Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.9028
Peer-review started: July 13, 2016
First decision: August 8, 2016
Revised: August 22, 2016
Accepted: September 12, 2016
Article in press: September 12, 2016
Published online: October 28, 2016
Processing time: 106 Days and 22.6 Hours
Gastric fundic gland polyps (FGPs) are common non-adenomatous gastric polyps arising from normal fundic mucosa without Helicobacter pylori (H. pylori) infection. Although systemic FGPs associated with familial adenomatous polyposis (FAP) often have dysplasia, there are few reports of dysplasia occurring in sporadic FGPs, especially when detected by magnifying endoscopy with narrow band imaging (ME-NBI). We experienced two cases of adenocarcinoma occurring in sporadic FGPs, and their ME-NBI findings were very useful for differentiating FGP with cancer from non-dysplastic FGP. A 68-year-old man and a 63-year-old woman were referred to our institution for medical checkup. H. pylori was negative in both patients. Endoscopic examination revealed a small reddish polypoid lesion on the anterior wall of the upper gastric body and several FGPs. ME-NBI showed an irregular microvascular architecture composed of closed loop- or open loop-type vascular components, plus an irregular microsurface structure composed of oval-type surface components which was different from that of FGPs. FAP was denied because of the absence of colon polyps and no familial history of FAP. Pathological diagnosis was adenocarcinoma occurring in sporadic FGP.
Core tip: Gastric fundic gland polyps (FGPs) are common non-adenomatous gastric polyps arising from normal fundic mucosa without Helicobacter pylori infection. Although systemic FGPs associated with familial adenomatous polyposis often have dysplasia, there are few reports of dysplasia occurring in sporadic FGPs, especially when detected by magnifying endoscopy with narrow band imaging (ME-NBI). We experienced two cases of adenocarcinoma occurring in sporadic FGPs. ME-NBI showed an irregular microvascular architecture plus an irregular microsurface structure which was different from that of FGPs. ME-NBI findings were very useful for differentiating FGP with cancer from non-dysplastic FGP.
- Citation: Togo K, Ueo T, Yonemasu H, Honda H, Ishida T, Tanabe H, Yao K, Iwashita A, Murakami K. Two cases of adenocarcinoma occurring in sporadic fundic gland polyps observed by magnifying endoscopy with narrow band imaging. World J Gastroenterol 2016; 22(40): 9028-9034
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/9028.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.9028
Gastric fundic gland polyps (FGPs) are the most common non-adenomatous gastric polyps composed of cystically dilated fundic glands beneath a normal gastric foveolar epithelium without Helicobacter pylori (H. pylori) infection and atrophic gastritis[1]. FGPs were initially recognized as one of the gastric lesions in patients with familial adenomatous polyposis (FAP). In such systemic FGPs, foveolar dysplasia and rarely invasive gastric adenocarcinoma have been reported[2,3]. In contrast to FGPs without FAP, namely “sporadic FGPs”, it is extremely rare to encounter dysplasia, even though it is frequently encountered in sporadic FGPs in daily clinical practice[2,4-6].
Despite the strong link between H. pylori infection and gastric cancer, the new entity “adenocarcinoma of fundic gland type” has been increasingly reported as a representative neoplasia with no H. pylori infection[7]. An increased risk of FGPs has been reported with long term use of proton pump inhibitors (PPIs) therapy in H. pylori-negative patients with symptomatic gastroesophageal reflux disease[8]. Although it is rare, endoscopists must be vigilant for dysplasia from FGPs as one of representing neoplasias with no H. pylori infection. However, the endoscopic finding of FGPs with dysplasia, especially sporadic cases, has not been described. Furthermore, there has been no report of findings using magnifying endoscopy with narrow band imaging (ME-NBI).
We experienced two cases of adenocarcinoma occurring in sporadic FGP, and their ME-NBI findings were very useful for differentiating dysplastic from non-dysplastic FGP.
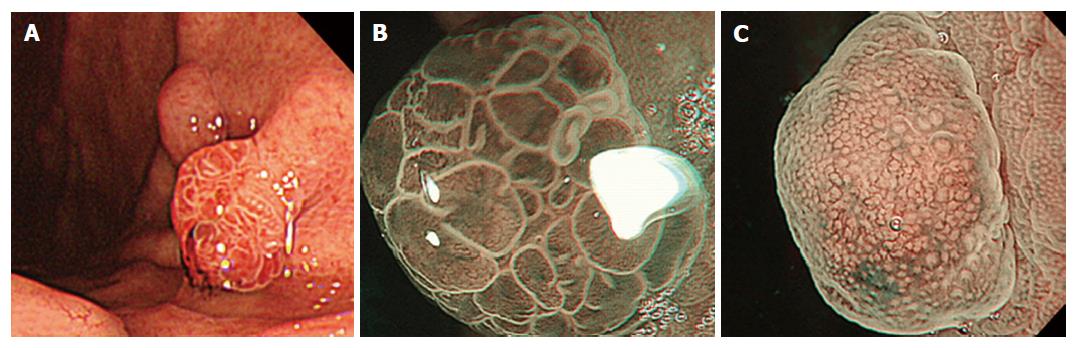
A 68-year-old man was referred to our institution for medical checkup. He had no medical history of PPIs use and no family history of FAP. H. pylori was negative by urea breath test and antibody in blood serum. Upper endoscopic examination revealed a reddish polypoid lesion of approximately 5 mm in size adjacent to an isochromatic small polyp that was thought to be FGP on the anterior wall of the upper gastric body (Figure 1A). In addition to these two polypoid lesions, there were several FGPs in non-atrophic background mucosa. ME-NBI of the reddish polypoid lesion showed an irregular microvascular (MV) architecture composed of closed loop- or open loop-type vascular components, with an irregular microsurface structure (MS) composed of oval-type surface components (vessels within epithelium pattern; Figure 1B). In contrast, ME-NBI of the adjacent isochromatic polyp showed regularly arranged round gastric pits with regularly arranged honeycomb-like microvessels (epithelium within vessels pattern; Figure 1C). Biopsy specimen from the reddish polypoid lesion was suspicious for gastric adenocarcinoma of differentiated type. From these findings, we suspected that the reddish polypoid lesion was an intramucosal adenocarcinoma of differentiated type, whereas the adjacent isochromatic polyp was a non-dysplastic FGP.
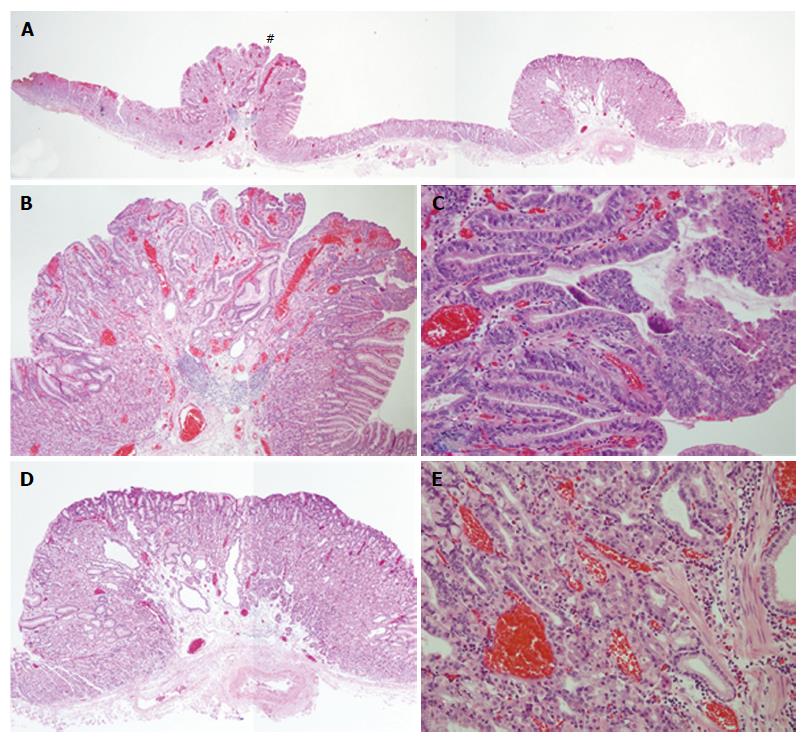
After obtaining informed consent, we resected these two polypoid lesions by endoscopic submucosal dissection (ESD). Histological examination of the en bloc specimen showed that the reddish polypoid lesion was very well-differentiated adenocarcinoma (Figure 2A and B). Irregularly branching tumor glands with atypical nuclei were found in the surface part of the lesion, and proliferation of fundic glands was observed with some cystic dilatation at the basal part of the lesion (Figure 2B and C). Immunohistochemical study showed that the tumor cells were positive for MUC5AC and negative for MUC6 pepsinogen-I, H, K-ATPase, CD10, MUC2 and p53. Ki67 was diffusely present in tumor cells. Beta-catenin was positive on the cell membrane of both neoplastic and non-neoplastic cells. The final pathological diagnosis was very well-differentiated adenocarcinoma occurring in FGP (4 mm × 4 mm in size, tub1, pT1a (M), ly0, v0, pHM0, pVM0). In contrast, histological examination of the adjacent isochromatic polyp showed proliferation of fundic glands with some cystic dilatation, and was diagnosed as FGP without dysplasia (Figure 2A, D and E). FAP was denied because of the absence of colon polyps and no familial history of FAP.
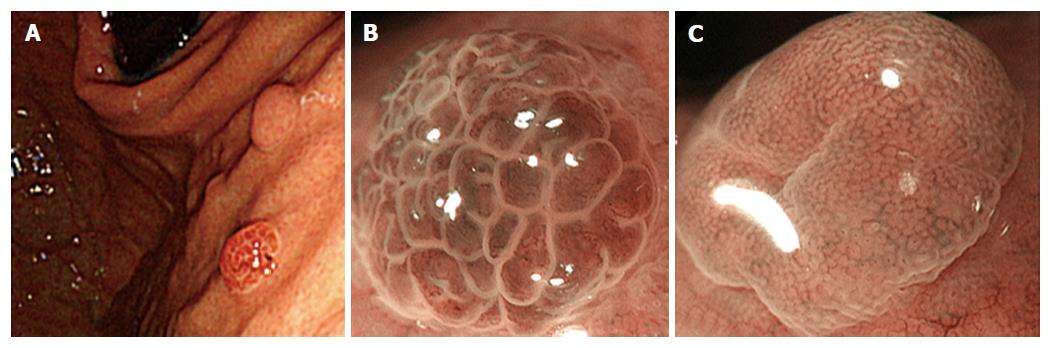
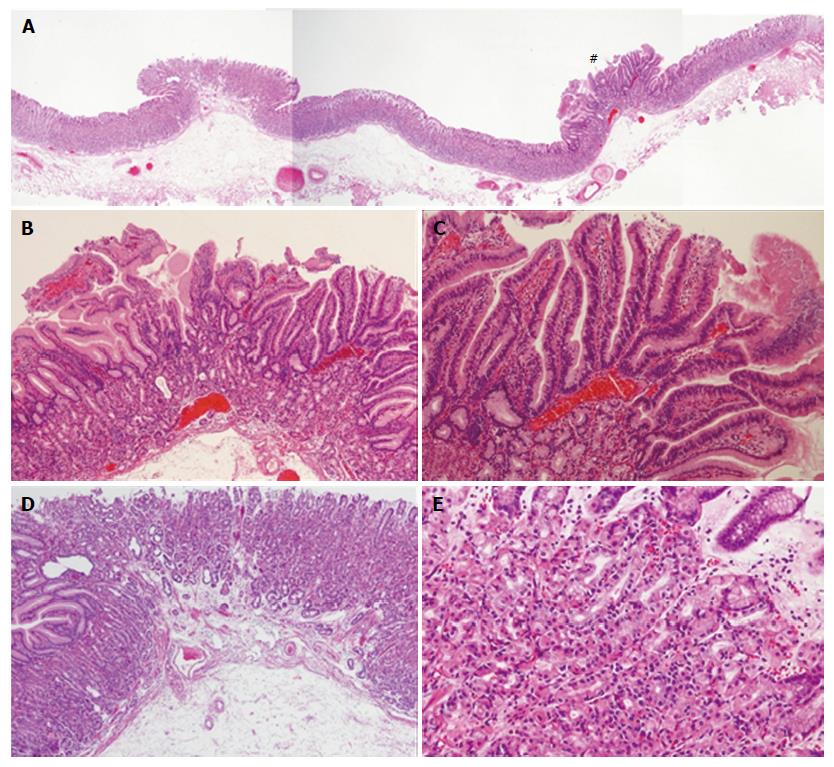
A 63-year-old woman was referred to our institution for management of a newly diagnosed gastric tumor. She had no medical history of PPIs use and no family history. H. pylori was negative by antibody in blood serum and antigen in stool. Upper endoscopic examination revealed a reddish polypoid lesion approximately 3 mm in size adjacent to an isochromatic small polyp that was thought to be FGP on the anterior wall of the upper gastric body (Figure 3A). There were many additional FGPs in non-atrophic background mucosa as well. ME-NBI of the reddish polypoid lesion and adjacent isochromatic polyp were almost the same as described for Case 1. Briefly, the reddish polypoid lesion showed an irregular MV pattern and irregular MS pattern with demarcation (vessels within epithelium pattern; Figure 3B), while the adjacent isochromatic polyp showed a regular MV pattern and regular MS pattern with demarcation (epithelium within vessels pattern; Figure 3C). Although biopsy specimen from the reddish polypoid lesion was suspicious for adenoma, we suspected that the reddish polypoid lesion was an adenocarcinoma occurring in FGP as we experienced in Case 1, whereas the adjacent isochromatic polyp was non-dysplastic FGP. After obtaining informed consent, we resected these two polypoid lesions by ESD. Histological examination of the en bloc specimen showed that the reddish polypoid lesion was very well-differentiated adenocarcinoma (Figure 4A and B). Irregularly branching tumor glands with atypical nuclei were found at the surface part of the lesion, and proliferation of fundic glands with some cystic dilatation were observed at the basal part of the lesion (Figure 4B and C). Immunohistochemical study showed that the tumor cells were positive for MUC5AC and negative for MUC6, CD10, MUC2 and p53. Ki67 was diffusely distributed in tumor cells. Beta-catenin was positive on the cell membrane of both neoplastic and non-neoplastic cells. The final pathological diagnosis was very well-differentiated adenocarcinoma occurring in FGP [3 mm × 3 mm in size, tub1, pT1a (M), ly0, v0, pHM0, pVM0]. In contrast, histological examination of the adjacent isochromatic polyp showed proliferation of fundic glands with some cystic dilatation, and was diagnosed FGP without dysplasia (Figure 4A, D and E). FAP was denied because of the absence of colon polyps and no familial history of FAP.
FGPs are the most common types of gastric polyps. Stolte et al[9] reported that FGPs were found in 47.0% of all types of gastric polyps[9]. Genta et al[10] reported that FGPs were found in up to 5.9% of adults undergoing upper endoscopic examination. Because of its high prevalence, FGPs have been thought to be an incidental finding with little clinical significance in most patients. However, FGPs have important clinical significance in that they basically occur in gastric mucosa without atrophic gastritis or H. pylori infection[10,11]. This is quite different from other gastric polyps such as hyperplastic polyps or adenomas which have a strong link to H. pylori infection. When we find multiple FGPs in the stomach, we must be careful to rule out FAP, since FGPs arise in both sporadic and systemic clinical settings associated with FAP patients. Moreover, systemic FGPs are more likely to be multiple polyps compared to the sporadic setting[12]. Although multiple FGPs were observed in the present two cases, we ruled out FAP by negative colonoscopy findings and no family history of FAP in either case.
Systemic FGPs often contain low-grade dysplasia in superficial foveolar epithelium with an incidence rate of 25%[2], but high-grade dysplasia and rarely invasive gastric adenocarcinoma have been reported[3,13]. In contrast, sporadic FGPs have been thought to be benign lesions with less malignant potential. Low-grade dysplasia was diagnosed in only 1% of sporadic FGPs (3 of 270 cases)[2]. There are only two reports showing that sporadic FGPs had high-grade dysplasia or adenocarcinoma[4,5].
Because it is extremely rare, there are few endoscopic findings of sporadic FGPs with dysplasia, and no reports of findings using ME-NBI.
Regarding the conventional endoscopic finding of tumor color in the present cases, it was only the FGPs with carcinoma that showed a reddish color, while several FGPs located at another site all showed isochromatic color. Maki et al[14] reported that reddish coloration by conventional endoscopy was a useful objective marker for differentiating cancerous lesions from superficial elevated lesions of the stomach. The appearance of reddish coloration in conventional endoscopy may therefore be useful for detecting and differentiating cancerous lesions from non-dysplastic FGPs. Furthermore, the ME-NBI findings of FGPs with cancer were completely different from those of FGPs without dysplasia. FGPs with cancer showed an irregular MV pattern plus irregular MS pattern with demarcation (vessels within epithelium pattern), whereas FGPs without dysplasia showed a regular MV pattern and regular MS pattern with demarcation (epithelium within vessels pattern). These ME-NBI findings may accurately reflect the surface structure and tumor vessels of the adenocarcinoma component of foveolar type pathologically.
Considering the fate of this well-differentiated adenocarcinoma from sporadic FGP, there is no report of advanced adenocarcinoma from sporadic FGP. Therefore, we speculate that such cancer has less malignant potential, and may not increase in size or even diminish spontaneously. Furthermore, a recent report from systematic review and meta-analysis revealed that long-term use of PPIs therapy increase the risk of FGPs[15]. Although it is unclear whether dysplasia occurs in such PPIs associated FGPs[15], PPIs have been widely used in clinical practice. It is expected that we encounter FGPs more frequently in future, and may encounter FGPs with cancer in such cases.
In summary, we reported two cases of adenocarcinoma occurring in sporadic FGP. Reddish coloration by conventional endoscopy and ME-NBI findings were very useful for differentiating FGP with cancer from non-dysplastic FGP.
A 68-year-old man and a 63-year-old woman without apparent symptoms were referred to our institution. They had no medical history of proton pump inhibitor use and no family history.
Gastric fundic gland polyps (FGPs).
Gastric hyperplastic polyps.
Helicobacter pylori was negative by antibody in blood serum.
Suspicious for gastric adenocarcinoma of differentiated type by magnifying endoscopy with narrow band imaging (ME-NBI).
The final pathological diagnosis was very well-differentiated adenocarcinoma occurring in FGP.
Resected by endoscopic submucosal dissection in both cases.
There are only two reports showing that sporadic FGPs had high-grade dysplasia or adenocarcinoma.
FGPs are initially recognized as one of the gastric lesions in patients with familial adenomatous polyposis (FAP). In contrast, sporadic FGPs which are not associated with FAP, we frequently encounter in daily clinical practice.
Reddish coloration by conventional endoscopy and ME-NBI findings were very useful for differentiating FGP with cancer from non-dysplastic FGP.
This is a well written manuscript describing the significance of endoscopic findings of sporadic FGPs with malignant potential.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kishikawa H, Viola LA S- Editor: Qi Y L- Editor: A E- Editor: Zhang FF
| 1. | Lee RG, Burt RW. The histopathology of fundic gland polyps of the stomach. Am J Clin Pathol. 1986;86:498-503. [PubMed] |
| 2. | Wu TT, Kornacki S, Rashid A, Yardley JH, Hamilton SR. Dysplasia and dysregulation of proliferation in foveolar and surface epithelia of fundic gland polyps from patients with familial adenomatous polyposis. Am J Surg Pathol. 1998;22:293-298. [PubMed] |
| 3. | Zwick A, Munir M, Ryan CK, Gian J, Burt RW, Leppert M, Spirio L, Chey WY. Gastric adenocarcinoma and dysplasia in fundic gland polyps of a patient with attenuated adenomatous polyposis coli. Gastroenterology. 1997;113:659-663. [PubMed] |
| 4. | Jalving M, Koornstra JJ, Götz JM, van der Waaij LA, de Jong S, Zwart N, Karrenbeld A, Kleibeuker JH. High-grade dysplasia in sporadic fundic gland polyps: a case report and review of the literature. Eur J Gastroenterol Hepatol. 2003;15:1229-1233. [PubMed] |
| 5. | Kawase R, Nagata S, Onoyama M, Nakayama N, Honda Y, Kuwahara K, Kimura S, Tsuji K, Ohgoshi H, Sakatani A. A case of gastric adenocarcinoma arising from a fundic gland polyp. Clin J Gastroenterol. 2009;2:279-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Tazaki S, Nozu F, Yosikawa N, Imawari M, Suzuki N, Tominaga K, Hoshino M, Suzuki S, Hayashi K. Sporadic fundic gland polyp-related adenomas occurred in non-atrophic gastric mucosa without helicobacter pylori infection. Dig Endosc. 2011;23:182-186. [PubMed] |
| 7. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 8. | Freeman HJ. Proton pump inhibitors and an emerging epidemic of gastric fundic gland polyposis. World J Gastroenterol. 2008;14:1318-1320. [PubMed] |
| 9. | Stolte M, Sticht T, Eidt S, Ebert D, Finkenzeller G. Frequency, location, and age and sex distribution of various types of gastric polyp. Endoscopy. 1994;26:659-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 136] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Genta RM, Schuler CM, Robiou CI, Lash RH. No association between gastric fundic gland polyps and gastrointestinal neoplasia in a study of over 100,000 patients. Clin Gastroenterol Hepatol. 2009;7:849-854. [PubMed] |
| 11. | Kishikawa H, Kaida S, Takarabe S, Miyoshi J, Matsukubo T, Miyauchi J, Tanaka Y, Miura S, Nishida J. Fundic gland polyps accurately predict a low risk of future gastric carcinogenesis. Clin Res Hepatol Gastroenterol. 2014;38:505-512. [PubMed] |
| 12. | Iida M, Yao T, Watanabe H, Itoh H, Iwashita A. Fundic gland polyposis in patients without familial adenomatosis coli: its incidence and clinical features. Gastroenterology. 1984;86:1437-1442. [PubMed] |
| 13. | Sekine S, Shimoda T, Nimura S, Nakanishi Y, Akasu T, Katai H, Gotoda T, Shibata T, Sakamoto M, Hirohashi S. High-grade dysplasia associated with fundic gland polyposis in a familial adenomatous polyposis patient, with special reference to APC mutation profiles. Mod Pathol. 2004;17:1421-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Maki S, Yao K, Nagahama T, Beppu T, Hisabe T, Takaki Y, Hirai F, Matsui T, Tanabe H, Iwashita A. Magnifying endoscopy with narrow-band imaging is useful in the differential diagnosis between low-grade adenoma and early cancer of superficial elevated gastric lesions. Gastric Cancer. 2013;16:140-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 15. | Tran-Duy A, Spaetgens B, Hoes AW, de Wit NJ, Stehouwer CD. Use of Proton Pump Inhibitors and Risks of Fundic Gland Polyps and Gastric Cancer: Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 147] [Article Influence: 16.3] [Reference Citation Analysis (1)] |