginine amelioration prevails (attenuated esophageal and gastric lesions) or they counteract each other (L-NAME + L-arginine); with the addition of BPC 157 (L-NAME + L-arginine + BPC 157), there was a marked beneficial effect. BPC 157 treatment for esophagogastric anastomosis, along with NOS-blocker L-NAME and/or NOS substrate L-arginine, demonstrated an innate NO-system disability (as observed with L-arginine effectiveness). BPC 157 distinctively affected corresponding events: worsening (obtained with L-NAME administration that was counteracted); or amelioration (L-arginine + BPC 157-rats correspond to BPC 157-rats).
Innate NO-system disability for esophagogastric anastomoses, including L-NAME-worsening, suggests that these effects could be corrected by L-arginine and almost completely eliminated by BPC 157 therapy.
Core tip: In rats underwent esophagogastric anastomosis, BPC 157 (given intraperitoneally or in drinking water) fully counteracted an otherwise serious disease course since very beginning (i.e., with BPC 157 bath blood vessels remained present at the gastric surface after anastomosis creation) and eliminated mortality. Additionally, BPC 157 treatment, along with L-NAME, nullified any effect of L-NAME that otherwise intensified the regular course. Consistently, with worsening (with L-NAME administration) and amelioration (with L-arginine), either L-arginine-amelioration prevails (i.e., esophageal and gastric lesions are attenuated) or they counteract each other (L-NAME + L-arginine), an effect which is further reversed toward a marked beneficial effect with the addition of BPC 157 (L-NAME + L-arginine + BPC 157).
- Citation: Djakovic Z, Djakovic I, Cesarec V, Madzarac G, Becejac T, Zukanovic G, Drmic D, Batelja L, Zenko Sever A, Kolenc D, Pajtak A, Knez N, Japjec M, Luetic K, Stancic-Rokotov D, Seiwerth S, Sikiric P. Esophagogastric anastomosis in rats: Improved healing by BPC 157 and L-arginine, aggravated by L-NAME. World J Gastroenterol 2016; 22(41): 9127-9140
- URL: https://www.wjgnet.com/1007-9327/full/v22/i41/9127.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i41.9127
We acknowledged curative treatment of esophagogastric anastomosis in rats with stable gastric pentadecapeptide BPC 157 (an anti-ulcer peptide stable in human gastric juice), as a novel mediator of Robert’s cytoprotection that was effective in the entire gastrointestinal tract, which was originally tested in clinical trials for ulcerative colitis and multiple sclerosis[1-7]. Additionally, BPC 157 treatment of esophagogastric anastomosis along with a NO-synthase (NOS) blocker, L-NAME, and/or NOS substrate L-arginine would evidence an innate NO-system disability, and investigate the effect on the corresponding worsening (obtained with L-NAME administration) or amelioration (due to L-arginine).
In general, in the curative treatment of esophageal cancer, the most feared complication is the highest rate of anastomotic leakage[8] compared with anastomoses involving other parts of the gastrointestinal tract[9].
Likely, BPC 157 exhibits some favorable effects for esophagogastric anastomosis healing. Together, intestinal anastomosis[10-14] and fistulas[15-20] healing, esophagitis and gastric lesion healing, alongside with rescued sphincter function[10,11,17,18,20-25] could certainly improve the possible curative peptides therapy for rat esophagogastric anastomosis. Until now, only to improve anastomosis healing, tested were keratinocyte growth factor-2 (KGF-2) (shown to be ineffective given intraperitoneally)[26] (regardless to therapeutic efficacy of a mutant of KGF-2 on trinitrobenzene sulfonic acid-induced rat model of Crohn’s disease[27]) and FGF-beta (effective given topically[28]).
Additionally, unlike other anastomoses, the esophagogastric anastomosis should not only resist leakage, it should maintain some “sphincter” function at the anastomosis site, a point that thus far been unappreciated for standard peptide growth factors[26,28]. As a result, these standard peptide growth factors[26,28] only support the particular perilous course of the esophagogastric anastomosis and show little improvement in rats[29,30]. On the other hand, these combined BPC 157 effects may be more useful for both anastomosis healing and sphincter function rescue[10-14,17,18,20-25]. As a result, the esophagogastric anastomosis healing should both resist leakage and maintain some “sphincter” function at the anastomosis site. These effects may be the maintained esophageal and stomach integrity, and they counteracted the development of both esophagitis and gastric lesions.
Another point may be the perilous effect of ischemia[31-33]. To accelerate anastomosis healing, several studies implicate the positive effect of the induced angiogenesis that follows partial devascularization of the stomach after a certain period (i.e., two-week period)[34-37]. As a very active cytoprotective agent, BPC 157[6], confronted with an injurious course, rapidly induces strong endothelium protection[38] as with standard cytoprotective agents[39], but it has a more prominent angiogenic effect[40] that may significantly contribute to healing in esophagogastric anastomosis. Finally, with BPC 157 designated as a “wound healing therapy”[1-7], these were attributed to the stimulation of the early growth response-1 (EGR1) gene and its co-repressor nerve growth factor 1-A binding protein-2 (NAB2), which affected cytokine and growth factor generation and, thereby, early extracellular matrix (collagen) and blood vessel formation[41]. As a result, a particular feedback-process for the simultaneous healing of different tissues was suggested, leading to both internal and external wound healing, anastomosis and fistulas[1-7]. Others correlated the BPC 157 beneficial effects with the activation of a cellular FAK-paxillin signaling pathway and, subsequently, demonstrated that BPC 157 dose- and time-dependently increased the expression of growth hormone receptor, Janus kinase 2, which belongs to the downstream signal pathway of growth hormone receptor and may interact with other molecular pathways[42-44].
Additionally, BPC 157, based on the beneficial activities noted[1,5,7,17,18,19,45-51], would have particular effects on the NO-system (for review[1-7]), as observed in different models and species[1,5,7,17,18,19,45-51], but it has not previously been tested in anastomosis healing. Likewise, the NO-system plays a particular role in the gastrointestinal lesion healing[1]. It has been more frequently investigated in gastric lesions[1] than in esophagitis lesions[18,52]; despite inconsistencies, L-arginine has a beneficial effect, while L-NAME has an ulcerogenic effect[1], and they have not been investigated in esophagogastric anastomosis.
For practical purposes, the stable gastric pentadecapeptide BPC 157, was given daily, intraperitoneally or orally, in drinking water, using the previous efficacious regimens[7,15-25]. In addition to these effects, the possible simultaneous healing (stable gastric pentadecapeptide BPC 157, along with both NOS-blockade, L-NAME, and NOS-substrate L-arginine application[1]) would define the esophagogastric anastomosis healing, define esophagitis and gastric defects healing, rescue “sphincter” pressure at the site of anastomosis and preserve pyloric sphincter pressure, as a new NO-system related phenomenon.
Wistar Albino male rats (200 g b.w.) were randomly assigned to the experiments (at least 10 animals per experimental group). All experiments were approved by the Local Ethics Committee. Furthermore, all experiments were performed under a blind protocol, and the effect was assessed by examiners who were blinded to the given protocol.
Pentadecapeptide BPC 157 (GEPPPGKPADDAGLV, M.W. 1419), (Diagen, Ljubljana, Slovenia) dissolved in saline, was used in all experiments. BPC 157, a peptide, is part of the sequence of human gastric juice protein BPC, and it is freely soluble in water at pH 7.0 and saline. The peptide was prepared, as described previously[15-25], with 99% high pressure liquid chromatography (HPLC) purity, expressing 1-des-Gly peptide as an impurity. L-NAME (Sigma, United States) and L-arginine (Sigma, United States) were used accordingly[1,5,7,17-19,45-51].
In deeply anaesthetized rats, an esophagogastric anastomosis (PDS 6.0 suture, Johnson & Johnson, USA) was created at the apical part of the forestomach and distal part of the cut and transferred esophagus.
BPC 157 was given perorally, in drinking water (10 μg/kg, 10 ng/kg, 0.16 μg/mL, 0.16 ng/mL, and 12 mL/rat per day) until sacrifice, or it was administered intraperitoneally (10 μg/kg and 10 ng/kg) with the first application at 30 min after surgery, once daily, and the last at 24 h before sacrifice.
Combination studies: L-NAME (5 mg/kg intraperitoneally) and/or L-arginine (100 mg/kg intraperitoneally) were given alone or together with the first application at 30 min after surgery, once daily, and the last at 24 h before sacrifice. BPC 157 (10 μg/kg and 10 ng/kg intraperitoneally) was given with L-NAME (5 mg/kg intraperitoneally) and/or L-arginine (100 mg/kg intraperitoneally). Controls simultaneously received an equal volume of saline (5.0 mL/kg ip) or water alone. The full assessment was performed at days 1, 2, 3, and 4, as follows (due to subsequent mortality).
To demonstrate the direct effect of BPC 157 administration on the blood vessel presentation immediately after the creation of esophagogastric anastomosis, a bath containing 2 μg/mL of BPC 157 or a corresponding volume of saline was applied to the ventral surface of the stomach.
A precise caliper was used to verify the final size of the stomach lesions and largest diameter of the gastric lesions (mm)[53-55]. The esophagitis scoring[20-23] was modified to a 0-5 scoring system, normal, glistening mucosa (score 0); edematous mucosa with focal hemorrhagic spots (score 1); multiple erosions with hematins attached (score 2); tiny esophagus with hemorrhagic and linear yellowish lesions (score 3); tiny esophagus with coalesced hemorrhagic and yellowish lesions (score 4); and tiny esophagus with coalesced hemorrhagic, yellowish lesions and dehiscent anastomosis (score 5), which was also photographed and further verified using the program ISSA (VAMSTEC Software Company, Zagreb, Croatia), as described previously[1-7]. The tissue was placed in 10% formalin and used for histopathological examination, and processed for further microscopic analysis[1-7].
To assess anastomosis leakage, a separate group of animals received a volume of water intragastrically to induce leakage[17].
As described previously[17,18,20-23], manometrical evaluation (cm H2O) was performed in all rats, with a water manometer connected to the drainage port of the Foley catheter, as previously described (values of 68-76 cm H2O for the lower esophageal sphincter, and 68-74 cm H2O for the pyloric sphincter, were considered normal)[17,18,20-23]. The proximal side of the esophageal incision, or distal side of the duodenal incision, was ligated to prevent regurgitation[17,18,20-23].
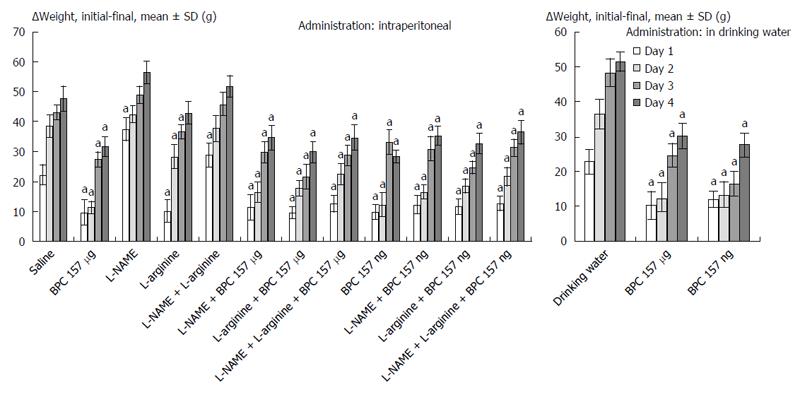
As described in prior works[13,18], animals were weighed before surgery, once daily thereafter, and before sacrifice. Weight loss (g) was presented as the Δ between the initial and final weight[13,18].
In separate group of animals, mortality was assessed daily until post-operative day 7, as described previously[13,18].
For disappearance and presentation of blood vessels at the stomach surface in rats that underwent esophagogastric anastomosis, we described blood vessel presentation at the ventral stomach surface (scored 0-5) throughout stomach distension and/or alcohol instillation into the stomach[53], as follows: presentation completely reduced, only the main tree of the left gastric artery present (score 0); thin vessels present in the fore stomach only (score 1); thin vessels present in the entire stomach (score 2); moderate vessels present in the whole stomach (score 3); prominent vessels present in the fore stomach (score 4); and prominent vessels present in the entire stomach (score 5). Continuous camera (Veho discovery VMS-004 deluxe) recordings (5 cm above the tissue; magnification 30-100 x) were used in deeply anesthetized rats for the next 15 min.
Statistical analysis was performed by a non-parametric Kruskal-Wallis ANOVA test and, later, a Mann-Whitney U-test, to compare groups. The Fisher exact probability test was used for mortality assessment. Values of P < 0.05 were considered statistically significant.
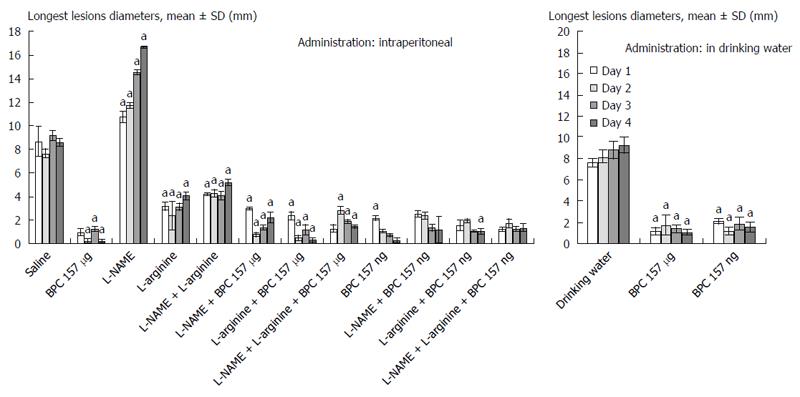
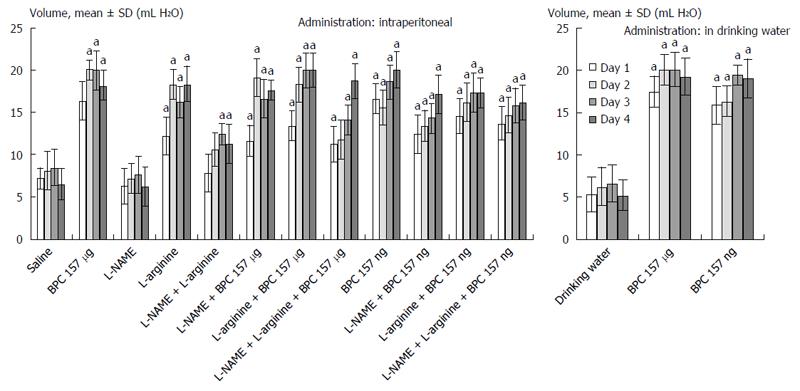
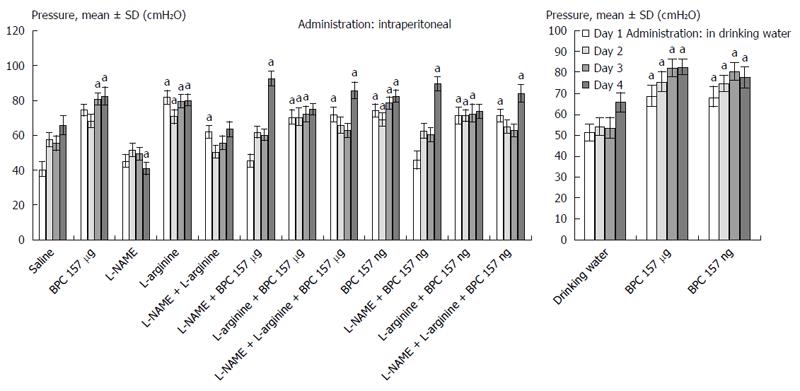
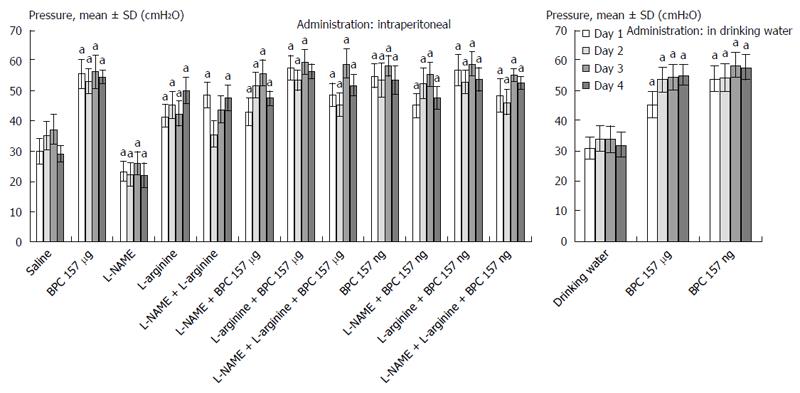
In general, since the beginning, the rats that underwent esophagogastric anastomosis without medication suffered a very severe course (as assessed until post-operative day 4) that would eventually be lethal (at post-operative day 5). These rats had relatively small gastric lesions (Figure 1) compared with severe esophagitis lesions (Table 1) and poor anastomosis (constantly small water volume that could be sustained before leakage) (Figure 2). Considering the esophagus at the site of the anastomosis (Figure 3) and pyloric sphincter (Figure 4), the pyloric pressure seems to be more affected (constantly low pyloric sphincter pressure) than the esophageal pressure at the anastomotic site. The esophageal pressure was initially considerably lower that the lower esophageal pressure in normal rats; however, on the fourth day, the esophageal pressure approached to that values. These changes, however, shortly preceded the lethal outcome on post-operative day 5. Meanwhile, these rats suffered considerable weight loss.
| Medication (/kg) given ip (once time daily) or continuously in drinking water (po) after the creation of esophagogastric anastomosis in rats | Esophagitis score (0-5) Min/Med/Max in rats that underwent esophagogastric anastomosis | |||
| 1 d | 2 d | 3 d | 4 d | |
| Min/Med/Max | Min/Med/Max | Min/Med/Max | Min/Med/Max | |
| Saline 5 mL | 4/4/4 | 4/4/4 | 4/4/4 | 4/4/4 |
| BPC 157 10 μg | 0/1/2a | 0/1/2a | 0/1/2a | 0/1/2a |
| L-NAME 5 mg | 5/5/5a | 5/5/5a | 5/5/5a | 5/5/5a |
| L-arginine 100 mg | 0/1/2a | 1/2/3a | 1/2/3a | 1/2/3a |
| L-NAME 5 mg + L-arginine 100 mg | 2/3/4a | 0/1/2a | 1/2/3a | 1/2/3a |
| L-NAME 5 mg + BPC 157 10 μg | 1/2/3a | 1/2/3a | 0/1/2a | 0/1/2a |
| L-arginine 100 mg + BPC 157 10 μg | 0/1/2a | 0/1/2a | 0/1/2a | 0/1/2a |
| L-NAME 5 mg + L-arginine 100 mg +BPC 157 10 μg | 0/1/2a | 0/1/2a | 1/2/3a | 0/1/2a |
| BPC 157 10 ng | 0/1/2a | 0/1/2a | 0/1/2a | 0/1/2a |
| L-NAME 5 mg + BPC 157 10 ng | 1/2/3a | 2/3/4a | 1/2/3a | 0/1/2a |
| L-arginine 100 mg + BPC 157 10 ng | 0/1/2a | 0/1/2 a | 0/1/2a | 0/1/2a |
| L-NAME 5 mg + L-arginine 100 mg +BPC 157 10 ng | 1/2/3a | 0/1/2a | 1/2/3a | 0/1/2a |
| Drinking water | 4/4/4 | 4/4/4 | 4/4/4 | 4/4/4 |
| 12 mL/d per rat | ||||
| BPC 157 10 μg | 0/1/2a | 0/1/2a | 0/1/2a | 0/1/2a |
| BPC 157 10 ng | 0/1/2a | 0/1/2a | 0/1/2a | 0/1/2a |
BPC 157 therapy: On the other hand, the effect of BPC 157 (both μg- and ng regimens, intraperitoneal and drinking water applications) seems to be important considering the severe and perilous course without it, after rats underwent esophagogastric anastomosis. Gastric lesions (Figure 1) and esophagitis lesions (Table 1) were attenuated, and anastomoses were strengthened (water volume before anastomosis leakage was more than two times that in the controls) (Figure 2); the pressure in the esophagus at the site of the anastomosis (Figure 3) and pyloric sphincter (Figure 4) markedly increased. Lethal outcomes were completely avoided (Fisher exact probability test vs control P < 0.05). Weight loss was attenuated (Figure 5).
L-arginine therapy: Rats that underwent esophagogastric lesions and were treated with L-arginine had an attenuated course. Gastric (Figure 1) and esophagitis lesions (Table 1) were attenuated and anastomosis was strengthened (more water volume before anastomosis leakage than in controls) (Figure 2). The pressures in the esophagus at the site of the anastomosis (Figure 3) and pyloric sphincter (Figure 4) markedly increased. Lethal outcomes were completely avoided (Fisher exact probability test vs control, P < 0.05). Weight loss was continuously attenuated before the last day (Figure 5).
L-NAME therapy: Rats that underwent esophagogastric lesions and treated with L-NAME had an aggravated course. Gastric (Figure 1) and esophagitis lesions (Table 1) constantly worsened; other disturbances were less expressed. For instance, weakened anastomosis constantly sustained a small volume water before anastomosis leakage such as in controls (Figure 2); there was small pressure in the esophagus at the site of the anastomosis (Figure 3) and in the pyloric sphincter (Figure 4); and weight loss increased on post-operative days 1 and 4 (Figure 5). However, they could not survive post-operative day 4 (Fisher exact probability test vs control P < 0.05, at least).
Combined therapies: L-NAME-induced worsening was commonly reversed with medication combinations (L-NAME + L-arginine; L-NAME + BPC 157; and L-NAME + L-arginine + BPC 157). Gastric (Figure 1) and esophagitis lesions (Table 1) were constantly attenuated. However, in particular, L-NAME + L-arginine rats do not immediately have increased anastomosis strength (they develop it later), and pressure in the esophagus at anastomosis site and in pyloric sphincter is only occasionally increased (Figures 2, 3 and 4). Weight loss initially remained increased; then, it was reversed to control values (Figure 5). In rats that additionally received BPC 157 (L-NAME + BPC 157 and L-NAME + L-arginine + BPC 157), as well as rats treated with L-arginine + BPC 157), these parameters were constantly improved. Lethal outcomes were commonly avoided (Fisher exact probability test vs control P < 0.05, at least).
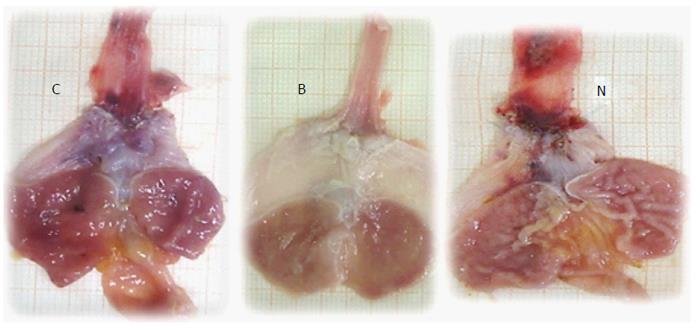
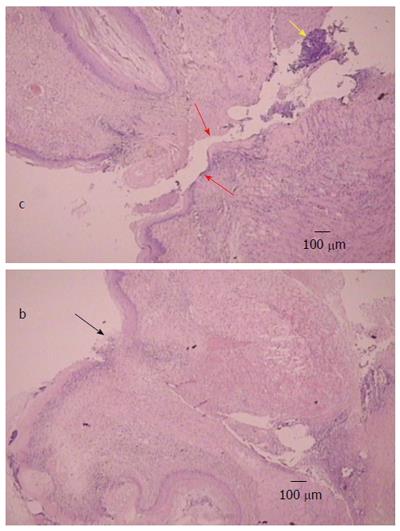
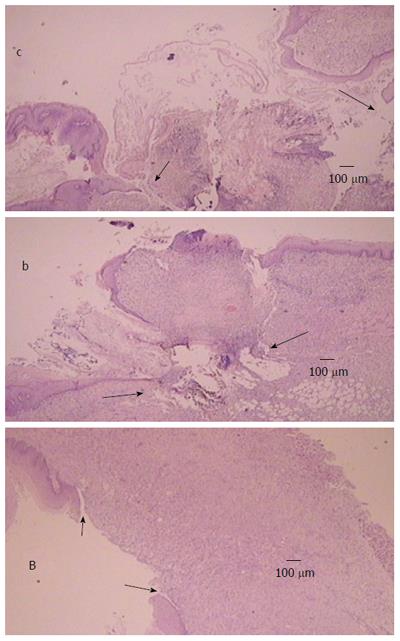
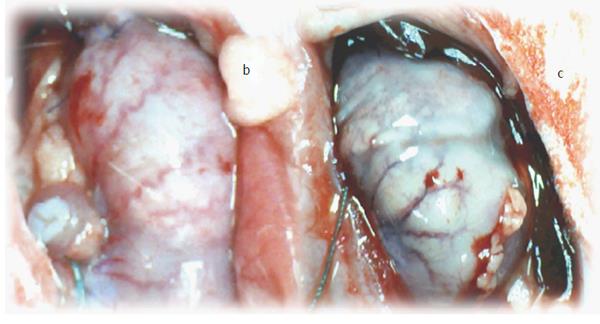
Generally, the described macroscopical healing (Figure 6) is along with microscopic presentation followed and thereby counteracted as described above (Figures 7 and 8). In the period after esophagogastric anastomosis creation, at the site of anastomosis, the control animals showed severe necrosis along the anastomosis line, including a large necrotic area of the superficial epithelium and broad band of necrotic subcutaneous tissue and muscle. Abundant, predominantly polymorphonuclear infiltration was present along the anastomosis. The inflammation extended to the adipose tissue. Grossly, regular confluent hemorrhagic and yellowish lesions appear in advanced esophagitis; microscopically, ulcerations with pronounced subepithelial and muscular edema, mononuclear infiltration, thinner epithelium and superficial corneal layers are present. Gastric mucosal lesions mostly presented with hemorrhagic lesions that were surrounded by edema of the lamina propria and submucosa with a mixed inflammatory reaction. However, some presented with extensive necrosis to all parts of the mucosa, and they had sharp edges with infiltrated granulocytes at the bases.
Very early findings: The following very early findings could likely be illustrative of all post-operative courses. In control rats that underwent esophagogastric anastomosis immediately after anastomosis creation and saline bath application at the ventral side of the stomach, blood vessels disappeared from the rat gastric surface, lasting for at least 15 min (scored Min/Med/Max 0/0/0), a period that was carefully monitored (Figure 9). Conversely, immediately after anastomosis creation and BPC 157 bath application, blood vessels did not disappear from the rat gastric surface; these vessels remained present, at least during the next 15 min of monitoring (scored Min/Med/Max 5/5/5, vs control P < 0.05) (Figure 9).
We report on the curative treatment of esophagogastric anastomosis in rats with stable gastric pentadecapeptide BPC 157[1-7]. It was highly successful against a perilous and mortal course even when it had to be markedly aggravated by L-NAME application. Namely, as observed before, rats undergoing esophagogastric anastomosis are severely affected[29,30]. They exhibited failed anastomosis healing[30,31], but they also presented with progressive esophagitis and gastric lesions, leakage, failed pressure within the anastomosis site that was markedly below values noted in the rat’s lower esophageal sphincter, a dysfunctional pyloric sphincter, weight loss, a short-life, and inescapable lethal outcomes.
As mentioned, BPC 157 treatment along with an NO-synthase (NOS) blocker, L-NAME, nullified any effect of L-NAME that would otherwise markedly intensify the regular course. Consistently, with worsening (obtained with L-NAME administration) and amelioration (with L-arginine), either L-arginine-amelioration prevails (i.e., esophageal and gastric lesions attenuated) or they counteract each other (L-NAME + L-arginine) with an effect that was further reversed toward a marked beneficial effect by the addition of BPC 157 (L-NAME + L-arginine + BPC 157). Together, these provide evidence for an innate NO-system disability (L-NAME-worsening) that could be corrected by the administration of a NOS substrate, such as L-arginine, and almost completely eliminated by BPC 157 therapy. Accordingly, in various models and species[1,5,7,17,18,20,45-51], BPC 157 counteracted the L-NAME effect better than L-arginine[1,5,7,17,18,20,45-51] as well as induced NO-release in the gastric mucosa from rat stomach tissue homogenates, even in conditions in which L-arginine is not working[50,56]. No further beneficial effect was observed when BPC 157 and L-arginine were co-administered[1,5,7,17,18,20,45-51].
In the rats that underwent esophagogastric anastomosis, the particular point of BPC 157 effectiveness involving both anastomosis healing and sphincter rescue was the realized anastomosis creation already in controls that at least partly rescued the sphincter function at the site of anastomosis, while pressure in the pyloric sphincter remains constantly low. Of note, pylorus sphincter failure was thought to reflect lower esophageal sphincter failure[17,18,20-23]. This was further additionally improved in rats that underwent BPC 157 therapy, and pressure in the pyloric sphincter is also rescued, which is an important point now reported.
Previously, we demonstrated that BPC 157 maintains sphincter function (lower esophageal, pyloric[17,18,20-23], urethral[24], and pupil[25]). Specifically, simultaneous lower esophageal and pyloric sphincter function assessment, as a hallmark of reinstated function and tissue integrity[17,18,20-23], demonstrates that when there are more lesions present, the sphincter pressure is lower[17,18,20-23]. Of note, BPC 157 therapy regularly counteracted the disability of the lower esophageal and pyloric sphincter[17,18,20-23], induced in various ways (i.e., stretching sphincters with temporal tube insertion[21-23], potassium chloride overdose application[20], bile duct ligation-induced pancreatitis[21], esophagocutaneous[18] or duodenocutaneous fistula creation[17], and lower esophageal sphincter dysfunction instantly induced pyloric sphincter failure, and vice versa[21-23]). In fistula conditions, this was shown to be a NO-system related phenomenon[7,17,18]. With respect to the outcome of esophagogastric anastomosis, an interesting anastomosis analogy could be made, providing that these surgically created fistulas are actually anastomosis between two different tissues (i.e., esophagus and skin[17]; duodenum and skin[18]; colon and skin[7]) and, thereby, sphincter function rescue could be observed along with anastomoses healing.
Of note, indicatively, anastomosis creation that better rescued the sphincter function at the site of anastomosis (as well as the pyloric sphincter function) could be also obtained in L-arginine-treated rats. Additionally, sphincter failure is proposed as a hallmark of ongoing injury[17,18,20-23] along with an injurious effect of L-NAME itself[1,5,7,17,18,20,45-51] that overrides previous considerations about NO-sphincter relationships[57] while being unrelated to injurious conditions (i.e., in dogs, ferrets and muscle strips[58-60]). In rats that underwent esophagogastric anastomosis and L-NAME therapy, the final drop of pressure within the esophagus at the site of anastomosis on day 4 occurs just prior to death. Here, moreover, we have to assume dysfunction of the nitrergic pathway; for instance, excision-immediate heavy loss of endothelium cells from the vascular wall results in a lower NO-production ability[61], which has different action for the damaged tissue integrity.
Thereby, in rats with esophagogastric anastomosis that were treated with L-NAME, the degree of sphincter failure was higher, in accordance with the worst esophageal and gastric lesions, and accelerated lethal outcomes.
Finally, it is reasonable to assume also in the esophagogastric anastomosis studies that constant vessel presentation could predict the beneficial effect of the applied agent[53]. Thereby, it is interesting to note the perilous effect of ischemia[31-33] and, conversely, angiogenesis in improving esophagogastric anastomosis healing triggered in the conditioned stomach (partial stomach devascularization)[34-37], as evidenced in a period of one week[34-37]. These observations have to be further corroborated with the noted beneficial effect of BPC 157 in rats with esophagogastric anastomosis. Namely, BPC 157 exhibits a rapid, beneficial effect (since the first day), and BPC 157 is a cytoprotective agent[1-7,38,53] that rapidly induces strong endothelium protection[38] and prominent angiogenic effects (seen when placed in the classic sponge inserted into the rat’s back or through various tissues healing[2,40,62] with VGEF expression[2,40,62]). As a result, BPC 157 obviously has an additional, more direct beneficial effect on blood vessel presentation[1-7,38,40,53,62].
The constant vessel presentation synergizes the beneficial effect of BPC 157[53], and it is worth noting that after pentadecapeptide BPC 157 instillation into the stomach following distension or alcohol instillation into the stomach, the vessel presentation remains constant, while left gastric artery blood vessels clearly disappear at the serosal site, indicative of loss in the integrity and function within a minute[53]. These findings[53] correlate with the findings noted immediately after the creation of esophagogastric anastomosis in rats, wherein left gastric artery blood vessels clearly disappear at the serosal site, unlike the constant vessel presentation in rats that underwent BPC 157 therapy. This may be an early, essential point for achieving the further full healing effect.
The esophagogastric anastomosis point provides the anastomosis strength (i.e., with various anastomosis leakage, the highest rates belong to this anastomotic leakage alone[8,9]). Since the very beginning, stable gastric pentadecapeptide BPC 157 significantly improved all parameters of anastomotic wound healing in rats with esophagogastric anastomosis, as has previously been shown with various intestinal anastomoses[10-14] (note BPC 157 also improves the blood vessel and peripheral nerve anastomoses[63,64]), and with both external and internal fistulas[7,15-19], which were originally created as the surgical anastomoses between various tissues[7,15-19]. As a result, BPC 157 especially improves the anastomotic strength. Furthermore, we noted comparable, complex functional and biomechanical improvement of various tissues[65-68], as well as their suitable healing and functional restoration (i.e., increased tensile breaking force, relative elongation of the burned skin[65,66], failure of the load of the transected tendon[67] or muscle[68], improved walking[67,68], and absent post-injury contracture[67,68]). Therefore, since these results were obtained with the same dosage regimen, increased tensile strength of the anastomosis is a direct reflection of the successful repair process[69], and an essential healing point[1-7,53] could be achieved along with the stimulation of the early growth response-1 (EGR-1) gene and its co-repressor nerve growth factor 1-A binding protein-2 (NAB2), cytokine and growth factor generation and thereby, early extracellular matrix (collagen) and blood vessel formation[41], and other molecular pathways[41-44].
These processes may be involved in a particular feedback-process for the simultaneous healing of different tissues, which can improve esophagogastric anastomosis healing and counteract all consequences of an otherwise fatal injury course.
In addition, for a new NO-system phenomenon, stable gastric pentadecapeptide BPC 157, along with NOS-blockade, L-NAME, and NOS-substrate L-arginine application[1], would favorably define esophagogastric anastomosis healing, esophagitis and gastric defect healing, as well as rescue the “sphincter” pressure at the site of anastomosis while preserving the pyloric sphincter pressure. These approaches should be used to counteract the frequently dangerous course after esophagogastric anastomosis creation.
BPC 157 treatment of esophagogastric anastomosis along with a NO-synthase (NOS) blocker, L-NAME, and/or NOS substrate L-arginine would evidence an innate NO-system disability, and investigate the effect on the corresponding worsening (obtained with L-NAME administration) or amelioration (due to L-arginine).
BPC 157 treatment of esophagogastric anastomosis along with a NO-synthase (NOS) blocker, L-NAME, and/or NOS substrate L-arginine would evidence an innate NO-system disability, and investigate the effect on the corresponding worsening (obtained with L-NAME administration) or amelioration (due to L-arginine).
The stable gastric pentadecapeptide BPC 157, was given daily, intraperitoneally or orally, in drinking water, using the previous efficacious regimens. In addition to these effects, the possible simultaneous healing (stable gastric pentadecapeptide BPC 157, along with both NOS-blockade, L-NAME, and NOS-substrate L-arginine application) would define the esophagogastric anastomosis healing, define esophagitis and gastric defects healing, rescue “sphincter” pressure at the site of anastomosis and preserve pyloric sphincter pressure, as a new NO-system related phenomenon.
A new NO-system phenomenon, stable gastric pentadecapeptide BPC 157, along with NOS-blockade, L-NAME, and NOS-substrate L-arginine application[1], would favorably define esophagogastric anastomosis healing, esophagitis and gastric defect healing, as well as rescue the “sphincter” pressure at the site of anastomosis while preserving the pyloric sphincter pressure. These approaches should be used to counteract the frequently dangerous course after esophagogastric anastomosis creation.
This manuscript presents stable gastric pentadecapeptide BCP157, along with NOS-blockade, L-NAME, and NOS-substrate L-argnine application would favorably define the esophagogastric anastomosis healing, esophagitis and gastric defects healing and rescued sphincter pressure.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Garcia-Olmo D, Fukuchi M S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20:1126-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | Seiwerth S, Brcic L, Vuletic LB, Kolenc D, Aralica G, Misic M, Zenko A, Drmic D, Rucman R, Sikiric P. BPC 157 and blood vessels. Curr Pharm Des. 2014;20:1121-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19:76-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17:1612-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Sikiric P, Seiwerth S, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D. Revised Robert’s cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16:1224-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Klicek R, Sever M, Radic B, Drmic D, Kocman I, Zoricic I, Vuksic T, Ivica M, Barisic I, Ilic S. Pentadecapeptide BPC 157, in clinical trials as a therapy for inflammatory bowel disease (PL14736), is effective in the healing of colocutaneous fistulas in rats: role of the nitric oxide-system. J Pharmacol Sci. 2008;108:7-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Kechagias A, van Rossum PS, Ruurda JP, van Hillegersberg R. Ischemic Conditioning of the Stomach in the Prevention of Esophagogastric Anastomotic Leakage After Esophagectomy. Ann Thorac Surg. 2016;101:1614-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Morse BC, Simpson JP, Jones YR, Johnson BL, Knott BM, Kotrady JA. Determination of independent predictive factors for anastomotic leak: analysis of 682 intestinal anastomoses. Am J Surg. 2013;206:950-95; discussion 950-95;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Sikiric P, Jadrijevic S, Seiwerth S, Sosa T, Deskovic S, Perovic D, Aralica G, Grabarevic Z, Rucman R, Petek M. Long-lasting cytoprotection after pentadecapeptide BPC 157, ranitidine, sucralfate or cholestyramine application in reflux oesophagitis in rats. J Physiol Paris. 1999;93:467-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Sikirić P, Seiwerth S, Desković S, Grabarević Z, Marović A, Rucman R, Petek M, Konjevoda P, Jadrijević S, Sosa T. New model of cytoprotection/adaptive cytoprotection in rats: endogenous small irritants, antiulcer agents and indomethacin. Eur J Pharmacol. 1999;364:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Klicek R, Kolenc D, Suran J, Drmic D, Brcic L, Aralica G, Sever M, Holjevac J, Radic B, Turudic T. Stable gastric pentadecapeptide BPC 157 heals cysteamine-colitis and colon-colon-anastomosis and counteracts cuprizone brain injuries and motor disability. J Physiol Pharmacol. 2013;64:597-612. [PubMed] |
| 13. | Sever M, Klicek R, Radic B, Brcic L, Zoricic I, Drmic D, Ivica M, Barisic I, Ilic S, Berkopic L. Gastric pentadecapeptide BPC 157 and short bowel syndrome in rats. Dig Dis Sci. 2009;54:2070-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Vuksic T, Zoricic I, Brcic L, Sever M, Klicek R, Radic B, Cesarec V, Berkopic L, Keller N, Blagaic AB. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL14736, Pliva, Croatia) heals ileoileal anastomosis in the rat. Surg Today. 2007;37:768-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Grgic T, Grgic D, Drmic D, Sever AZ, Petrovic I, Sucic M, Kokot A, Klicek R, Sever M, Seiwerth S. Stable gastric pentadecapeptide BPC 157 heals rat colovesical fistula. Eur J Pharmacol. 2016;780:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Baric M, Sever AZ, Vuletic LB, Rasic Z, Sever M, Drmic D, Pavelic-Turudic T, Sucic M, Vrcic H, Seiwerth S. Stable gastric pentadecapeptide BPC 157 heals rectovaginal fistula in rats. Life Sci. 2016;148:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Skorjanec S, Kokot A, Drmic D, Radic B, Sever M, Klicek R, Kolenc D, Zenko A, Lovric Bencic M, Belosic Halle Z. Duodenocutaneous fistula in rats as a model for “wound healing-therapy” in ulcer healing: the effect of pentadecapeptide BPC 157, L-nitro-arginine methyl ester and L-arginine. J Physiol Pharmacol. 2015;66:581-590. [PubMed] |
| 18. | Cesarec V, Becejac T, Misic M, Djakovic Z, Olujic D, Drmic D, Brcic L, Rokotov DS, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol. 2013;701:203-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Skorjanec S, Dolovski Z, Kocman I, Brcic L, Blagaic Boban A, Batelja L, Coric M, Sever M, Klicek R, Berkopic L. Therapy for unhealed gastrocutaneous fistulas in rats as a model for analogous healing of persistent skin wounds and persistent gastric ulcers: stable gastric pentadecapeptide BPC 157, atropine, ranitidine, and omeprazole. Dig Dis Sci. 2009;54:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Barisic I, Balenovic D, Klicek R, Radic B, Nikitovic B, Drmic D, Udovicic M, Strinic D, Bardak D, Berkopic L. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul Pept. 2013;181:50-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Petrovic I, Dobric I, Drmic D, Sever M, Klicek R, Radic B, Brcic L, Kolenc D, Zlatar M, Kunjko K. BPC 157 therapy to detriment sphincters failure-esophagitis-pancreatitis in rat and acute pancreatitis patients low sphincters pressure. J Physiol Pharmacol. 2011;62:527-534. [PubMed] |
| 22. | Dobric I, Drvis P, Petrovic I, Shejbal D, Brcic L, Blagaic AB, Batelja L, Sever M, Kokic N, Tonkic A. Prolonged esophagitis after primary dysfunction of the pyloric sphincter in the rat and therapeutic potential of the gastric pentadecapeptide BPC 157. J Pharmacol Sci. 2007;104:7-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Petrovic I, Dobric I, Drvis P, Shejbal D, Brcic L, Blagaic AB, Batelja L, Kokic N, Tonkic A, Mise S. An experimental model of prolonged esophagitis with sphincter failure in the rat and the therapeutic potential of gastric pentadecapeptide BPC 157. J Pharmacol Sci. 2006;102:269-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Jandric I, Vrcic H, Jandric Balen M, Kolenc D, Brcic L, Radic B, Drmic D, Seiwerth S, Sikiric P. Salutary effect of gastric pentadecapeptide BPC 157 in two different stress urinary incontinence models in female rats. Med Sci Monit Basic Res. 2013;19:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Kokot A, Zlatar M, Stupnisek M, Drmic D, Radic R, Vcev A, Seiwerth S, Sikiric P. NO system dependence of atropine-induced mydriasis and L-NAME- and L-arginine-induced miosis: Reversal by the pentadecapeptide BPC 157 in rats and guinea pigs. Eur J Pharmacol. 2016;771:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Cui Y, Urschel JD, Petrelli NJ. The effect of keratinocyte growth factor-2 on esophagogastric anastomotic wound healing in rats. Int J Surg Investig. 1999;1:307-309. [PubMed] |
| 27. | Wang J, Chen H, Wang Y, Cai X, Zou M, Xu T, Wang M, Wang J, Xu D. Therapeutic efficacy of a mutant of keratinocyte growth factor-2 on trinitrobenzene sulfonic acid-induced rat model of Crohn’s disease. Am J Transl Res. 2016;8:530-543. [PubMed] |
| 28. | Cunha Medeiros A, Mota HJ, Neto TA, Filho AMD, Brito Macedo LM, Melo NMC. Effect of fibroblast growth factor-beta on esophageal anastomosis healing. Rev Col Bras Cir. 2004;31:21-26. |
| 29. | Cui Y, Urschel JD, Petrelli NJ. Esophagogastric anastomoses in rats--an experimental model. J Invest Surg. 1999;12:295-298. [PubMed] |
| 30. | Cui Y, Urschel JD. Esophagogastric anastomotic wound healing in rats. Dis Esophagus. 1999;12:149-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Park SY, Kang WJ, Cho A, Chae JR, Cho YL, Kim JY, Lee JW, Chung KY. 64Cu-ATSM Hypoxia Positron Emission Tomography for Detection of Conduit Ischemia in an Experimental Rat Esophagectomy Model. PLoS One. 2015;10:e0131083. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Wang ZG, Huang YD, Cheng KL, Cai XB, Wu Z, Zhan JD. [Influence of blood supply of the esophageal and gastric stumps on anastomotic healing after esophagogastrostomy in rabbits]. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:345-36, 351. [PubMed] |
| 33. | Alfabet C, Montero EF, Paes Leme LF, Higashi VS, Sallum Fo CF, Fagundes DJ, Gomes PO. Progressive gastric perfusion in rats: role of ischemic conditioning. Microsurgery. 2003;23:513-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Lamas S, Azuara D, de Oca J, Sans M, Farran L, Alba E, Escalante E, Rafecas A. Time course of necrosis/apoptosis and neovascularization during experimental gastric conditioning. Dis Esophagus. 2008;21:370-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Mittermair C, Klaus A, Scheidl S, Maglione M, Hermann M, Margreiter R, Nguyen N, Weiss H. Functional capillary density in ischemic conditioning: implications for esophageal resection with the gastric conduit. Am J Surg. 2008;196:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Urschel JD, Antkowiak JG, Delacure MD, Takita H. Ischemic conditioning (delay phenomenon) improves esophagogastric anastomotic wound healing in the rat. J Surg Oncol. 1997;66:254-256. [PubMed] |
| 37. | Urschel JD. Ischemic conditioning of the stomach may reduce the incidence of esophagogastric anastomotic leaks complicating esophagectomy: a hypothesis. Dis Esophagus. 1997;10:217-219. [PubMed] |
| 38. | Sikiric P, Seiwerth S, Grabarevic Z, Petek M, Rucman R, Turkovic B, Rotkvic I, Jagic V, Duvnjak M, Mise S. The beneficial effect of BPC 157, a 15 amino acid peptide BPC fragment, on gastric and duodenal lesions induced by restraint stress, cysteamine and 96% ethanol in rats. A comparative study with H2 receptor antagonists, dopamine promotors and gut peptides. Life Sci. 1994;54:PL63-PL68. [PubMed] |
| 39. | Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88:228-236. [PubMed] |
| 40. | Sikiric P, Separovic J, Anic T, Buljat G, Mikus D, Seiwerth S, Grabarevic Z, Stancic-Rokotov D, Pigac B, Hanzevacki M. The effect of pentadecapeptide BPC 157, H2-blockers, omeprazole and sucralfate on new vessels and new granulation tissue formation. J Physiol Paris. 1999;93:479-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | Tkalcević VI, Cuzić S, Brajsa K, Mildner B, Bokulić A, Situm K, Perović D, Glojnarić I, Parnham MJ. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007;570:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 42. | Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985). 2011;110:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 43. | Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19:19066-19077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 44. | Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, Mu N, Gu J, Zhang W, Wang Y. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485-2499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 45. | Zemba M, Cilic AZ, Balenovic I, Cilic M, Radic B, Suran J, Drmic D, Kokot A, Stambolija V, Murselovic T. BPC 157 antagonized the general anaesthetic potency of thiopental and reduced prolongation of anaesthesia induced by L-NAME/thiopental combination. Inflammopharmacology. 2015;23:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Balenovic D, Barisic I, Prkacin I, Horvat I, Udovicic M, Uzun S, Strinic D, Pevec D, Drmic D, Radic B. Mortal furosemide-hypokalemia-disturbances in rats NO-system related. Shorten survival by L-NAME. Therapy benefit with BPC 157 more than with L-arginine. J Clin Exp Cardiolog. 2012;3:201. |
| 47. | Stupnisek M, Kokot A, Drmic D, Hrelec Patrlj M, Zenko Sever A, Kolenc D, Radic B, Suran J, Bojic D, Vcev A. Pentadecapeptide BPC 157 Reduces Bleeding and Thrombocytopenia after Amputation in Rats Treated with Heparin, Warfarin, L-NAME and L-Arginine. PLoS One. 2015;10:e0123454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 48. | Balenovic D, Bencic ML, Udovicic M, Simonji K, Hanzevacki JS, Barisic I, Kranjcevic S, Prkacin I, Coric V, Brcic L. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: a relation with NO-system. Regul Pept. 2009;156:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 49. | Boban-Blagaic A, Blagaic V, Romic Z, Jelovac N, Dodig G, Rucman R, Petek M, Turkovic B, Seiwerth S, Sikiric P. The influence of gastric pentadecapeptide BPC 157 on acute and chronic ethanol administration in mice. The effect of N(G)-nitro-L-arginine methyl ester and L-arginine. Med Sci Monit. 2006;12:BR36-BR45. [PubMed] |
| 50. | Sikirić P, Seiwerth S, Grabarević Z, Rucman R, Petek M, Jagić V, Turković B, Rotkvić I, Mise S, Zoricić I. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur J Pharmacol. 1997;332:23-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 51. | Grabarevic Z, Tisljar M, Artukovic B, Bratulic M, Dzaja P, Seiwerth S, Sikiric P, Peric J, Geres D, Kos J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J Physiol Paris. 1997;91:139-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 52. | Nagahama K, Nishio H, Yamato M, Takeuchi K. Orally administered L-arginine and glycine are highly effective against acid reflux esophagitis in rats. Med Sci Monit. 2012;18:BR9-B15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Sikiric P, Seiwerth S, Brcic L, Blagaic AB, Zoricic I, Sever M, Klicek R, Radic B, Keller N, Sipos K. Stable gastric pentadecapeptide BPC 157 in trials for inflammatory bowel disease (PL-10, PLD-116, PL 14736, Pliva, Croatia). Full and distended stomach, and vascular response. Inflammopharmacology. 2006;14:214-221. [PubMed] |
| 54. | Ilic S, Drmic D, Zarkovic K, Kolenc D, Brcic L, Radic B, Djuzel V, Blagaic AB, Romic Z, Dzidic S. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur J Pharmacol. 2011;667:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Ilic S, Drmic D, Franjic S, Kolenc D, Coric M, Brcic L, Klicek R, Radic B, Sever M, Djuzel V. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011;88:535-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 56. | Turkovic B, Sikiric P, Seiwerth S, Mise S, Anic T, Petek M, Rucman R. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology. 2004;126:287. |
| 57. | Sidhu AS, Triadafilopoulos G. Neuro-regulation of lower esophageal sphincter function as treatment for gastroesophageal reflux disease. World J Gastroenterol. 2008;14:985-990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 58. | Sanmiguel CP, Hagiike M, Mintchev MP, Cruz RD, Phillips EH, Cunneen SA, Conklin JL, Soffer EE. Effect of electrical stimulation of the LES on LES pressure in a canine model. Am J Physiol Gastrointest Liver Physiol. 2008;295:G389-G394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Niedringhaus M, Jackson PG, Evans SR, Verbalis JG, Gillis RA, Sahibzada N. Dorsal motor nucleus of the vagus: a site for evoking simultaneous changes in crural diaphragm activity, lower esophageal sphincter pressure, and fundus tone. Am J Physiol Regul Integr Comp Physiol. 2008;294:R121-R131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Braverman AS, Vegesna AK, Miller LS, Barbe MF, Tiwana M, Hussain K, Ruggieri MR. Pharmacologic specificity of nicotinic receptor-mediated relaxation of muscarinic receptor precontracted human gastric clasp and sling muscle fibers within the gastroesophageal junction. J Pharmacol Exp Ther. 2011;338:37-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Berra-Romani R, Avelino-Cruz JE, Raqeeb A, Della Corte A, Cinelli M, Montagnani S, Guerra G, Moccia F, Tanzi F. Ca2 -dependent nitric oxide release in the injured endothelium of excised rat aorta: a promising mechanism applying in vascular prosthetic devices in aging patients. BMC Surg. 2013;13 Suppl 2:S40. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Brcic L, Brcic I, Staresinic M, Novinscak T, Sikiric P, Seiwerth S. Modulatory effect of gastric pentadecapeptide BPC 157 on angiogenesis in muscle and tendon healing. J Physiol Pharmacol. 2009;60 Suppl 7:191-196. [PubMed] |
| 63. | Hrelec M, Klicek R, Brcic L, Brcic I, Cvjetko I, Seiwerth S, Sikiric P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J Physiol Pharmacol. 2009;60 Suppl 7:161-165. [PubMed] |
| 64. | Gjurasin M, Miklic P, Zupancic B, Perovic D, Zarkovic K, Brcic L, Kolenc D, Radic B, Seiwerth S, Sikiric P. Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Regul Pept. 2010;160:33-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | Sikiric P, Seiwerth S, Mise S, Staresinic M, Bedekovic V, Zarkovic N, Borovic S, Gjurasin M, Boban-Blagaic A, Batelja L. Corticosteroid-impairment of healing and gastric pentadecapeptide BPC-157 creams in burned mice. Burns. 2003;29:323-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Mikus D, Sikiric P, Seiwerth S, Petricevic A, Aralica G, Druzijancic N, Rucman R, Petek M, Pigac B, Perovic D. Pentadecapeptide BPC 157 cream improves burn-wound healing and attenuates burn-gastric lesions in mice. Burns. 2001;27:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 67. | Staresinic M, Sebecic B, Patrlj L, Jadrijevic S, Suknaic S, Perovic D, Aralica G, Zarkovic N, Borovic S, Srdjak M. Gastric pentadecapeptide BPC 157 accelerates healing of transected rat Achilles tendon and in vitro stimulates tendocytes growth. J Orthop Res. 2003;21:976-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Staresinic M, Petrovic I, Novinscak T, Jukic I, Pevec D, Suknaic S, Kokic N, Batelja L, Brcic L, Boban-Blagaic A. Effective therapy of transected quadriceps muscle in rat: Gastric pentadecapeptide BPC 157. J Orthop Res. 2006;24:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Ilhan YS, Bulbuller N, Kirkil C, Ozercan R, Seckin D. The effect of an angiotensin converting enzyme inhibitor on intestinal wound healing. J Surg Res. 2005;128:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |