Published online Nov 7, 2016. doi: 10.3748/wjg.v22.i41.9039
Peer-review started: July 21, 2016
First decision: August 29, 2016
Revised: August 31, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: November 7, 2016
Processing time: 111 Days and 13.9 Hours
Nonalcoholic fatty liver disease (NAFLD) is the commonest chronic liver disease and its prevalence is increasing driven by the pandemic of obesity and type 2 diabetes mellitus. NAFLD can progress to cirrhosis and is associated with increased risk for cardiovascular disease and hepatocellular cancer. Diet and exercise are limited by suboptimal long-term adherence in patients with NAFLD. On the other hand, current pharmacological treatment of NAFLD has limited efficacy and unfavorable safety profile. In this context, obeticholic acid (OCA), a selective agonist of the farnesoid X receptors, might represent a useful option in these patients. Preclinical studies suggest that OCA improves hepatic steatosis, inflammation and fibrosis. A proof-of-concept study and the randomized, placebo-controlled Farnesoid X Receptor Ligand Obeticholic Acid in non-alcoholic steatohepatitis Treatment (FLINT) trial also showed improvements in liver histology in patients with NAFLD who received OCA. Weight loss and reduction in blood pressure were also observed. However, the effects of OCA on insulin resistance are conflicting and the lipid profile is adversely affected by this agent. In addition, pruritus is frequently observed during treatment with OCA and might lead to treatment discontinuation. However, given the limitations of existing treatments for NAFLD, OCA might represent a useful therapeutic option in selected patients with NAFLD.
Core tip: Nonalcoholic fatty liver disease (NAFLD) is the commonest chronic liver disease in Western countries, can progress to cirrhosis and is associated with increased all-cause and cardiovascular disease mortality risk. Current pharmacological treatment of NAFLD has limited efficacy and therefore, there is a pressing need to develop more effective and safe agents for this common and life-threatening disease. Obeticholic acid (OCA), a selective agonist of the farnesoid X receptors, might be a useful agent in the management of NAFLD. In the Farnesoid X Receptor Ligand Obeticholic Acid in non-alcoholic steatohepatitis (NASH) Treatment (FLINT) trial in patients with NASH, OCA administration was associated with improvements in liver histology, while weight loss and reduction in blood pressure were also observed. Although its adverse effects on the lipid profile and insulin sensitivity are worrisome, given the increased cardiovascular risk of this population, OCA might be considered in selected patients with NAFLD/NASH, particularly in those with adequately controlled glucose and lipid levels.
- Citation: Makri E, Cholongitas E, Tziomalos K. Emerging role of obeticholic acid in the management of nonalcoholic fatty liver disease. World J Gastroenterol 2016; 22(41): 9039-9043
- URL: https://www.wjgnet.com/1007-9327/full/v22/i41/9039.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i41.9039
Nonalcoholic fatty liver disease (NAFLD) is defined as hepatic steatosis in the absence of secondary hepatic fat accumulation such as significant alcohol consumption, use of steatogenic medication or hereditary disorders. NAFLD is asymptomatic in most patients but can progress to cirrhosis and hepatocellular carcinoma. The prevalence of NAFLD is steadily increasing and is currently 20%-30% in Western countries and 5%-18% in Asia[1-3]. NAFLD is the commonest cause of elevated liver enzymes and is even more prevalent in patients with metabolic diseases such as obesity and type 2 diabetes mellitus (T2DM)[4]. Consequently, its prevalence is expected to increase in the near future as an aftermath of the increasing adoption of a sedentary lifestyle and an unhealthy diet[3,4].
Patients with NAFLD have higher all-cause mortality risk than general population[5]. Moreover, cardiovascular disease represents the leading cause of death in these patients, whereas liver-related mortality is less frequent[5,6]. Lifestyle modifications, including diet and exercise, are imperative for achieving weight loss and reducing insulin resistance and hepatic steatosis /inflammation in patients with NAFLD[7,8]. Despite the short-term effectiveness of such measures, adherence to lifestyle changes wanes with time underlining the need for pharmacological therapy[7,9]. The therapeutic interventions that are used for the treatment of NAFLD aim at the underpinning pathophysiologic mechanisms of the disease but are hampered by suboptimal efficacy and safety[1,10]. Therefore, there is a pressing need to develop more effective and safe agents for this common and life-threatening disease.
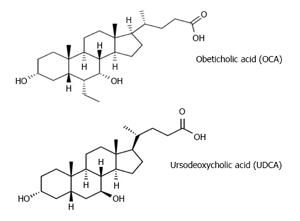
Farnesoid X receptors (FXRs) represent an attractive target for the management of NAFLD. FXRs are nuclear receptors that are abundantly expressed and play several roles including regulation of bilirubin, carbohydrate and lipid metabolism and modulation of liver growth[11-14]. Activation of FXRs ameliorates hyperlipidemia, glucose intolerance and insulin resistance, protects against cholestasis-induced liver injury and induces hepatocyte regeneration[11-14]. Bile acids are the natural ligands of the FXRs. Obeticholic acid (OCA, 6α-ethyl-chenodeoxycholic acid) is a semi-synthetic bile acid analogue of chenodeoxycholic acid (CDCA) with a 100-fold higher affinity for FXR, compared to CDCA, and represents the first selective FXR agonist to be used in human studies[15-17]. Similarly to ursodeoxycholic acid (UDCA), OCA has anti-apoptotic action and increases bile flow and the concentrations of bile acid transport proteins, such as multidrug resistance-associated protein 2. However, UDCA is a very weak FXR agonist[13,15-17] (Figure 1).
Accumulating data suggest that OCA might be a useful agent in the management of NAFLD[18,19]. In a rabbit model of NAFLD, OCA induced weight loss and improved glucose tolerance[20]. In a rat model of NAFLD [Zucker (fa/fa) rats], in which a loss of function mutation leads to T2DM, visceral adiposity and hepatic steatosis[21,22], OCA ameliorated these consequences by reducing hepatic expression of genes involved in fatty acid synthesis, lipogenesis and gluconeogenesis[21]. In other animal models of NAFLD, OCA exerted anti-inflammatory and antifibrotic effects[23-26]. However, opposite effects have also been reported; indeed, OCA induced hepatic steatosis, ballooning and inflammation in an animal study[27]. The effects of OCA on liver carcinogenesis are also controversial. In animal study, activation of FXR inhibited hepatocarcinogenesis by regulating nuclear factor κB-mediated hepatic inflammatory reactions[28]. In contrast, in another recent report using 3 mouse models, loss of FXR was associated with liver carcinogenesis in diabetic animals[29].
Regarding human studies, OCA in patients with NAFLD was first evaluated in a double-blind, placebo-controlled, proof-of-concept study[19]: patients with NAFLD and T2DM were randomly assigned to receive 25 mg OCA (n = 20), 50 mg OCA (n = 21) or placebo (n = 23) for 6 weeks[19]. Insulin resistance was evaluated using hyperinsulinemic-euglycemic clamp[19]. Insulin sensitivity improved by 28.0% and 20.1% in patients treated with 25 and 50 mg OCA, respectively, whereas it worsened in the placebo group (a 5.5% reduction)[19]. A dose-dependent weight loss was observed in patients treated with OCA[19]. Moreover, alanine transaminase (ALT) and γ-glutamyltransferase (γGT) levels declined in both OCA groups[19]. The Enhanced Liver Fibrosis test, a non-invasive marker of hepatic fibrosis, improved in patients treated with 25 mg OCA and remained stable in patients treated with 50 mg OCA[19]. On the other hand, aspartate transaminase (AST) levels remained stable and alkaline phosphatase (ALP) levels increased in both OCA groups[19]. In addition, serum low-density lipoprotein cholesterol (LDL-C) levels increased with both OCA doses and serum high-density lipoprotein cholesterol (HDL-C) decreased in patients under 50 mg OCA[19]. Serum triglyceride levels also decreased in the 50 mg OCA group[19]. These lipid effects have also been reported in animal studies and were attributed to inhibition of conversion of cholesterol to bile acids and to reduction of intestinal cholesterol absorption[30]. Regarding OCA safety, adverse reactions were comparable in all groups[19].
More recently, the Farnesoid X Receptor Ligand Obeticholic Acid in NASH Treatment (FLINT) trial, a multicentre, double-blind, placebo-controlled clinical trial in patients with non-alcoholic steatohepatitis (NASH) but without cirrhosis, was published[31]. In this trial, 283 patients were randomized to receive OCA 25 mg daily or placebo for 72 wk[31]. The primary outcome was a decrease in NAFLD activity score by at least 2 points without deterioration of fibrosis. Fifty of 110 patients in the OCA group (45%) met the primary endpoint at 72 weeks compared with 23 of 109 patients in the placebo group (21%; P = 0.0002)[31]. These results did not change after pre-specified sensitivity analyses with adjustment for confounders, including weight loss[31]. Moreover, 35% of patients treated with OCA had reduction in fibrosis, compared with 19% of patients treated with placebo (P = 0.04)[31]. However, the rates of resolution of NASH did not differ between the two groups (22% vs 13%, respectively, P = 0.08)[31]. Treatment with OCA resulted in a reduction in AST, ALT and γGT levels but increased ALP levels[31]. OCA induced weight loss and lowered systolic blood pressure but increased glucose levels and insulin resistance[31]. Its contrasting effects on insulin resistance in the FLINT trial and in the earlier proof-of-concept study[19] might be due to differences in the study population (only patients with T2DM in the latter study[19], patients with and without T2DM in FLINT trial[31]). Notable, insulin resistance was evaluated using homeostasis model in the FLINT trial, which is less accurate than hyperinsulinemic-euglycemic clamp[31]. Regarding the effects on lipid profile, OCA increased serum LDL-C and reduced HDL-C levels whereas TG levels did not change at 72 wk[31]. A study in healthy subjects also reported that OCA treatment for 14-20 d increased LDL-C and decreased HDL-C levels regardless of dose (5, 10 or 25 mg daily)[32]. After treatment discontinuation, differences in liver function tests, lipid profile and insulin resistance between groups were no longer apparent[31]. Finally, side effects were non-severe and occurred at similar rates, but a higher frequency of pruritus was observed in OCA group, compared to placebo group (23% vs 6%)[31]. These high rates of pruritus have also been reported in patients using OCA for other liver diseases, including primary biliary cirrhosis[33].
In light of these findings, how does OCA fit into the management of NAFLD? According to current guidelines, pharmacological treatment is recommended only in non-diabetic patients with biopsy-proven NASH[8]. Vitamin E is recommended as first-line agent whereas pioglitazone could also be used[8]. These recommendations are primarily based on the results of Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis (PIVENS) trial[34], which randomized 247 non-diabetic patients with NASH to receive vitamin E, pioglitazone or placebo. Both vitamin E and pioglitazone reduced hepatic steatosis and inflammation but not fibrosis[34]. In contrast, OCA improved all histological features of NAFLD (steatosis, inflammation and fibrosis)[31]. Moreover, FLINT trial included patients with T2DM (52% of the study population, n = 149) and OCA was equally effective in both patients with and without T2DM[31]. On the other hand, vitamin E has no substantial metabolic effects whereas pioglitazone reduces insulin resistance and improves the lipid profile but induces weight gain[34]. OCA appears to reduce body weight but might aggravate insulin resistance and dyslipidemia[32]. However, adverse lipid effects of OCA can be mitigated using statins, which are frequently required for the management of dyslipidemia in patients with NAFLD and appear to be safe and to reduce cardiovascular morbidity in this population[8,35]. Finally, long-term studies in other populations suggest an increased risk for all-cause mortality in patients treated with vitamin E[36] and an increased risk for edema, heart failure and bone fractures in patients treated with pioglitazone[37,38]. On the other hand, the long-term safety of treatment with OCA is unknown.
OCA appears to represent a promising treatment for patients with NAFLD, since it improves fibrosis, induces weight loss and appears to be effective in patients with T2DM. On the other hand, the adverse effects on the lipid profile and insulin sensitivity are worrisome, given the increased cardiovascular risk of this population. Therefore, and given the suboptimal efficacy and safety of other pharmacotherapies in NAFLD, lifestyle changes should be recommended as first-line management in these patients whereas OCA might be considered in selected patients, particularly those with T2DM and adequately controlled glucose and lipid levels.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Balaban YH, Ikura Y, Park YM S- Editor: Yu J L- Editor: A E- Editor: Wang CH
| 1. | Dajani A, AbuHammour A. Treatment of nonalcoholic fatty liver disease: Where do we stand? an overview. Saudi J Gastroenterol. 2016;22:91-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 2. | Masarone M, Federico A, Abenavoli L, Loguercio C, Persico M. Non alcoholic fatty liver: epidemiology and natural history. Rev Recent Clin Trials. 2014;9:126-133. [PubMed] |
| 3. | Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol. 2011;26 Suppl 1:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 4. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2405] [Cited by in RCA: 2290] [Article Influence: 163.6] [Reference Citation Analysis (0)] |
| 5. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 561] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 6. | Yatsuji S, Hashimoto E, Tobari M, Taniai M, Tokushige K, Shiratori K. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 239] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 7. | Bellentani S, Dalle Grave R, Suppini A, Marchesini G. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology. 2008;47:746-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 176] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2611] [Article Influence: 200.8] [Reference Citation Analysis (1)] |
| 9. | Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52:79-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 437] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 10. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 259] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 11. | Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38-S53. [PubMed] |
| 12. | Lee FY, Kast-Woelbern HR, Chang J, Luo G, Jones SA, Fishbein MC, Edwards PA. Alpha-crystallin is a target gene of the farnesoid X-activated receptor in human livers. J Biol Chem. 2005;280:31792-31800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, Orlandi S, Pruzanski M, Morelli A, Pellicciari R. A farnesoid x receptor-small heterodimer partner regulatory cascade modulates tissue metalloproteinase inhibitor-1 and matrix metalloprotease expression in hepatic stellate cells and promotes resolution of liver fibrosis. J Pharmacol Exp Ther. 2005;314:584-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Mencarelli A, Renga B, Distrutti E, Fiorucci S. Antiatherosclerotic effect of farnesoid X receptor. Am J Physiol Heart Circ Physiol. 2009;296:H272-H281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 152] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 15. | Makishima M, Okamoto AY, Repa JJ, Tu H, Learned RM, Luk A, Hull MV, Lustig KD, Mangelsdorf DJ, Shan B. Identification of a nuclear receptor for bile acids. Science. 1999;284:1362-1365. [PubMed] |
| 16. | Wang H, Chen J, Hollister K, Sowers LC, Forman BM. Endogenous bile acids are ligands for the nuclear receptor FXR/BAR. Mol Cell. 1999;3:543-553. [PubMed] |
| 17. | Adorini L, Pruzanski M, Shapiro D. Farnesoid X receptor targeting to treat nonalcoholic steatohepatitis. Drug Discov Today. 2012;17:988-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 202] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 18. | Ali AH, Carey EJ, Lindor KD. Recent advances in the development of farnesoid X receptor agonists. Ann Transl Med. 2015;3:5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 102] [Reference Citation Analysis (0)] |
| 19. | Mudaliar S, Henry RR, Sanyal AJ, Morrow L, Marschall HU, Kipnes M, Adorini L, Sciacca CI, Clopton P, Castelloe E. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology. 2013;145:574-582.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 656] [Cited by in RCA: 734] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 20. | Vignozzi L, Morelli A, Filippi S, Comeglio P, Chavalmane AK, Marchetta M, Toce M, Yehiely-Cohen R, Vannelli GB, Adorini L. Farnesoid X receptor activation improves erectile function in animal models of metabolic syndrome and diabetes. J Sex Med. 2011;8:57-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 347] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 22. | Varela-Rey M, Embade N, Ariz U, Lu SC, Mato JM, Martínez-Chantar ML. Non-alcoholic steatohepatitis and animal models: understanding the human disease. Int J Biochem Cell Biol. 2009;41:969-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 23. | Mencarelli A, Renga B, Migliorati M, Cipriani S, Distrutti E, Santucci L, Fiorucci S. The bile acid sensor farnesoid X receptor is a modulator of liver immunity in a rodent model of acute hepatitis. J Immunol. 2009;183:6657-6666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Wang XX, Jiang T, Shen Y, Adorini L, Pruzanski M, Gonzalez FJ, Scherzer P, Lewis L, Miyazaki-Anzai S, Levi M. The farnesoid X receptor modulates renal lipid metabolism and diet-induced renal inflammation, fibrosis, and proteinuria. Am J Physiol Renal Physiol. 2009;297:F1587-F1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 25. | Gadaleta RM, van Erpecum KJ, Oldenburg B, Willemsen EC, Renooij W, Murzilli S, Klomp LW, Siersema PD, Schipper ME, Danese S. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut. 2011;60:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 637] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 26. | Vavassori P, Mencarelli A, Renga B, Distrutti E, Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251-6261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 481] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 27. | Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 28. | Wang YD, Chen WD, Wang M, Yu D, Forman BM, Huang W. Farnesoid X receptor antagonizes nuclear factor kappaB in hepatic inflammatory response. Hepatology. 2008;48:1632-1643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 495] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 29. | Zhang Y, Ge X, Heemstra LA, Chen WD, Xu J, Smith JL, Ma H, Kasim N, Edwards PA, Novak CM. Loss of FXR protects against diet-induced obesity and accelerates liver carcinogenesis in ob/ob mice. Mol Endocrinol. 2012;26:272-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 30. | Zhang Y, Yin L, Anderson J, Ma H, Gonzalez FJ, Willson TM, Edwards PA. Identification of novel pathways that control farnesoid X receptor-mediated hypocholesterolemia. J Biol Chem. 2010;285:3035-3043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Neuschwander-Tetri BA, Loomba R, Sanyal AJ, Lavine JE, Van Natta ML, Abdelmalek MF, Chalasani N, Dasarathy S, Diehl AM, Hameed B. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:956-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1726] [Cited by in RCA: 1793] [Article Influence: 179.3] [Reference Citation Analysis (3)] |
| 32. | Pencek R, Marmon T, Roth JD, Liberman A, Hooshmand-Rad R, Young MA. Effects of obeticholic acid on lipoprotein metabolism in healthy volunteers. Diabetes Obes Metab. 2016;18:936-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 33. | Silveira MG, Lindor KD. Obeticholic acid and budesonide for the treatment of primary biliary cirrhosis. Expert Opin Pharmacother. 2014;15:365-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, Neuschwander-Tetri BA, Lavine JE, Tonascia J, Unalp A. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2642] [Cited by in RCA: 2466] [Article Influence: 164.4] [Reference Citation Analysis (2)] |
| 35. | Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 36. | Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst Rev. 2012;CD007176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 37. | Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3109] [Cited by in RCA: 2958] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 38. | Zhu ZN, Jiang YF, Ding T. Risk of fracture with thiazolidinediones: an updated meta-analysis of randomized clinical trials. Bone. 2014;68:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |