Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1721
Peer-review started: April 20, 2015
First decision: June 23, 2015
Revised: July 14, 2015
Accepted: November 9, 2015
Article in press: November 9, 2015
Published online: January 28, 2016
Processing time: 290 Days and 12.2 Hours
AIM: To systematically review the survival outcomes relating to extramural venous invasion in rectal cancer.
METHODS: A systematic review was conducted using PRISMA guidelines. An electronic search was carried out using MEDLINE, EMBASE, CINAHL, Cochrane library databases, Google scholar and PubMed until October 2014. Search terms were used in combination to yield articles on extramural venous invasion in rectal cancer. Outcome measures included prevalence and 5-year survival rates. These were graphically displayed using Forest plots. Statistical analysis of the data was carried out.
RESULTS: Fourteen studies reported the prevalence of extramural venous invasion (EMVI) positive patients. Prevalence ranged from 9%-61%. The pooled prevalence of EMVI positivity was 26% [Random effects: Event rate 0.26 (0.18, 0.36)]. Most studies showed that EMVI related to worse oncological outcomes. The pooled overall survival was 39.5% [Random effects: Event rate 0.395 (0.29, 0.51)].
CONCLUSION: Historically, there has been huge variation in the prevalence of EMVI through inconsistent reporting. However the presence of EMVI clearly leads to worse survival outcomes. As detection rates become more consistent, EMVI may be considered as part of risk-stratification in rectal cancer. Standardised histopathological definitions and the use of magnetic resonance imaging to identify EMVI will improve detection rates in the future.
Core tip: Extramural venous invasion (EMVI) has been shown to be an adverse risk factor in rectal cancer. Historical studies have shown a wide range of prevalence which has made survival risk difficult to interpret. This has been due to lack of standardised detection methods. As these methods improve, we are more likely to be able to identify those patients with evidence of EMVI and thus offer patients optimal treatment.
- Citation: Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol 2016; 22(4): 1721-1726
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1721.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1721
Venous invasion is considered a prognostic factor in rectal cancer[1-7] however the exact effect on survival outcomes and disease recurrence remains unknown. The current nomenclature refers to extramural venous invasion (EMVI) and specifically describes tumour cells within the veins outside the muscularis propria of the bowel wall[8]. This distinction from other descriptions of venous or vascular invasion is helpful in the modern management of rectal cancer where risk of recurrence needs to be accurately defined for individual patients, to determine whether they would benefit from neo-adjuvant and adjuvant therapies.
The existing literature generally examines venous invasion based on pathology specimens and combine colon and rectal cancer. There are only a limited number of reports focusing on rectal cancer with clear methods and definitions used for identification of EMVI. As a result there is a large variation in reporting EMVI between pathologists[9,10]. Furthermore, the reliance on pathology for identification of EMVI particularly after neoadjuvant treatment (CRT) may lead to under-detection[11].
More recently, magnetic resonance imaging (MRI) has been shown to accurately detect EMVI before and after CRT and identify cases which are missed on routine pathological assessment[11]. These inconsistencies in the definition and specific histological methods applied have led to challenging interpretation of the true incidence and risk associated with EMVI; leading to its lack of mandatory consideration for oncological treatment. If EMVI is shown to have prognostic implications but is being under-detected by traditional histopathological methods alternatives such as MRI may be considered to avoid the risk of disease recurrence.
The aim of this review is to critically examine the evidence for the prognostic importance of venous invasion; specifically EMVI on histopathology, on the survival outcomes of rectal cancer.
An electronic search was carried out using MEDLINE (1965-2014), EMBASE (1980-2014), CINAHL (1982-2014) and the Cochrane library databases. Google scholar and PubMed were used to search articles prior to 1965. Medical subject heading terms and keywords were used: “rectal cancer”; “venous invasion”; “vascular invasion”; “extramural”; and “EMVI”. The “related articles” function was used to broaden the search and all abstracts, studies, and citations retrieved were scanned for subject relevance. The latest date of this search was October 2014. All potentially relevant manuscripts were retrieved and evaluated for inclusion. Reference lists of all relevant publications were hand-searched for additional studies, and cross referenced until no further relevant publications were identified.
Study methodology was carried out in accordance with the “Preferred Reporting for Systematic Reviews and Meta-Analyses” guidelines. We included all studies in English reporting on outcomes of venous invasion or EMVI in curative rectal cancer. Adult patients over the age of 18 were included. Where multiple studies describing the same patient population were identified, the most recent publication was used. Case reports were excluded. Studies of colorectal cancer were included where data for rectal cancer could be extracted. We included studies if they reported on outcomes such as disease recurrence and overall survival. Quality assessment of eligible studies was carried out by two independent reviewers.
Pooling of prevalence rates was performed using Comprehensive Meta-Analysis[12] and forest plots were used as a graphical display.
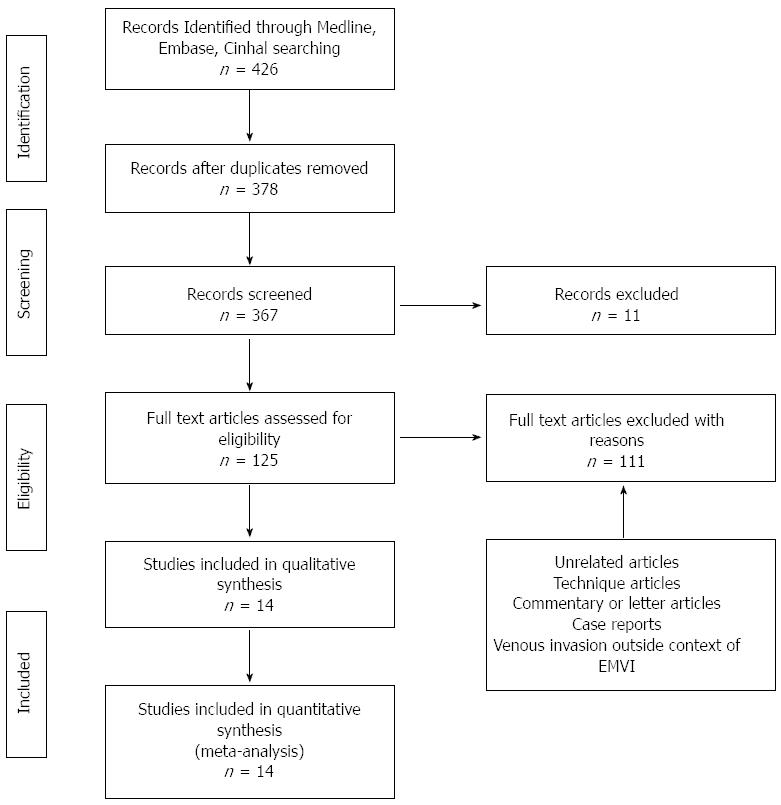
Three hundred and sixty-four publications were initially identified with potential relevance (Figure 1). Thirty-two articles included patients who did not undergo surgery; 30 articles described techniques only; 25 articles were case reports; 24 articles described venous invasion outside the context of cancer. Further screening identified 14 studies published between 1935 and 2014 which were included in this review. This is shown in Table 1.
| Author | Year | No. patients | Study design | No. of pathologists, blinding | Stain | Tumoursite | Elastin stain | No. EMVI +ve pts | Prev VI +ve | EMVI +ve 5 yr survival | Independent prognosticator |
| Brown et al[29] | 1938 | 170 | Retrospective | Unspecified | H + E | Rectum | Yes | 104 | 61% | No 5 yr data | No comment |
| Dukes et al[30] | 1941 | 689 | Prospective | Unspecified | No stain | Rectum | No | 107 | 17% | No 5 yr data | No comment |
| Seefeld et al[13] | 1943 | 100 | Prospective | 1, blinded | H + E, Gieson’s | Rectum | Yes | 20 | 20% | 5% | No comment |
| Madison et al[31] | 1954 | 42 | Prospective | Unspecified | Brominol, H + E, Gieson’s | Rectum | Yes | 19 | 43% | No 5 yr data | No comment |
| Carroll[17] | 1963 | 1996 | Retrospective | Unspecified | H + E | Rectum | No | 240 | 11.8% | 36% | No comment |
| Khankhanian et al[32] | 1977 | 143 | Retrospective | Unspecified | Not stated | Rectum | No | 70 | 19% (BVI + LVI) | Data not usable | EMVI is +ve IPS |
| Talbot et al[6] | 1980 | 706 | Prospective | 2, blinded | H + E, elastin | Rectum | Yes | 366 | 52% | 33% EMVI+ve | No comment |
| Rich et al[15] | 1983 | 142 | Prospective | 1, blinded | H + E | RS/Rectum | No | 23 | 17% | No 5 yr data | No comment |
| Freedman et al[4] | 1984 | 494 | Retrospective | Unspecified | No comment | Rectum | Yes | 89 | 36% | 31% EMVI+ve | No comment |
| Jass et al[16] | 1986 | 447 | Prospective | 1, blinded | H + E | Rectum | No | 116 | 26% (extramural only) | 41% EMVI+ve | EMVI - No IPS |
| Sasaki et al[33] | 1987 | 774 | Retrospective | Unspecified | H + E | Rectum | No | 163 | 21% (extramural only) | No 5 yr data | No comment |
| Minsky et al[14] | 1988 | 168 | Retrospective | 1, blinded | H + E, elastin | RS/Rectum | Yes | 81 | 48% | 33% EMVI+ve | EMVI - No IPS |
| Harrison et al[2] | 1994 | 348 | Retrospective | 2, blinded | H + E, elastin | Rectum | Yes | 74 | 21.2% | 21% EMVI+ve | EMVI has IPS |
| Ptok et al[18] | 2006 | 1043 | Retrospective | Unspecified | Not state | Rectum | No | 75 | 9% | 80.7% LVI +ve | EMVI - No IPS |
A total of 7262 patients in 14 studies were involved in this review. The patient cohort spanned 1938-2006. Six studies were retrospective. Only 6 studies commented on number of pathologists and blinding status[2,6,13-16]. There was clinical heterogeneity in the stains used, the common ones being used were H + E, Gieson’s, elastin and Brominol. All the studies we included examined rectal tumours however 2 papers also incorporated rectosigmoid tumours[14,15].
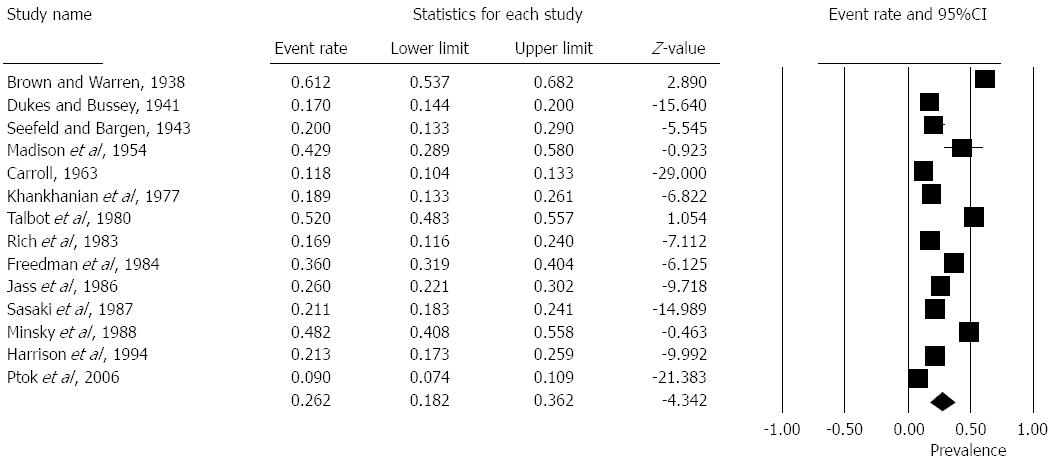
Prevalence of EMVI positive patients ranged from 9%-61% in the studies. The pooled overall prevalence from fourteen studies was 26% [Random effects: Event rate 0.26 (0.18, 0.36), z = -4.3, Q = 787, I2 = 98%] (Figure 2).
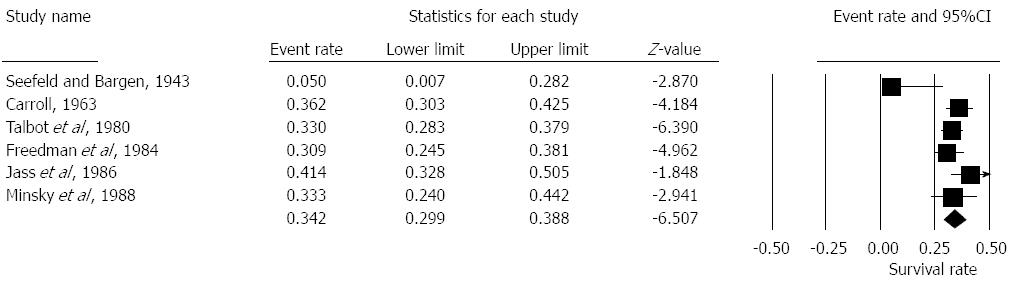
Seven studies reported on 5 year survival rates in patients with EMVI positive histology[4,6,13,14,16-18]. The pooled overall survival was 39.5% [Random effects: Event rate 0.395 (0.29, 0.51), z = -1.9, Q = 58.06, I2 = 90%] (Figure 3).
The results of the present study have shown an association between venous invasion and poor prognosis. Patients that demonstrate evidence of venous invasion have worse overall survival. However the most striking finding is the variation in histopathological detection rates. The prevalence ranges from 9%-61% reflecting the inconsistent nature of recognition and detection. The overall prevalence is around 25% which is consistent with guidance from the Royal College of Pathologists.
The present study has shown that venous invasion generally, and EMVI more specifically, is associated with worse survival outcomes. Despite this, EMVI is not considered a mandatory factor for the use of adjuvant treatment. Indeed the current position on EMVI is variable and has been recently investigated[19]. Many clinicians rely on the EMVI status to make decisions on treatment and it has become a mandatory part of the pathology reporting dataset in the United Kingdom. The reasons behind this variability on behalf of clinicians are unknown. This may be due to inconsistent detection rates shown above or may be that it is rare to find EMVI without the association of more traditional adverse features such as nodal disease or increased T-stage. However, the evolution of rectal cancer management may lead to a change in attitude towards EMVI if a more selective approach is taken to neoadjuvant treatment in the light of clinical trial evidence. For example, the universal policy of irradiating all T3 tumours or any tumour that has local nodal disease may be over-treating a proportion of patients. There is accumulating evidence that not all T3 tumours behave the same and that it is depth of penetration through the mesorectum (T3 sub-stage) that is prognostic[20,21]. Further, in the presence of optimal TME surgery nodal disease may not be prognostic for local recurrence[22]. In these situations whereby early T3 tumours or those with N1 disease may not benefit from neoadjuvant chemoradiotherapy, it may be EMVI which tips the balance towards pre-operative treatment. Another consideration is that stage II tumours are a heterogenous group and it is those which demonstrate EMVI that have a much higher risk of disease recurrence and may ultimately benefit from adjuvant chemotherapy[23].
The more consistent detection rates, found in more recent reports usually use the terminology of “extramural venous invasion”. Messenger et al[24] have offered suggestions which may help pathologists improve detection rates - the use of elastin stains to identify cases where there is uncertainty; and to reference imaging studies such as MRI to guide sampling.
MRI can accurately identify EMVI both before and after CRT[8,25,26]. It has also been shown to detect cases of EMVI which have been “missed” on routine pathology[25]. A further benefit of MRI is that it is able to visualise the entire rectum in-situ whereas the analysis of a small sample of the tumour is dependent on macroscopic assessment by the pathologist in the first instance, to ensure a representative area has been evaluated.
Current multicentre studies such as BACCHUS (Bevacizumab And Combination Chemotherapy in rectal cancer Until Surgery)[27] and MARVEL (Molecular And Radiological EValuation of Extramural venous invasion in RectaL Cancer)[28], may help in resolving some of these issues and future results will be highly anticipated.
In conclusion, the presence of EMVI leads to worse survival outcomes. As detection rates become more consistent, EMVI may be considered as part of risk-stratification in rectal cancer. Standardised histopathological definitions and MRI may improve detection rates in the future.
Extramural venous invasion is a poor prognostic factor in rectal cancer. Many of the historical studies investigating extramural venous invasion (EMVI) did not use a standardised method of detection. With modern techniques in both pathology and radiology we have been able to identify EMVI more consistently and confidently. This has helped clinicians to offer patients optimal treatment.
Despite good historical evidence EMVI remains a contentious prognostic factor with many clinicians outside Europe.
This systematic review adds further high-quality evidence to the clinical importance of EMVI in rectal cancer and may influence future treatment decision making.
EMVI should be specifically sought by radiologists and pathologists to offer patients a more accurate prognosis of rectal cancer and aid clinicians in treatment decision making, specifically for adjuvant chemotherapy.
This manuscript is good paper, well written and well presented.
P- Reviewer: Paydas S, Zhou LM S- Editor: Gong ZM L- Editor: A E- Editor: Zhang DN
| 1. | Michelassi F, Block GE, Vannucci L, Montag A, Chappell R. A 5- to 21-year follow-up and analysis of 250 patients with rectal adenocarcinoma. Ann Surg. 1988;208:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 88] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 2. | Harrison JC, Dean PJ, el-Zeky F, Vander Zwaag R. From Dukes through Jass: pathological prognostic indicators in rectal cancer. Hum Pathol. 1994;25:498-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Blenkinsopp WK, Stewart-Brown S, Blesovsky L, Kearney G, Fielding LP. Histopathology reporting in large bowel cancer. J Clin Pathol. 1981;34:509-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 196] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 4. | Freedman LS, Macaskill P, Smith AN. Multivariate analysis of prognostic factors for operable rectal cancer. Lancet. 1984;2:733-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 132] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Knudsen JB, Nilsson T, Sprechler M, Johansen A, Christensen N. Venous and nerve invasion as prognostic factors in postoperative survival of patients with resectable cancer of the rectum. Dis Colon Rectum. 1983;26:613-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 103] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Talbot IC, Ritchie S, Leighton MH, Hughes AO, Bussey HJ, Morson BC. The clinical significance of invasion of veins by rectal cancer. Br J Surg. 1980;67:439-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 285] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Horn A, Dahl O, Morild I. Venous and neural invasion as predictors of recurrence in rectal adenocarcinoma. Dis Colon Rectum. 1991;34:798-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G. Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer. Br J Surg. 2008;95:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 328] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 9. | Littleford SE, Baird A, Rotimi O, Verbeke CS, Scott N. Interobserver variation in the reporting of local peritoneal involvement and extramural venous invasion in colonic cancer. Histopathology. 2009;55:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Messenger DE, Driman DK, McLeod RS, Riddell RH, Kirsch R. Current practice patterns among pathologists in the assessment of venous invasion in colorectal cancer. J Clin Pathol. 2011;64:983-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Shihab OC, Taylor F, Salerno G, Heald RJ, Quirke P, Moran BJ, Brown G. MRI predictive factors for long-term outcomes of low rectal tumours. Ann Surg Oncol. 2011;18:3278-3284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Borenstein MHL, Higgins J, Rothstein H, editors . Comprehensive Meta-analysis Version 2. NJ: Englewood NJ 2005; . |
| 13. | Seefeld PH, Bargen JA. The spread of carcinoma of the rectum: invasion of lymphatics, veins and nerves. Ann Surg. 1943;118:76-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 14. | Minsky BD, Mies C, Recht A, Rich TA, Chaffey JT. Resectable adenocarcinoma of the rectosigmoid and rectum. II. The influence of blood vessel invasion. Cancer. 1988;61:1417-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Rich T, Gunderson LL, Lew R, Galdibini JJ, Cohen AM, Donaldson G. Patterns of recurrence of rectal cancer after potentially curative surgery. Cancer. 1983;52:1317-1329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Jass JR, Atkin WS, Cuzick J, Bussey HJ, Morson BC, Northover JM, Todd IP. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 1986;10:437-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 414] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Carroll SE. The prognostic significance of gross venous invasion in carcinoma of the rectum. Can J Surg. 1963;6:281-288. [PubMed] |
| 18. | Ptok H, Meyer F, Steinert R, Vieth M, Ridwelski K, Lippert H, Gastinger I. No prognostic impact of isolated lymphovascular invasion after radical resection of rectal cancer--results of a multicenter observational study. Int J Colorectal Dis. 2007;22:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Chand M, Swift RI, Chau I, Heald RJ, Tekkis PP, Brown G. Adjuvant therapy decisions based on magnetic resonance imaging of extramural venous invasion and other prognostic factors in colorectal cancer. Ann R Coll Surg Engl. 2014;96:543-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Merkel S, Mansmann U, Siassi M, Papadopoulos T, Hohenberger W, Hermanek P. The prognostic inhomogeneity in pT3 rectal carcinomas. Int J Colorectal Dis. 2001;16:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 21. | Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29:3753-3760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 501] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 22. | Chand M, Heald RJ, Brown G. The importance of not overstaging mesorectal lymph nodes seen on MRI. Colorectal Dis. 2013;15:1201-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Chand M, Bhangu A, Wotherspoon A, Stamp GW, Swift RI, Chau I, Tekkis PP, Brown G. EMVI-positive stage II rectal cancer has similar clinical outcomes as stage III disease following pre-operative chemoradiotherapy. Ann Oncol. 2014;25:858-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Messenger DE, Driman DK, Kirsch R. Developments in the assessment of venous invasion in colorectal cancer: implications for future practice and patient outcome. Hum Pathol. 2012;43:965-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 25. | Chand M, Evans J, Swift RI, Tekkis PP, West NP, Stamp G, Heald RJ, Brown G. The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer. Ann Surg. 2015;261:473-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 119] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Yu SK, Tait D, Chau I, Brown G. MRI predictive factors for tumor response in rectal cancer following neoadjuvant chemoradiation therapy--implications for induction chemotherapy? Int J Radiat Oncol Biol Phys. 2013;87:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Bevacizumab And Combination Chemotherapy in rectal cancer Until Surgery: A Phase II, Multicentre, Open-label, Randomised Study of Neoadjuvant Chemotherapy and Bevacizumab in Patients with MRI defined High-Risk Cancer of the Rectum. UK Clinical Network Portfolio Database online. 2010, cited 2015-04-04. Available from: http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=12897. |
| 28. | Molecular pathologic and MRI investigation of the prognostic and redictive importance of extramural venous invasion in rectaL cancer. UK Clinical Network Portfolio Database online. 2013, cited 2015-04-04. Available from: http://public.ukcrn.org.uk/search/StudyDetail.aspx?StudyID=13993. |
| 29. | Brown C, Warren S. Visceral metastases from rectal carcinoma. Surg Gynaecol Obstet. 1938;66:611-21. |
| 30. | Dukes CE, Bussey HJ. Venous Spread in Rectal Cancer: (Section of Proctology). Proc R Soc Med. 1941;34:571-573. [PubMed] |
| 31. | Madison MS, Dockerty MB, Waugh JM. Venous invasion in carcinoma of the rectum as evidenced by venous radiography. Surg Gynecol Obstet. 1954;99:170-178. [PubMed] |
| 32. | Khankhanian N, Mavligit GM, Russell WO, Schimek M. Prognostic significance of vascular invasion in colorectal cancer of Dukes’ B class. Cancer. 1977;39:1195-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 33. | Sasaki O, Atkin WS, Jass JR. Mucinous carcinoma of the rectum. Histopathology. 1987;11:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 2.4] [Reference Citation Analysis (0)] |