Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.8869
Peer-review started: June 30, 2016
First decision: August 8, 2016
Revised: August 27, 2016
Accepted: September 28, 2016
Article in press: September 28, 2016
Published online: October 28, 2016
Processing time: 119 Days and 3.9 Hours
Improved surgical techniques and greater efficacy of new anti-rejection drugs have significantly improved the survival of patients undergoing orthotopic liver transplantation (OLT). This has led to an increased incidence of metabolic disorders as well as cardiovascular and cerebrovascular diseases as causes of morbidity and mortality in OLT patients. In the last decade, several studies have examined which predisposing factors lead to increased cardiovascular risk (i.e., age, ethnicity, diabetes, NASH, atrial fibrillation, and some echocardiographic parameters) as well as which factors after OLT (i.e., weight gain, metabolic syndrome, immunosuppressive therapy, and renal failure) are linked to increased cardiovascular mortality. However, currently, there are no available data that evaluate the development of atherosclerotic damage after OLT. The awareness of high cardiovascular risk after OLT has not only lead to the definition of new but generally not accepted screening of high risk patients before transplantation, but also to the need for careful patient follow up and treatment to control metabolic and cardiovascular pathologies after transplant. Prospective studies are needed to better define the predisposing factors for recurrence and de novo occurrence of metabolic alterations responsible for cardiovascular damage after OLT. Moreover, such studies will help to identify the timing of disease progression and damage, which in turn may help to prevent morbidity and mortality for cardiovascular diseases. Our preliminary results show early occurrence of atherosclerotic damage, which is already present a few weeks following OLT, suggesting that specific, patient-tailored therapies should be started immediately post OLT.
Core tip: Due to better immunosuppressive therapies, the survival of liver transplantation recipients is improved, but an increased incidence of metabolic disorders as well as cardiovascular and cerebrovascular diseases as causes of morbidity and mortality is observed. This review analyzes risk factors [before orthotopic liver transplantation (OLT) and occurring de novo after OLT] leading to cardiovascular diseases and the current tools to identify high risk patients. We also provide preliminary data from one of the first prospective studies on the evolution of cardiovascular damage in adult patients submitted to OLT.
- Citation: Pisano G, Fracanzani AL, Caccamo L, Donato MF, Fargion S. Cardiovascular risk after orthotopic liver transplantation, a review of the literature and preliminary results of a prospective study. World J Gastroenterol 2016; 22(40): 8869-8882
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/8869.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.8869
Orthotopic liver transplantation (OLT) represents the only therapy for several end stage liver diseases of different etiology. In Europe as well as in United States nearly 6000 patients/year are submitted to liver transplantation[1,2].
According to the United Network for Organ Sharing registry, the survival rate at 1, 5 and 10 years after OLT is respectively of 85%, 70% and 50%[2]. Similarly, according to the European Liver Transplant Registry[1], the survival rate at 1, 5, 10, 15, 20 years after OLT is of 82%, 71%, 61%, 51% and 43%. However, various diseases have emerged as possible causes of post OLT complications. During the first 6 mo post-transplant, the highest risk of death was observed (11% mortality rate), while between 6 mo and 8 years post OLT, it rated at 2.5%-5%. Such mortality value increased again after 8 years to 6%-7%.
In a recent study including 798 transplanted subjects followed for a median of 10 years[3], in which 327 deaths were reported, malignity was the first cause of death followed by cardiovascular causes, infective diseases and renal failure, accounting for 22%, 11%, 9% and 6% of death, respectively.
As cardiovascular diseases emerged as a leading cause of death, several studies have been proposed in order to understand the predisposing factors leading to cardiovascular disease, as well as the post-OLT conditions facilitating cardiovascular morbidity (de novo diseases). However, scanty data are available in prospective studies.
Pre-existing cardio-metabolic pathologies and de novo occurrence, partly associated with immunosuppressive therapy, are considered the main causes of post-transplant cardiovascular complications[4].
The relevance of cardiometabolic pathologies reflects the ongoing epidemic of obesity and diabetes in the United States[5,6] and all over the Western countries. This is further documented by the marked increase in the prevalence of diabetes among candidates to OLT independently of etiology. Another metabolic disease recently recognized to have a strong role in OLT is non-alcoholic fatty liver disease (NAFLD), considered a manifestation of the metabolic syndrome (MS) or even suggested to precede MS, of which insulin resistance is the hallmark. NAFLD is the most frequent cause of liver disease in Western countries and is likely to become the most common indication for OLT over the next decade[7,8].
Non-alcoholic steatohepatitis (NASH) represents 20%-25% of all NAFLD and may potentially evolve to cirrhosis and hepatocellular carcinoma, besides carrying all cardiovascular risks typically associated to MS[9]. Interestingly, Targher et al[10] reported that NASH-affected patients are at increased risk of atrial fibrillation, which was recently identified as a severe risk factor for OLT[11]. In an analysis performed by Van Wagner et al[12], atrial fibrillation was one of the factors independently associated with major adverse cardiovascular events, especially in patients with a previous history of NASH and alcoholic cirrhosis. Indeed, a positive history of atrial fibrillation before liver transplantation was significantly more frequent in patients with major adverse cardiovascular events than in those without a previous episode of atrial fibrillation[13].
Several studies pointed out the relationship between NAFLD and cardiovascular mortality in patients submitted to OLT. Overweight/obesity, dyslipidemia, hypertension and glucose metabolism abnormalities are typical alterations detected with a high frequency in patients with NAFLD and they also define MS., notably, they have all been associated with high morbidity and mortality in patients submitted to OLT[14,15]. In addition, patients with NAFLD have been reported to be at risk for chronic kidney disease, which is another known risk factor for CVD[16,17]. The strong association between NAFLD and chronic kidney disease suggests that NAFLD could be used to stratify patients undergoing liver or kidney transplantation for a better evaluation of CV risk[18].
In line with the importance of NAFLD in the history of patients undergoing OLT, Laish et al[19] found in a retrospective analysis that pretransplant NAFLD, body mass index, diabetes, and triglycerides levels were predisposing factors for the recurrence of post-transplant MS and that post-transplant MS was associated with cardiovascular morbidity and mortality.
Alteration of glucose metabolism, one of the major complications of NAFLD/MS, is already recognized to be associated with a worse prognosis (increased risk of cirrhosis and hepatocellular carcinoma occurrence) in patients with chronic liver disease, independently from the etiology (HCV chronic hepatitis, NAFLD). In particular, its presence is also associated with a worse clinical history of transplanted patients. Several studies performed in large cohorts of transplanted patients reported that both patients and graft survival was negatively associated with pre-existing diabetes or with de novo occurrence of diabetes after OLT[20,21].
In addition, it has been reported that subjects with type 1 diabetes had a significantly lower survival than subjects with type 2 diabetes and, in turn the latters had a reduced survival compared to patients without diabetes[20].
The role of diabetes and NAFLD in the natural history of OLT is further demonstrated by the evidence that patients undergoing OLT for NASH related cirrhosis showed significantly higher risk of CVD, either in the first 30 d[22] or within 3 years post OLT, compared with patients undergoing transplantation for other chronic liver diseases such as primary biliary and sclerosing cholangitis[23]. However, overall survival did not differ. In addition, a strong association between adverse CVD events and post-transplant hypertension and diabetes was observed, with a double risk of CVD if both comorbidities coexisted. However, conflicting data were recently obtained in a large prospective study performed in United Kingdom, including almost 4000 subjects recipients of liver transplant, with diabetes having no impact on mortality at any time after OLT[24-29]. The Authors have speculated that the intensive screenings for cardiovascular complications in the diabetic liver transplant candidates of the cohort studied could explain these unexpected results. Thus, they emphasize the importance of a careful screening and selection of the candidates to OLT, of their follow up as well as of an active diabetes management after transplantation. Such procedures could lead recipients with diabetes to have outcomes comparable to those of recipients without diabetes.
Although malnutrition is commonly observed in patients with end stage liver disease, obesity is a metabolic problem that impacts negatively both on immediate and long-term survival. Most patients in the United States who underwent liver transplantations between 1988 and 1996 were overweight, reflecting the epidemic of obesity. Obesity was more common in women and in patients with cryptogenic cirrhosis, suggesting that the so called cryptogenic cirrhosis was in truth a metabolic cirrhosis. Severe obesity (BMI > 40 kg/m2) was associated with decreased 30-d, 1-year, and 2-year survival, while five-year survival was reduced even in patients with BMI > 35 kg/m2[30].
It is very well known that recurrence or de novo occurrence of NAFLD post OLT as well as after kidney transplantation may facilitate MS happening[21,31-37]. However it is difficult to define the prevalence of MS after OLT due to malnutrition, which is usually present in most cirrhotic candidates to OLT, and which ameliorates after transplantation. This is followed, in the first years after OLT, by a marked increase in body weight, which in almost 20% of cases reaches the level of obesity with an increase of BMI of 60%-70% compared to the pretransplant one. The increase in weight is almost always associated with the development of NAFLD, which in turn is accompanied by insulin resistance, the hallmark of MS and of NAFLD, present in 20%-58% of cases, glucose intolerance/diabetes, altered lipids metabolism in 50%-70%, and very often hypertension in 60%-70%[21,36,38-41]. Altogether, this also contributes to the induction of systemic and renal vasoconstrition, as well as impaired sodium excretion when treated with immunosuppressive drugs.
Interestingly, obesity in liver donors is also a predictor of liver steatosis in the liver recipients, and the presence of steatosis in the donor liver is strongly related to decreased allograft function and patient survival, with a high probability of NASH development. This has led to the decision that grafts with steatosis greater than 60% cannot be used for liver transplant[22].
New-onset diabetes mellitus, a well described complication following solid organ transplantation (liver, lung and kidney), occurs in 2% to 53% of all solid organ transplants, in 4% to 25% of renal transplant recipients and in 2.5% to 25% of liver-transplants[42-48] and is associated with an increased risk of cardiovascular morbidity and infection, as well as reduced quality of life, impaired graft function and lower patient survival[21,43,48,49]. De novo diabetes after OLT has been associated with hepatitis C virus infection, pre-existing NAFLD, increased BMI, use of tacrolimus (as opposed to ciclosporine), steroids, age, and ethnicity[43,44,50-52]. In kidney transplant recipients variables predictors of new onset diabetes were similar to those of OLT, being crucial the early detection and management. Table 1 lists risk factors leading to metabolic syndrome and its different clinical manifestations after OLT.
| Disease | Incidence/prevalence | Risk factors | Ref. |
| Diabetes mellitus | 9%-21% (incidence) | Male gender | [65,105-107] |
| High pre-LT BMI | |||
| Family history | |||
| Hepatitis C | |||
| Older age immunosuppressants rapamycin gene polymorphisms | |||
| TCF7L2 gene polymorphisms (donor) | |||
| Hyperlipidemia | 45%-69% (prevalence) | Diet | [38,108-110] |
| Older age | |||
| High BMI | |||
| DM | |||
| Renal impairment, immunosuppressants | |||
| low-density lipoprotein receptor gene polymorphism (donor) | |||
| Arterial hypertension | 60%-70% (prevalence) | Obesity | [106,111,112] |
| Older age | |||
| Impaired glycemia | |||
| Immunosuppressants | |||
| Overweight-obesity | 24%-31% (prevalence) | High BMI before LT | [113-116] |
| Diet | |||
| Immunosuppressants | |||
| Metabolic syndrome | 40%-60% (prevalence) | Older age | [33,106,117,118] |
| Obesity and increased BMI | |||
| pre-LT DM | |||
| Genetic polymorphisms in the living donor | |||
| High-dosage immunosuppressive drugs | |||
| Changes in intestinal microbiota | |||
| NAFLD/NASH | 18%-100% (incidence of NAFLD in NASH and cryptogenic recipients) | DM | [18,33,80,119-124] |
| 0%-14% (incidence of NASH in NASH and cryptogenic recipients) | Obesity and weight gain, dyslipidemia | ||
| 10%-40% (incidence of NAFLD in non-NASH or cyptogenic recipients) | Genetic predisposition (presence of the rs738409-G allele of the Patatin-like phospholipase) | ||
| Arterial hypertension | |||
| Immunosuppressant | |||
| pre-LT alcoholic cirrhosis | |||
| Liver graft steatosis |
The use of highly effective anti-rejection medications has led to improved survival, albeit with evidence of well-recognized side effects such as metabolic derangements, and an overall increase in MS and insulin resistance after OLT.
Steroids decrease insulin production by beta-cells, increase gluconeogenesis and reduce glucose utilization, thus strongly contributing to the occurrence of diabetes and weight gain. Tacrolimus and cyclosporine A, the calcineurin inhibitors (CNIs), facilitate de novo occurrence of diabetes by decreasing insulin production and inducing insulin resistance, which is followed by hyperinsulinemia, the effect being more severe for tacrolimus. In addition to these effects increased oxidative stress and lipid peroxidation also occur, followed by hypertension, dyslipidemia and kidney damage[36,53-64].
Among other immunosuppressive drugs demonstrated to exert negative cardiovascular effects is sirolimus, which was reported to be complicated by serious adverse events including hepatic artery thrombosis and wound healing complications within the first 30 d after OLT[65]. Thus, sirolimus was not approved in liver transplantation, although recent studies using lower doses showed an improved safety profile[66,67]. Viceversa, everolimus provides a new therapeutic option for liver transplant recipients, when introduced early after liver transplantation[68] particularly with respect to posttransplant nephrotoxicity and other adverse events associated with long- term administration of CNIs.
Several studies[69-71] showed that hyperlipidemia was more frequent in the everolimus-treated patients than in those treated with CNIs. The relationship between dyslipidemia during mTOR inhibitor administration and cardiovascular outcomes has not been systematically evaluated, and thus the clinical effect of these adverse events is not fully understood. However, the proportion of patients receiving lipid lowering treatment was similar when everolimus associated to a reduced doses of tacrolimus or the standard-of-care tacrolimus treatment were given[72]. Furthermore, the incidence of cardiovascular events after 24 mo did not differ between the two treatment groups[70]. The relationship between high rates of dyslipidemia and mTOR inhibitor use (sirolimus and everolimus), either in conjunction with or instead of CNIs, may be due to altered insulin signaling pathways that result in excess triglyceride production and secretion.
Thus, it is evident that the type of immunosuppressive therapy may strongly influence the occurrence of metabolic complications (Table 2).
| Factor | Metabolic consequences | Ref. |
| Steroid | Increased fat deposition with truncal fat distribution | [36,53-55] |
| Decreased fat oxidation | ||
| Increased gluconeogenesis | ||
| Obesity | ||
| Decreased glucose utilization | ||
| Decreased b-cell insulin production | ||
| Increased proteolysis, | ||
| Reduced protein synthesis | ||
| Insulin resistance | ||
| Diabetes, NAFLD | ||
| Mineralocorticoids effects | ||
| Sodium retention | ||
| Hypertension | ||
| Hyperlipemia | ||
| Calcineurin inhibitor | Tacrolimus: | [58-65] |
| b-cell toxicity | ||
| Decreased insulin secretion | ||
| Insulin resistance, | ||
| Diabetes (more than cyclosporine) | ||
| Cyclosporine: | ||
| Decreased energy metabolism and muscle mass obesity | ||
| Weight gain | ||
| Decreased cholesterol transport into bile hyperlipidemia | ||
| Occupy LDL receptor | ||
| (more than tacrolimus) | ||
| Renal vasoconstriction | ||
| Hypertension | ||
| (more than tacrolimus) | ||
| mTOR inhibitor | Increase insulin response | [68,125-129] |
| Block b-cell proliferation | ||
| Alter insulin signaling | ||
| Decreased diabetes | ||
| Increased diabetes | ||
| Increased triglyceride production pathways | ||
| And secretion | ||
| Increased adipose tissue lipase activity | ||
| Hyperlipidemia | ||
| Decreased Lipoprotein lipase activity | ||
| Anti-metabolites | Mycophenolate mofetil: | [145-149] |
| No nephrotoxity | ||
| No effect on lipid profile, hypertention or diabetes mellitus | ||
| Azatioprine: | ||
| Vascular calcification | ||
| Arteriosclerosis | ||
| Monoclonal antibodies | Basiliximab | [150] |
| No nephrotoxity | ||
| Rare effect on lipid profile, hypertension and diabetes mellitus |
In the last 10 years a marked increase of OLT for NASH cirrhosis was observed while that of HCV remained stable[36]. Thus NAFLD is expected to become the most common indication for OLT over the next decade, given that HCV related morbidity will progressively decrease. Post-transplant NAFLD can be due to the recurrence of pretransplant MS and NAFLD, but often develops de novo because of modified metabolic conditions and use of the immunosuppressive drugs. NAFLD recurrence post-liver transplantation may progress to end-stage disease with liver failure and a need for retransplantation[73-76]. NAFLD incidence after liver transplantation ranges from 18 to 40% and that of NASH between 9%-13%. In addition, in patients transplanted for cryptogenic cirrhosis, the time-dependent risk of developing allograft steatosis is 100% over 5 years[40,75,77,78], indirectly confirming that cryptogenic cirrhosis is an evolution of NASH.
It is also worth noting that genetic background seems to play a role in allograft steatosis, since post-transplant NAFLD risk is linked to a polymorphism in adiponectrin (PNPLA3), which mediates triglyceride hydrolysis and has been reported to be the strongest genetic factor for liver steatosis, independently of insulin resistance. This polymorphism has been repeatedly reported to be associated with more severe fibrosis in patients with NASH and is also associated with pre-transplant obesity risk and presence of steatosis in the donor graft[79,80].
The natural history of post-transplant de novo NAFLD is poorly understood, but it may contribute to increased CVD mortality, since NAFLD is an independent risk factor for CVD even in non-cirrhotic patients[34,77,81].
It is very likely that the mediator of these processes is insulin resistance, which is linked to weight gain and high-dose steroid use post-transplantation, and is reflected by worsening of glucose tolerance, and underlies all manifestations of MS[76,81,82]. Overall, the main consequences of post-transplant MS appear to be NAFLD recurrence/development, higher incidence of adverse CVD events, and chronic transplant nephropathy[64].
Taken together, these evidence clearly demonstrate that a strict selection of patients with a complete cardiovascular assessment is necessary before listing patients for OLT in order to optimize resources and start early therapy to prevent complications.
In patients with cirrhosis, a clinical syndrome named cirrhotic cardiomyopathy has been noticed. This pathology is defined as a blunted contractile responsiveness to physiologic, pathologic, or pharmacologic stress and/or altered diastolic relaxation with electrophysiological abnormalities but with normal increased cardiac output and contractility at rest, in the absence of known cardiac disease and irrespective of the causes of cirrhosis[83]. Strict diagnostic criteria are lacking and this syndrome often goes unrecognized.
Van Wagner, as previously reported, showed that non coronary incidents represent the major adverse cardiovascular events after OLT, including atrial fibrillation, heart failure, thromboembolism and stroke[11].
This suggests that some OLT candidates may have subclinical CVD and may not be identified as patients at high risk when using standard risk algorithms. A study designed to evaluate the association between the presence of segmental myocardial perfusion defects pre-OLT by using myocardial perfusion scintigraphy and the occurrence of post-OLT complications and 1-year mortality after OLT, showed that even the presence of a single reversible perfusion defect was significantly related to an increased incidence of 1-year all-cause mortality. Due to these results the authors suggest the use of myocardial perfusion scintigraphy in the work up process[84]. Other studies point out the attention to pre-transplant pathology detected by echocardiography, including valve regurgitation, pulmonary artery pressure, right and left ventricular size, systolic function and left ventricular ejection fraction[85-87]. One study found a positive association between left ventricular hypertrophy and post- transplant death[85], whereas others yielded conflicting results regarding tricuspid regurgitation and post-transplant death[86,87].
Bushyhead et al[88] tried to determine if specific findings in pre-transplant echocardiography were associated with post-transplant survival and the development of cardiovascular and renal disease. The results of this study showed that increasing pulmonary artery systolic pressure was associated with significantly increased risk of hospitalization for myocardial infarction or heart failure, while increased left ventricular ejection fraction, a possible expression of cirrhotic cardiomyopathy, was associated with a non-significant increased risk of stage 4 or 5 chronic kidney disease.
Thus, because of the high risk of cardiovascular complications after OLT, careful preoperative evaluation of coronary risk is assessed in every transplant center. However, there is not yet a general agreement on a standard cardiovascular screening in OLT candidates. European guidelines suggest that electrocardiogram and echocardiography should be performed in all liver transplant candidates. If the patient has multiple cardiovascular risk factors, and is older than 50 years, a more extensive work up has to be assessed, including a cardiopulmonary exercise test to uncover asymptomatic ischaemic heart disease[89]. If the target heart rate is not achieved during a standard exercise test, a pharmacological stress test is the test of choice. If coronary disease is suspected, coronary angiography should be performed[90].
After OLT, to prevent cardiovascular events it is necessary to plan a follow up and a therapy focused on the control of metabolic syndrome manifestations, including control of blood pressure, blood glucose, lipid levels and weight, in addition to encouraging physical activity, and a correct diet. Individualized immunosuppressive therapy should also be designed. Furthermore, it is important to assess the presence of early vascular and cardiac damage, and to recognize their progression by carotid ultrasound and echocardiography, in order to be able to start specific therapy and prevent CV events in the future.
Given the high risk of developing NAFLD after OLT, therapy to prevent its occurrence and/or to treat it, if already developed, should be started. However, currently the only exploitable therapy for NAFLD is diet (Mediterranean diet is recommended[91]) and physical activity, although all available data suggest that improving insulin sensitivity could reduce the risk of post OLT NAFLD recurrence or de novo development. Drugs as thiazolidinediones (PPARγ agonist with insulin sensitizing effects), metformin, incretin-mimetics (liraglutide), antioxidants (vitamin E), angiotensin converting enzyme inhibitors have given promising results in patients with NAFLD. Several other pharmacological therapies for NAFLD are being studied, such as obeticolic (a syntetic farnesoid X receptor agonist), n-3 polynsaturated fatty acids (PUFA), and novel agents with anti-inflammatory, anti-fibrotic or insulin sensitizing properties [dual PPAR ά/δ agonists, dual chemokine receptor (CCR)2/CCR5 agonists and fatty acid/bile acid conjugates] and antifibrotic anti-lysil oxidase-like (anti-LOXL2) monoclonal antibodies[92]. While data on pentoxyphilline and orlistat have provided limited or inconclusive results, as well as those on lipid lowering drugs (ezetimibe and statins), no clinical trials have been conducted in the post-transplantation setting[34,76,82,93,94].
Thus, at present the only effective approaches for avoiding cardiovascular disease in the post-transplant setting are to prevent and manage MS and its manifestations. Table 3 presents the current available therapies to control metabolic syndrome manifestations.
| Disease | Suggested therapy | Contraindicated therapy | Ref. |
| Diabetes mellitus | Insuline: in the early post-operative setting | Metformin: not usable with renal failure (lactic acidosis) | [130-133,151-157] |
| Life-style modification (diet, physical activity) | Thiazolidinediones: may be associated to hepato and cardiotoxicity and are adipogenic | ||
| Oral hypoglicemic agent (after steroids tapering): | Second generation sulfonylureas: determine weight gain, hypoglycaemia, may increase CNI level | ||
| Metformin: less weight gain and hypoglicemia | Meglitinides: determine weight gain, hypoglycemia (only with renal insuff), CNI may increase repaglinide level, are expensive | ||
| Thiazolidinediones: well tolerated, may improve post-LT NAFLD | Alpha-glucosidase inhibitors: determine gastrointestinal side effects,are less effective, are expensive | ||
| Dypeptyl peptidase-4 (DPP4) inhibitors, well tolerate, no weight gain, no hypoglicemia, potential anti-inflammation, antihypertension, antiapoptosis effects and immunomodulation on the heart, vessels, and kidney, independent of their hypoglicemic effect | Selective renal sodium glucose co-transporter 2 (SGLT 2): dapagliflozin, canagliflozin, empagliflozin, well tolerated but reported hepato-toxicity, contraindicated in patients with renal impairment | ||
| Hyperlipidemia | Hypercholesterolemia responds to: | Statins (except pravastatin and flestatin) are metabolized by cytochrome P-450 3A4, the same that metabolize CNIs and sirolimus so they must be used with caution because of myotoxicity | [134-138] |
| HMGCoA inibitors (statins): pravastatine is the most studied and used but also atorvastatin, simvastatin, lovastatin, cerivastatin and fluvastatin are used | If used with statins fibrates may increase calcineurin inibitors levels | ||
| Diet rich in omega 3 fatty acids, fruits, vegetables and dietary fiber | |||
| Hypertrigliceridemia responds to: | |||
| Fish oil (omega 3) | |||
| Fibric acid derivates (gemfibrosil, clofibrate, fenofibrate) | |||
| Arterial hypertension | First line agents: calcium channels blockers (amlodipine, isradipine, felodipine) | Nifedipine may increase CNI levels and may cause leg edema | [139-141] |
| Second line agents: specific β-blockers, ACE inibitors, angiotensin receptors blockers and loop diuretics | ACE inibitors and angiotensin receptors blockers may exacerbate CNI-induced hyperkalemia, but may provide anti-fibrotic properties and possibly protect against calcineurin induced renal injury | ||
| Thiazides and other diuretics must be used with close follow-up because of potentiation of electrolyte abnormalities, hyperuricemia and renal dysfunction | |||
| Obesity | Bariatric surgery: well tolerated and successful but require a complex reoperation | Orlistat (tetrahydrolipstatin), inhibitor of pancreatic lipase has limited efficacy and possibly interferes with immunosuppressive therapy | [141-144] |
| Gastric banding at the time of liver transplant procedure seems successful and well tolerate | Gastric bypass surgery can affect intestinal drug absorption |
Also renal dysfunction plays a relevant role in the occurrence of cardiovascular disease and death after transplantation. Therapeutic strategies should be focused to minimize renal injury, particularly in NAFLD patients, for example, by reducing exposure to CNIs[95]. This can be accomplished by reducing or withdrawing CNIs after the stable introduction of mycophenolate mofetil, introducing non-CNIs-based immunosuppressive protocols with mTOR inhibitors (sirolimus and everolimus) or reducing the CNIs dose in combination with mTOR inhibitors. The use of such protocols will require further prospective studies within the context of liver transplantation[95].
The onset of cardiovascular modifications after OLT remains poorly understood, the timing in which these modifications occur after OLT is still being debated. Only a few studies (based on paediatric population and prevalently on kidney transplant) and a meta-analysis[96-103] have shown that after solid organ transplantation there was a rapid increase of subclinical atherosclerosis evaluated by aortic stiffness and carotid intima-media thickness.
A recent study[104] demonstrated that at 1 year post-transplant, independently of the indication to OLT, LT recipients have similar pro-atherosclerotic profiles as patients with NASH, as measured by endothelial biomarkers and inflammatory cytokines, even when conventional cardiovascular risk factors, such as obesity or elevated Hs-CRP or/and high FRS, are not observed.
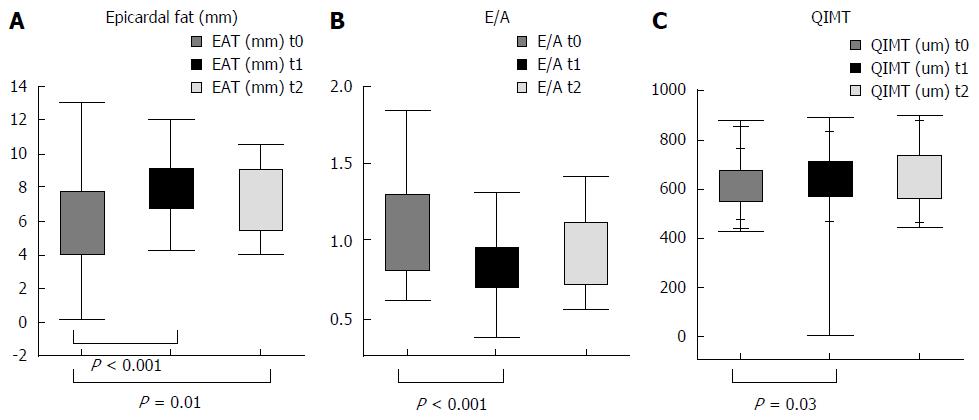
We are conducting a prospective study aimed to understand the types of cardiovascular modifications and their time of development after OLT. Seventy-nine patients in a liver transplant list, were enrolled from 2014 and followed for 2 years after transplant. In these patients cardiovascular, biochemical and anthropometrical parameters were assessed at admission to the transplant waiting list, and at 6, 12, and 18 mo after transplant. The cardiovascular study included: evaluation of cIMT, presence of plaques by carotid ultrasound, diastolic function (E/A), interventricular septum, ventricular mass, and epicardial fat thickness evaluation by echocardiography. Preliminary data showed that cIMT progressively increased during follow up, starting as early as the 6th month, while prevalence of plaques was similar pre and post-transplant. A significant decrease of diastolic function (E/A) and an increase of inter-ventricular septum was observed from enrollment to 6 mo, which then remained stable over time. A progressive increase of epicardial fat was observed during follow up, while ejection function, and ventricular mass did not significantly differ. These preliminary results are shown in Figure 1.
It is yet to be determined if different immunosuppressive therapies influence these early changes and/or if other predisposing factors contribute to cardiac and vascular damage.
OLT candidates and recipients should be carefully evaluated and followed up not only for liver, but also for metabolic complications, optimizing the follow up by introducing blood tests and imaging approaches that are able to show early metabolic, cardiovascular and atherosclerotic alterations.
New parameters that are able to identify subjects at higher metabolic/cardiovascular risk should be identified to plan personalized therapy, including nutritional rules and physical activity.
Prospective studies aimed to evaluate the development of early atherosclerotic damage are needed to understand the timing in which a specific therapy should be started.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Keller F, Ramsay MA S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Adam R, Karam V, Delvart V, O’Grady J, Mirza D, Klempnauer J, Castaing D, Neuhaus P, Jamieson N, Salizzoni M. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). J Hepatol. 2012;57:675-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 643] [Article Influence: 49.5] [Reference Citation Analysis (2)] |
| 2. | Futagawa Y, Terasaki PI, Waki K, Cai J, Gjertson DW. No improvement in long-term liver transplant graft survival in the last decade: an analysis of the UNOS data. Am J Transplant. 2006;6:1398-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Watt KD, Pedersen RA, Kremers WK, Heimbach JK, Charlton MR. Evolution of causes and risk factors for mortality post-liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant. 2010;10:1420-1427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 617] [Cited by in RCA: 587] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 4. | Gonwa TA. Hypertension and renal dysfunction in long-term liver transplant recipients. Liver Transpl. 2001;7:S22-S26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Saab S, Lalezari D, Pruthi P, Alper T, Tong MJ. The impact of obesity on patient survival in liver transplant recipients: a meta-analysis. Liver Int. 2015;35:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 6. | Wong RJ, Cheung R, Perumpail RB, Holt EW, Ahmed A. Diabetes mellitus, and not obesity, is associated with lower survival following liver transplantation. Dig Dis Sci. 2015;60:1036-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 855] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 8. | Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148:547-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1211] [Cited by in RCA: 1384] [Article Influence: 138.4] [Reference Citation Analysis (1)] |
| 9. | Vanwagner LB, Bhave M, Te HS, Feinglass J, Alvarez L, Rinella ME. Patients transplanted for nonalcoholic steatohepatitis are at increased risk for postoperative cardiovascular events. Hepatology. 2012;56:1741-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Targher G, Mantovani A, Pichiri I, Rigolon R, Dauriz M, Zoppini G, Morani G, Vassanelli C, Bonora E. Non-alcoholic fatty liver disease is associated with an increased prevalence of atrial fibrillation in hospitalized patients with type 2 diabetes. Clin Sci (Lond). 2013;125:301-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Piccini JP, Hammill BG, Sinner MF, Jensen PN, Hernandez AF, Heckbert SR, Benjamin EJ, Curtis LH. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 439] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 12. | VanWagner LB, Serper M, Kang R, Levitsky J, Hohmann S, Abecassis M, Skaro A, Lloyd-Jones DM. Factors Associated With Major Adverse Cardiovascular Events After Liver Transplantation Among a National Sample. Am J Transplant. 2016;16:2684-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 143] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 13. | Coss E, Watt KD, Pedersen R, Dierkhising R, Heimbach JK, Charlton MR. Predictors of cardiovascular events after liver transplantation: a role for pretransplant serum troponin levels. Liver Transpl. 2011;17:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Ratziu V, Bellentani S, Cortez-Pinto H, Day C, Marchesini G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53:372-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 789] [Article Influence: 52.6] [Reference Citation Analysis (1)] |
| 15. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2613] [Article Influence: 201.0] [Reference Citation Analysis (1)] |
| 16. | VanWagner LB, Lapin B, Skaro AI, Lloyd-Jones DM, Rinella ME. Impact of renal impairment on cardiovascular disease mortality after liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Int. 2015;35:2575-2583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Bonora E, Targher G. Increased risk of cardiovascular disease and chronic kidney disease in NAFLD. Nat Rev Gastroenterol Hepatol. 2012;9:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 18. | Mikolasevic I, Orlic L, Hrstic I, Milic S. Metabolic syndrome and non-alcoholic fatty liver disease after liver or kidney transplantation. Hepatol Res. 2016;46:841-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Laish I, Braun M, Mor E, Sulkes J, Harif Y, Ben Ari Z. Metabolic syndrome in liver transplant recipients: prevalence, risk factors, and association with cardiovascular events. Liver Transpl. 2011;17:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 20. | Yoo HY, Thuluvath PJ. The effect of insulin-dependent diabetes mellitus on outcome of liver transplantation. Transplantation. 2002;74:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 21. | Moon JI, Barbeito R, Faradji RN, Gaynor JJ, Tzakis AG. Negative impact of new-onset diabetes mellitus on patient and graft survival after liver transplantation: Long-term follow up. Transplantation. 2006;82:1625-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Khan RS, Newsome PN. Non-alcoholic fatty liver disease and liver transplantation. Metabolism. 2016;65:1208-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Albeldawi M, Aggarwal A, Madhwal S, Cywinski J, Lopez R, Eghtesad B, Zein NN. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transpl. 2012;18:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Tovikkai C, Charman SC, Praseedom RK, Gimson AE, van der Meulen J. Time-varying impact of comorbidities on mortality after liver transplantation: a national cohort study using linked clinical and administrative data. BMJ Open. 2015;5:e006971. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Samuelson AL, Lee M, Kamal A, Keeffe EB, Ahmed A. Diabetes mellitus increases the risk of mortality following liver transplantation independent of MELD score. Dig Dis Sci. 2010;55:2089-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Volk ML, Hernandez JC, Lok AS, Marrero JA. Modified Charlson comorbidity index for predicting survival after liver transplantation. Liver Transpl. 2007;13:1515-1520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 119] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Aloia TA, Knight R, Gaber AO, Ghobrial RM, Goss JA. Analysis of liver transplant outcomes for United Network for Organ Sharing recipients 60 years old or older identifies multiple model for end-stage liver disease-independent prognostic factors. Liver Transpl. 2010;16:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 28. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 29. | Nair S, Vanatta JM, Arteh J, Eason JD. Effects of obesity, diabetes, and prior abdominal surgery on resource utilization in liver transplantation: a single-center study. Liver Transpl. 2009;15:1519-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 290] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Laryea M, Watt KD, Molinari M, Walsh MJ, McAlister VC, Marotta PJ, Nashan B, Peltekian KM. Metabolic syndrome in liver transplant recipients: prevalence and association with major vascular events. Liver Transpl. 2007;13:1109-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 237] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 32. | Pagadala M, Dasarathy S, Eghtesad B, McCullough AJ. Posttransplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15:1662-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 111] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 33. | Sprinzl MF, Weinmann A, Lohse N, Tönissen H, Koch S, Schattenberg J, Hoppe-Lotichius M, Zimmermann T, Galle PR, Hansen T. Metabolic syndrome and its association with fatty liver disease after orthotopic liver transplantation. Transpl Int. 2013;26:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Seo S, Maganti K, Khehra M, Ramsamooj R, Tsodikov A, Bowlus C, McVicar J, Zern M, Torok N. De novo nonalcoholic fatty liver disease after liver transplantation. Liver Transpl. 2007;13:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 35. | Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4273] [Cited by in RCA: 4503] [Article Influence: 225.2] [Reference Citation Analysis (0)] |
| 36. | Watt KD, Charlton MR. Metabolic syndrome and liver transplantation: a review and guide to management. J Hepatol. 2010;53:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 164] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 37. | Lai HM, Pawar R, Wolf DC, Aronow WS. Impact of Cardiovascular Risk Factors on Long-Term Mortality After Liver Transplantation. Am J Ther. 2016;23:e357-e362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Bianchi G, Marchesini G, Marzocchi R, Pinna AD, Zoli M. Metabolic syndrome in liver transplantation: relation to etiology and immunosuppression. Liver Transpl. 2008;14:1648-1654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 39. | Colle I, Van Vlierberghe H, Troisi R, De Hemptinne B. Transplanted liver: consequences of denervation for liver functions. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:924-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Anastácio LR, Lima AS, Toulson Davisson Correia MI. Metabolic syndrome and its components after liver transplantation: incidence, prevalence, risk factors, and implications. Clin Nutr. 2010;29:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Giusto M, Lattanzi B, Di Gregorio V, Giannelli V, Lucidi C, Merli M. Changes in nutritional status after liver transplantation. World J Gastroenterol. 2014;20:10682-10690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Baid S, Cosimi AB, Farrell ML, Schoenfeld DA, Feng S, Chung RT, Tolkoff-Rubin N, Pascual M. Posttransplant diabetes mellitus in liver transplant recipients: risk factors, temporal relationship with hepatitis C virus allograft hepatitis, and impact on mortality. Transplantation. 2001;72:1066-1072. [PubMed] |
| 43. | Khalili M, Lim JW, Bass N, Ascher NL, Roberts JP, Terrault NA. New onset diabetes mellitus after liver transplantation: the critical role of hepatitis C infection. Liver Transpl. 2004;10:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 44. | AlDosary AA, Ramji AS, Elliott TG, Sirrs SM, Thompson DM, Erb SR, Steinbrecher UP, Yoshida EM. Post-liver transplantation diabetes mellitus: an association with hepatitis C. Liver Transpl. 2002;8:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Saliba F, Lakehal M, Pageaux GP, Roche B, Vanlemmens C, Duvoux C, Dumortier J, Salamé E, Calmus Y, Maugendre D. Risk factors for new-onset diabetes mellitus following liver transplantation and impact of hepatitis C infection: an observational multicenter study. Liver Transpl. 2007;13:136-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 46. | Thuluvath PJ. Is there a link between hepatitis C virus and new onset of diabetes mellitus after liver transplantation? Liver Transpl. 2007;13:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 47. | John PR, Thuluvath PJ. Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl. 2002;8:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 126] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Pham PT, Pham PC, Lipshutz GS, Wilkinson AH. New onset diabetes mellitus after solid organ transplantation. Endocrinol Metab Clin North Am. 2007;36:873-890; vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Marchetti P. New-onset diabetes after liver transplantation: from pathogenesis to management. Liver Transpl. 2005;11:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 50. | Levy G, Grazi GL, Sanjuan F, Wu Y, Mühlbacher F, Samuel D, Friman S, Jones R, Cantisani G, Villamil F. 12-month follow-up analysis of a multicenter, randomized, prospective trial in de novo liver transplant recipients (LIS2T) comparing cyclosporine microemulsion (C2 monitoring) and tacrolimus. Liver Transpl. 2006;12:1464-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 51. | Vincenti F, Friman S, Scheuermann E, Rostaing L, Jenssen T, Campistol JM, Uchida K, Pescovitz MD, Marchetti P, Tuncer M. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant. 2007;7:1506-1514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 460] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 52. | Parikh CR, Klem P, Wong C, Yalavarthy R, Chan L. Obesity as an independent predictor of posttransplant diabetes mellitus. Transplant Proc. 2003;35:2922-2926. [PubMed] |
| 53. | Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23-43. [PubMed] |
| 54. | Klintmalm GB, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, Eckhoff DE, Netto GJ, Katz E. Corticosteroid-free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1-year interim results of the HCV-3 study. Liver Transpl. 2007;13:1521-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 55. | van den Ham EC, Kooman JP, Christiaans MH, van Hooff JP. Relation between steroid dose, body composition and physical activity in renal transplant patients. Transplantation. 2000;69:1591-1598. [PubMed] |
| 56. | van den Ham EC, Kooman JP, Christiaans MH, Leunissen KM, van Hooff JP. Posttransplantation weight gain is predominantly due to an increase in body fat mass. Transplantation. 2000;70:241-242. [PubMed] |
| 57. | Gitto S, Villa E. Non-Alcoholic Fatty Liver Disease and Metabolic Syndrome after Liver Transplant. Int J Mol Sci. 2016;17:490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Sakuma K, Yamaguchi A. The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol. 2010;2010:721219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 59. | Rabkin JM, Corless CL, Rosen HR, Olyaei AJ. Immunosuppression impact on long-term cardiovascular complications after liver transplantation. Am J Surg. 2002;183:595-599. [PubMed] |
| 60. | Manzarbeitia C, Reich DJ, Rothstein KD, Braitman LE, Levin S, Munoz SJ. Tacrolimus conversion improves hyperlipidemic states in stable liver transplant recipients. Liver Transpl. 2001;7:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 62. | Rossetto A, Bitetto D, Bresadola V, Lorenzin D, Baccarani U, De Anna D, Bresadola F, Adani GL. Cardiovascular risk factors and immunosuppressive regimen after liver transplantation. Transplant Proc. 2010;42:2576-2578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 63. | Li DW, Lu TF, Hua XW, Dai HJ, Cui XL, Zhang JJ, Xia Q. Risk factors for new onset diabetes mellitus after liver transplantation: A meta-analysis. World J Gastroenterol. 2015;21:6329-6340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 64. | Haddad EM, McAlister VC, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006;CD005161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 65. | Asrani SK, Wiesner RH, Trotter JF, Klintmalm G, Katz E, Maller E, Roberts J, Kneteman N, Teperman L, Fung JJ. De novo sirolimus and reduced-dose tacrolimus versus standard-dose tacrolimus after liver transplantation: the 2000-2003 phase II prospective randomized trial. Am J Transplant. 2014;14:356-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 66. | Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, Onaca N, Goldstein R, Levy M, Klintmalm GB. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 67. | McKenna GJ, Trotter JF, Klintmalm E, Onaca N, Ruiz R, Jennings LW, Neri M, O’Leary JG, Davis GL, Levy MF. Limiting hepatitis C virus progression in liver transplant recipients using sirolimus-based immunosuppression. Am J Transplant. 2011;11:2379-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Ganschow R, Pollok JM, Jankofsky M, Junge G. The role of everolimus in liver transplantation. Clin Exp Gastroenterol. 2014;7:329-343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | De Simone P, Metselaar HJ, Fischer L, Dumortier J, Boudjema K, Hardwigsen J, Rostaing L, De Carlis L, Saliba F, Nevens F. Conversion from a calcineurin inhibitor to everolimus therapy in maintenance liver transplant recipients: a prospective, randomized, multicenter trial. Liver Transpl. 2009;15:1262-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 70. | Saliba F, De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Jonas S, Sudan D, Fischer L, Duvoux C. Renal function at two years in liver transplant patients receiving everolimus: results of a randomized, multicenter study. Am J Transplant. 2013;13:1734-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 71. | Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, Settmacher U, Heyne N, Clavien PA, Muehlbacher F. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012;12:1855-1865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 72. | De Simone P, Nevens F, De Carlis L, Metselaar HJ, Beckebaum S, Saliba F, Jonas S, Sudan D, Fung J, Fischer L. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008-3020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 253] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 73. | Sutedja DS, Gow PJ, Hubscher SG, Elias E. Revealing the cause of cryptogenic cirrhosis by posttransplant liver biopsy. Transplant Proc. 2004;36:2334-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Ong J, Younossi ZM, Reddy V, Price LL, Gramlich T, Mayes J, Boparai N. Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transpl. 2001;7:797-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 167] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 75. | Vallin M, Guillaud O, Boillot O, Hervieu V, Scoazec JY, Dumortier J. Recurrent or de novo nonalcoholic fatty liver disease after liver transplantation: natural history based on liver biopsy analysis. Liver Transpl. 2014;20:1064-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 76. | Shaker M, Tabbaa A, Albeldawi M, Alkhouri N. Liver transplantation for nonalcoholic fatty liver disease: new challenges and new opportunities. World J Gastroenterol. 2014;20:5320-5330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 77] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 77. | Targher G, Chonchol MB, Byrne CD. CKD and nonalcoholic fatty liver disease. Am J Kidney Dis. 2014;64:638-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 160] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 78. | Contos MJ, Cales W, Sterling RK, Luketic VA, Shiffman ML, Mills AS, Fisher RA, Ham J, Sanyal AJ. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 79. | Singal AG, Manjunath H, Yopp AC, Beg MS, Marrero JA, Gopal P, Waljee AK. The effect of PNPLA3 on fibrosis progression and development of hepatocellular carcinoma: a meta-analysis. Am J Gastroenterol. 2014;109:325-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 80. | Finkenstedt A, Auer C, Glodny B, Posch U, Steitzer H, Lanzer G, Pratschke J, Biebl M, Steurer M, Graziadei I. Patatin-like phospholipase domain-containing protein 3 rs738409-G in recipients of liver transplants is a risk factor for graft steatosis. Clin Gastroenterol Hepatol. 2013;11:1667-1672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Said A. Non-alcoholic fatty liver disease and liver transplantation: outcomes and advances. World J Gastroenterol. 2013;19:9146-9155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | Angulo P. Nonalcoholic fatty liver disease and liver transplantation. Liver Transpl. 2006;12:523-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 83. | Møller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 84. | Oprea-Lager DE, Sorgdrager BJ, Jukema JW, Scherptong RW, Ringers J, Coenraad MJ, van Hoek B, Stokkel MP. Clinical value of myocardial perfusion scintigraphy as a screening tool in liver transplant candidates. Liver Transpl. 2011;17:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 85. | Batra S, Machicao VI, Bynon JS, Mehta S, Tanikella R, Krowka MJ, Zacks S, Trotter J, Roberts KE, Brown RS. The impact of left ventricular hypertrophy on survival in candidates for liver transplantation. Liver Transpl. 2014;20:705-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 86. | Kia L, Shah SJ, Wang E, Sharma D, Selvaraj S, Medina C, Cahan J, Mahon H, Levitsky J. Role of pretransplant echocardiographic evaluation in predicting outcomes following liver transplantation. Am J Transplant. 2013;13:2395-2401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 87. | Leithead JA, Kandiah K, Steed H, Gunson BK, Steeds RP, Ferguson JW. Tricuspid regurgitation on echocardiography may not be a predictor of patient survival after liver transplantation. Am J Transplant. 2014;14:2192-2193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 88. | Bushyhead D, Kirkpatrick JN, Goldberg D. Pretransplant echocardiographic parameters as markers of posttransplant outcomes in liver transplant recipients. Liver Transpl. 2016;22:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 89. | Bernal W, Martin-Mateos R, Lipcsey M, Tallis C, Woodsford K, McPhail MJ, Willars C, Auzinger G, Sizer E, Heneghan M. Aerobic capacity during cardiopulmonary exercise testing and survival with and without liver transplantation for patients with chronic liver disease. Liver Transpl. 2014;20:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 90. | Wray C, Scovotti JC, Tobis J, Niemann CU, Planinsic R, Walia A, Findlay J, Wagener G, Cywinski JB, Markovic D. Liver transplantation outcome in patients with angiographically proven coronary artery disease: a multi-institutional study. Am J Transplant. 2013;13:184-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | Abenavoli L, Milic N, Peta V, Alfieri F, De Lorenzo A, Bellentani S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J Gastroenterol. 2014;20:16831-16840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 77] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 92. | European Association for the Study of the Liver (EASL). Electronic address: easloffice@easloffice.eu; European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3181] [Article Influence: 353.4] [Reference Citation Analysis (4)] |
| 93. | Angelico F, Burattin M, Alessandri C, Del Ben M, Lirussi F. Drugs improving insulin resistance for non-alcoholic fatty liver disease and/or non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2007;CD005166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Barb D, Portillo-Sanchez P, Cusi K. Pharmacological management of nonalcoholic fatty liver disease. Metabolism. 2016;65:1183-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 95. | Houlihan DD, Armstrong MJ, Davidov Y, Hodson J, Nightingale P, Rowe IA, Paris S, Gunson BK, Bramhall SB, Mutimer DJ. Renal function in patients undergoing transplantation for nonalcoholic steatohepatitis cirrhosis: time to reconsider immunosuppression regimens? Liver Transpl. 2011;17:1292-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 96. | Al Nasser Y, Moura MC, Mertens L, McCrindle BW, Parekh RS, Ng VL, Church PC, Mouzaki M. Subclinical cardiovascular changes in pediatric solid organ transplant recipients: A systematic review and meta-analysis. Pediatr Transplant. 2016;20:530-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 97. | Siirtola A, Kallio T, Ala-Houhala M, Lehtimäki T, Solakivi T, Antikainen M, Salo MK, Holmberg C. Carotid intima-media thickness after pediatric renal or liver transplantation at high-resolution B-mode ultrasonography. Transplant Proc. 2010;42:1695-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 98. | Delucchi A, Dinamarca H, Gainza H, Whitttle C, Torrealba I, Iñiguez G. Carotid intima-media thickness as a cardiovascular risk marker in pediatric end-stage renal disease patients on dialysis and in renal transplantation. Transplant Proc. 2008;40:3244-3246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Mitsnefes MM, Kimball TR, Witt SA, Glascock BJ, Khoury PR, Daniels SR. Abnormal carotid artery structure and function in children and adolescents with successful renal transplantation. Circulation. 2004;110:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Krmar RT, Balzano R, Jogestrand T, Cedazo-Minguez A, Englund MS, Berg UB. Prospective analysis of carotid arterial wall structure in pediatric renal transplants with ambulatory normotension and in treated hypertensive recipients. Pediatr Transplant. 2008;12:412-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Bilginer Y, Ozaltin F, Basaran C, Aki TF, Karabulut E, Duzova A, Besbas N, Topaloglu R, Ozen S, Bakkaloglu M. Carotid intima-media thickness in children and young adults with renal transplant: Internal carotid artery vs. common carotid artery. Pediatr Transplant. 2007;11:888-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | Litwin M, Wühl E, Jourdan C, Trelewicz J, Niemirska A, Fahr K, Jobs K, Grenda R, Wawer ZT, Rajszys P. Altered morphologic properties of large arteries in children with chronic renal failure and after renal transplantation. J Am Soc Nephrol. 2005;16:1494-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 103. | Basiratnia M, Fazel M, Lotfi M, Hosseini Al-Hashemi G, Fallahzadeh MH, Derakhshan A, Salehipour M. Subclinical atherosclerosis and related risk factors in renal transplant recipients. Pediatr Nephrol. 2010;25:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 104. | Alvares-da-Silva MR, de Oliveira CP, Stefano JT, Barbeiro HV, Barbeiro D, Soriano FG, Farias AQ, Carrilho FJ, D’Albuquerque LA. Pro-atherosclerotic markers and cardiovascular risk factors one year after liver transplantation. World J Gastroenterol. 2014;20:8667-8673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Lane JT, Dagogo-Jack S. Approach to the patient with new-onset diabetes after transplant (NODAT). J Clin Endocrinol Metab. 2011;96:3289-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 106. | Parekh J, Corley DA, Feng S. Diabetes, hypertension and hyperlipidemia: prevalence over time and impact on long-term survival after liver transplantation. Am J Transplant. 2012;12:2181-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 107. | Ling Q, Xie H, Lu D, Wei X, Gao F, Zhou L, Xu X, Zheng S. Association between donor and recipient TCF7L2 gene polymorphisms and the risk of new-onset diabetes mellitus after liver transplantation in a Han Chinese population. J Hepatol. 2013;58:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 108. | Singh S, Watt KD. Long-term medical management of the liver transplant recipient: what the primary care physician needs to know. Mayo Clin Proc. 2012;87:779-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 109. | Morrisett JD, Abdel-Fattah G, Hoogeveen R, Mitchell E, Ballantyne CM, Pownall HJ, Opekun AR, Jaffe JS, Oppermann S, Kahan BD. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J Lipid Res. 2002;43:1170-1180. [PubMed] |
| 110. | Nikkilä K, Åberg F, Isoniemi H. Transmission of LDLR mutation from donor through liver transplantation resulting in hypercholesterolemia in the recipient. Am J Transplant. 2014;14:2898-2902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Hryniewiecka E, Zegarska J, Paczek L. Arterial hypertension in liver transplant recipients. Transplant Proc. 2011;43:3029-3034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 112. | Zheng J, Wang WL. Risk factors of metabolic syndrome after liver transplantation. Hepatobiliary Pancreat Dis Int. 2015;14:582-587. [PubMed] |
| 113. | Richards J, Gunson B, Johnson J, Neuberger J. Weight gain and obesity after liver transplantation. Transpl Int. 2005;18:461-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 224] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 114. | Richardson RA, Garden OJ, Davidson HI. Reduction in energy expenditure after liver transplantation. Nutrition. 2001;17:585-589. [PubMed] |
| 115. | Ferreira LG, Santos LF, Anastácio LR, Lima AS, Correia MI. Resting energy expenditure, body composition, and dietary intake: a longitudinal study before and after liver transplantation. Transplantation. 2013;96:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 116. | Anastácio LR, Ferreira LG, de Sena Ribeiro H, Lima AS, Vilela EG, Toulson Davisson Correia MI. Body composition and overweight of liver transplant recipients. Transplantation. 2011;92:947-951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 117. | Qin N, Yang F, Li A, Prifti E, Chen Y, Shao L, Guo J, Le Chatelier E, Yao J, Wu L. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1230] [Cited by in RCA: 1537] [Article Influence: 139.7] [Reference Citation Analysis (40)] |
| 118. | Fussner LA, Heimbach JK, Fan C, Dierkhising R, Coss E, Leise MD, Watt KD. Cardiovascular disease after liver transplantation: When, What, and Who Is at Risk. Liver Transpl. 2015;21:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 119. | Burra P, Germani G. Orthotopic liver transplantation in non-alcoholic fatty liver disease patients. Rev Recent Clin Trials. 2014;9:210-216. [PubMed] |
| 120. | Dureja P, Mellinger J, Agni R, Chang F, Avey G, Lucey M, Said A. NAFLD recurrence in liver transplant recipients. Transplantation. 2011;91:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 121. | Malik SM, Devera ME, Fontes P, Shaikh O, Sasatomi E, Ahmad J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transpl. 2009;15:1843-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 122. | Watt KD, Dierkhising R, Fan C, Heimbach JK, Tillman H, Goldstein D, Thompson A, Krishnan A, Charlton MR. Investigation of PNPLA3 and IL28B genotypes on diabetes and obesity after liver transplantation: insight into mechanisms of disease. Am J Transplant. 2013;13:2450-2457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 123. | Dumortier J, Giostra E, Belbouab S, Morard I, Guillaud O, Spahr L, Boillot O, Rubbia-Brandt L, Scoazec JY, Hadengue A. Non-alcoholic fatty liver disease in liver transplant recipients: another story of “seed and soil”. Am J Gastroenterol. 2010;105:613-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 124. | Malhi H, Allen AM, Watt KD. Nonalcoholic fatty liver: optimizing pretransplant selection and posttransplant care to maximize survival. Curr Opin Organ Transplant. 2016;21:99-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Vodenik B, Rovira J, Campistol JM. Mammalian target of rapamycin and diabetes: what does the current evidence tell us? Transplant Proc. 2009;41:S31-S38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 126. | Morrisett JD, Abdel-Fattah G, Kahan BD. Sirolimus changes lipid concentrations and lipoprotein metabolism in kidney transplant recipients. Transplant Proc. 2003;35:143S-150S. [PubMed] |
| 127. | Neff GW, Montalbano M, Tzakis AG. Ten years of sirolimus therapy in orthotopic liver transplant recipients. Transplant Proc. 2003;35:209S-216S. [PubMed] |
| 128. | Miyabara EH, Conte TC, Silva MT, Baptista IL, Bueno C, Fiamoncini J, Lambertucci RH, Serra CS, Brum PC, Pithon-Curi T. Mammalian target of rapamycin complex 1 is involved in differentiation of regenerating myofibers in vivo. Muscle Nerve. 2010;42:778-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 129. | Zimmermann A, Zobeley C, Weber MM, Lang H, Galle PR, Zimmermann T. Changes in lipid and carbohydrate metabolism under mTOR- and calcineurin-based immunosuppressive regimen in adult patients after liver transplantation. Eur J Intern Med. 2016;29:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 130. | Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, Balas B, Gastaldelli A, Tio F, Pulcini J. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1307] [Cited by in RCA: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 131. | Lutchman G, Modi A, Kleiner DE, Promrat K, Heller T, Ghany M, Borg B, Loomba R, Liang TJ, Premkumar A. The effects of discontinuing pioglitazone in patients with nonalcoholic steatohepatitis. Hepatology. 2007;46:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 132. | Bonkovsky HL, Azar R, Bird S, Szabo G, Banner B. Severe cholestatic hepatitis caused by thiazolidinediones: risks associated with substituting rosiglitazone for troglitazone. Dig Dis Sci. 2002;47:1632-1637. [PubMed] |
| 133. | Nathan DM. Rosiglitazone and cardiotoxicity--weighing the evidence. N Engl J Med. 2007;357:64-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 134. | Martin JE, Cavanaugh TM, Trumbull L, Bass M, Weber F, Aranda-Michel J, Hanaway M, Rudich S. Incidence of adverse events with HMG-CoA reductase inhibitors in liver transplant patients. Clin Transplant. 2008;22:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 135. | Asberg A. Interactions between cyclosporin and lipid-lowering drugs: implications for organ transplant recipients. Drugs. 2003;63:367-378. [PubMed] |
| 136. | Mück W, Neal DA, Boix O, Voith B, Hasan R, Alexander GJ. Tacrolimus/cerivastatin interaction study in liver transplant recipients. Br J Clin Pharmacol. 2001;52:213-215. [PubMed] |
| 137. | McKenney JM, Sica D. Role of prescription omega-3 fatty acids in the treatment of hypertriglyceridemia. Pharmacotherapy. 2007;27:715-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 138. | Lee S, Gura KM, Puder M. Omega-3 fatty acids and liver disease. Hepatology. 2007;45:841-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 139. | Neal DA, Brown MJ, Wilkinson IB, Byrne CD, Alexander GJ. Hemodynamic effects of amlodipine, bisoprolol, and lisinopril in hypertensive patients after liver transplantation. Transplantation. 2004;77:748-750. [PubMed] |
| 140. | Lubel JS, Herath CB, Burrell LM, Angus PW. Liver disease and the renin-angiotensin system: recent discoveries and clinical implications. J Gastroenterol Hepatol. 2008;23:1327-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 141. | Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright JT. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560-2572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13416] [Cited by in RCA: 13295] [Article Influence: 604.3] [Reference Citation Analysis (0)] |
| 142. | Campsen J, Zimmerman M, Shoen J, Wachs M, Bak T, Mandell MS, Kam I. Adjustable gastric banding in a morbidly obese patient during liver transplantation. Obes Surg. 2008;18:1625-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 143. | Butte JM, Devaud N, Jarufe NP, Boza C, Pérez G, Torres J, Pérez-Ayuso RM, Arrese M, Martínez J. Sleeve gastrectomy as treatment for severe obesity after orthotopic liver transplantation. Obes Surg. 2007;17:1517-1519. [PubMed] |
| 144. | Cassiman D, Roelants M, Vandenplas G, Van der Merwe SW, Mertens A, Libbrecht L, Verslype C, Fevery J, Aerts R, Pirenne J. Orlistat treatment is safe in overweight and obese liver transplant recipients: a prospective, open label trial. Transpl Int. 2006;19:1000-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 145. | Mele TS, Halloran PF. The use of mycophenolate mofetil in transplant recipients. Immunopharmacology. 2000;47:215-245. [PubMed] |
| 146. | Cicinnati VR, Yu Z, Klein CG, Sotiropoulos GC, Saner F, Malagó M, Frilling A, Gerken G, Broelsch CE, Beckebaum S. Clinical trial: switch to combined mycophenolate mofetil and minimal dose calcineurin inhibitor in stable liver transplant patients--assessment of renal and allograft function, cardiovascular risk factors and immune monitoring. Aliment Pharmacol Ther. 2007;26:1195-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 147. | Kriss M, Sotil EU, Abecassis M, Welti M, Levitsky J. Mycophenolate mofetil monotherapy in liver transplant recipients. Clin Transplant. 2011;25:E639-E646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 148. | Prüfer J, Schuchardt M, Tölle M, Prüfer N, Höhne M, Zidek W, van der Giet M. Harmful effects of the azathioprine metabolite 6-mercaptopurine in vascular cells: induction of mineralization. PLoS One. 2014;9:e101709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 149. | Chen H, Chen B. Clinical mycophenolic acid monitoring in liver transplant recipients. World J Gastroenterol. 2014;20:10715-10728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 150. | Liu CL, Fan ST, Lo CM, Chan SC, Ng IO, Lai CL, Wong J. Interleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: a protocol with early elimination of steroids and reduction of tacrolimus dosage. Liver Transpl. 2004;10:728-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 151. | Strøm Halden TA, Åsberg A, Vik K, Hartmann A, Jenssen T. Short-term efficacy and safety of sitagliptin treatment in long-term stable renal recipients with new-onset diabetes after transplantation. Nephrol Dial Transplant. 2014;29:926-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 152. | Haidinger M, Werzowa J, Hecking M, Antlanger M, Stemer G, Pleiner J, Kopecky C, Kovarik JJ, Döller D, Pacini G. Efficacy and safety of vildagliptin in new-onset diabetes after kidney transplantation--a randomized, double-blind, placebo-controlled trial. Am J Transplant. 2014;14:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 153. | Boerner BP, Miles CD, Shivaswamy V. Efficacy and safety of sitagliptin for the treatment of new-onset diabetes after renal transplantation. Int J Endocrinol. 2014;2014:617638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 154. | Werzowa J, Hecking M, Haidinger M, Lechner F, Döller D, Pacini G, Stemer G, Pleiner J, Frantal S, Säemann MD. Vildagliptin and pioglitazone in patients with impaired glucose tolerance after kidney transplantation: a randomized, placebo-controlled clinical trial. Transplantation. 2013;95:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 155. | Lim SW, Jin JZ, Jin L, Jin J, Li C. Role of dipeptidyl peptidase-4 inhibitors in new-onset diabetes after transplantation. Korean J Intern Med. 2015;30:759-770. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 156. | Sharif A, Cohney S. Post-transplantation diabetes-state of the art. Lancet Diabetes Endocrinol. 2016;4:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 157. | García-Compeán D, González-González JA, Lavalle-González FJ, González-Moreno EI, Maldonado-Garza HJ, Villarreal-Pérez JZ. The treatment of diabetes mellitus of patients with chronic liver disease. Ann Hepatol. 2015;14:780-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |