Published online Jan 28, 2016. doi: 10.3748/wjg.v22.i4.1701
Peer-review started: June 4, 2015
First decision: July 14, 2015
Revised: August 20, 2015
Accepted: October 23, 2015
Article in press: October 29, 2015
Published online: January 28, 2016
Processing time: 235 Days and 10.7 Hours
Nearly 2.5% of cross-sectional imaging studies will report a finding of a cystic pancreatic lesion. Even though most of these are incidental findings, it remains very concerning for both patients and treating clinicians. Differentiating and predicting malignant transformation in pancreatic cystic lesions is clinically challenging. Current evaluation of suspicious cystic lesions includes a combination of radiologic imaging, endoscopic ultrasound (EUS) and cyst fluid analyses. Despite these attempts, precise diagnostic stratification among non-mucinous, mucinous, and malignant cystic lesions is often not possible until surgical resection. EUS-guided needle based confocal laser endomicroscopy (nCLE) for evaluation of pancreatic cysts is emerging as a powerful technique with remarkable potential. Though limited imaging data from 3 large clinical trials (INSPECT, DETECT and CONTACT) are currently the reference standard for nCLE imaging, nonetheless these have not been validated in large studies. The aim of this review article is to review the evolving role of EUS-guided nCLE in management of pancreatic cystic lesions in terms of its significance, adverse events, limitations, and implications.
Core tip: Differentiating and predicting malignant transformation in pancreatic cystic lesions is clinically challenging. Endoscopic ultrasound-guided confocal laser endomicroscopy for evaluation of pancreatic cysts is emerging as a powerful technique with remarkable potential. The feasibility of visualization at the microscopic level enables in differentiating cystic pancreatic lesions, but with certain challenges. In keeping with the gastroenterologist’s motto of ‘seeing is believing’, this technology is poised for continued and expanded research.
- Citation: Krishna SG, Lee JH. Appraisal of needle-based confocal laser endomicroscopy in the diagnosis of pancreatic cysts. World J Gastroenterol 2016; 22(4): 1701-1710
- URL: https://www.wjgnet.com/1007-9327/full/v22/i4/1701.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i4.1701
Approximately 2.5% of cross sectional imaging studies will detect pancreatic cysts[1,2]. In patients over the age of 70, this number can be as high as 10%[1]. Even though most of these are incidental findings, nevertheless it remains very concerning for both patients and the clinicians. This is in large part due to the fact that pancreatic cancer being the 4th leading cause of cancer-related death in the United States has dismal treatment outcomes with 5-year survival being less than 5%[3]. The foremost reason for the low survival is difficulty in detection of its earliest stages.
The scenario has changed in the last two decades. Benign inflammatory pancreatic pseudocysts were the most common pancreatic cysts, however with advent of sophisticated imaging techniques and discovery of mucinous neoplastic pancreatic lesions, cysts with neoplastic potential at small (< 2 cm) sizes are frequently detected. In addition, endoscopic ultrasound (EUS)-guided fine needle aspiration (FNA) has evolved and established its role as the diagnostic procedure of choice for pancreatic lesions (solid and cystic). The overall complication rate remains low (1%-2%), which is similar to radiology assisted FNA biopsies[4].
The common differential diagnoses for incidental cystic pancreatic lesions (CPL) include pseudocysts, serous cystadenomas (SCA), mucinous cystic lesions [categorized into mucinous cystic neoplasms (MCN), branch-duct (BD)-intraductal papillary mucinous neoplasm (IPMN), and main-duct (MD)-IPMN], and cystic-neuroendocrine tumors. Current guidelines recommend surgical resection for all large (> 4 cm) MCNs (malignancy risk of 17.5%), all patients with MD-IPMN (malignancy risk of 61%) and BD-IPMNs with worrisome features (≥ 3 cm, thick cyst wall, mural nodules and positive cytology; malignancy risk of 25%)[5]. To evaluate cysts, a combination of clinical history, demographics, imaging and endosonographic features, cytology, and cyst fluid carcinoembryonic antigen (CEA) and amylase are used to identify mucinous cysts[5]. Further sub-typing of mucinous cysts is also possible with some limitations. Distinguishing MCN from BD-IPMN can prove to be difficult. Unusual cysts like, macrocystic SCA, atypical pseudocysts and lymphoepithelial cysts can pose challenges[6]. A solitary CPL begins as a diagnostic challenge and sometimes remains so after completion of available investigations. Cyst fluid molecular analyses involving KRAS and GNAS mutations, and microRNAs have been studied, but there is no recommendation for routine use[7-10]. While surgical resection is the choice of treatment for symptomatic cysts, frequently, a decision for surgical approach is taken for asymptomatic cysts where either a conclusive diagnosis was not reached or where a BD-IPMN lacking “worrisome features” was still concerning[5]. Pancreatic surgery for cystic neoplasms is a major operative procedure that carries significant morbidity. Even at high volume specialty centers, the morbidity rate remains concerning at 20%-40%[11,12].
There is a problem of resource utilization that involves patients seeking specialized care for all types of pancreatic cysts while some undergo premature and unrequited surgery. This is compounded by significant differences in technique and elucidation of cross sectional imaging, and inter-operator variations in technique, practices, and interpretation of endosonographic studies. At the crux of these issues involving pancreatic cysts, the challenge of achieving an accurate diagnosis and when diagnosed, risk stratification of mucinous pancreatic cysts, makes management difficult. A recent comprehensive technical review by the American Gastroenterology Association (AGA) reviewed all the available literature with an inference that there was insufficient evidence to make decisive recommendations based on patient risk vs benefit[10]. The low quality of evidence due to the dynamic and evolving science of pancreatic cysts contributed to the fact that seven of the ten AGA guidelines were conditional (low quality of evidence)[13].
The technical review summarized the pooled data from available studies where surgical histopathology was available: For predicting malignancy, a cyst size of > 3 cm had a sensitivity and specificity of 74% and 49% respectively, a dilated pancreatic duct reached a sensitivity and specificity of 32% and 80% respectively, and presence of an intracystic solid component had the most specificity of 91% but at the cost of a low sensitivity of 48%[10]. For EUS guided cyst aspiration, the review summarized data from 12 studies where histopathology was available: Cyst amylase concentration of < 250 U/L indicated either a SCA or mucinous cyst with a sensitivity of 44% and specificity of 98%. A CEA level < 5 ng/mL predicted pseudocyst or SCA with a sensitivity of 50% and a specificity of 95%[10]. While no absolute value of CEA predicted malignancy, values exceeding 800 ng/mL reached a high specificity of 98% (sensitivity 48%) in predicting a mucinous lesion. Cytology of the cyst fluid also performed poorly. In 11 studies utilizing histopathology as final diagnosis, the pooled sensitivity and specificity to differentiate mucinous from non-mucinous lesions were 63% and 88% respectively. Further cytology detected malignancy in only 48% of mucinous cancers[10].
While the current guidelines recommend resection of symptomatic cysts, the evidence however continues to remain unclear. The AGA technical review and guidelines does not support symptom based surgical resection, although symptoms should be considered with other cyst features in the decision making process[10,13].
Although there are multiple studies involving CPLs, most of these investigations do not provide histopathology as the standard for comparison in evaluating sensitivities and specificities. The overall trend including the recent AGA guidelines is more conservative management of CPLs. This might further limit the availability of “gold-standard” histopathology for CPLs; thus relying on “expert consensus”. A majority of the data involving the novel technology of confocal laser endomicroscopy (CLE) in evaluating CPLs is hence gleaned from consensus rather than diagnostic histopathology.
CLE is a real-time laser-assisted microscopic imaging of tissue where the system provides tissue-sequences with a high resolution (1-3.5 μm) facilitating in vivo histopathology. A low power laser illuminates the tissue through optical fibers in a miniprobe, this light is absorbed by fluorophores (either naturally occurring or applied), and the reflected fluorescence is transferred back to the laser-scanning unit through the miniprobe. These miniprobes come in various sizes with differing resolutions and field of view. The CLE probes are currently manufactured by Cellvizio, Mauna Kea Technologies, Paris, France. A fluorescent contrast is necessary for CLE imaging of tissue that does not contain naturally occurring fluorophores. Intravenous fluorescein is the most commonly used contrast agent for CLE imaging. Fluorescein stains vessels and delineates tissue structures. Since the nuclei are not stained, they appear as dark spots. By providing in vivo microscopic-resolution images of mucosal glands, goblet cells, and capillary patterns that may highlight dysplastic changes, CLE has the potential to replace the role of biopsies in specimen acquisition[14].
Initially, there were two CLE systems, an endoscope-integrated system and a probe-based system. Following several proof-of-concept studies, the former endoscope-based CLE is no longer commercially available. The more recent probe-based CLE (pCLE) uses a separate unit outside the endoscope, which emits the laser required for the imaging. This miniprobe can be introduced through the working channel of any endoscope, and thus we have the GastroFlexTM, CholangioFlexTM, and ColoFlexTM high-definition probes for respective parts of the gastrointestinal tract. For imaging using pCLE, inter-or intra-observer agreement has been largely favorable in the esophagus and colon[15,16].
A novel needle-based CLE (nCLE) miniprobe (AQ-Flex 19; Mauna Kea Technologies) has been developed that can be used during EUS. It is compatible with the 19-gauge (g) FNA needle. The AQ-Flex miniprobe has 10000 optical fibers, a diameter of 0.85 mm, a field of view of 320 μm, a lateral resolution of 3.5 μm, and a length of 4 m.
Cystoscopy and direct visualization of the pancreatic cyst has also been achieved using through-the-needle SpyGlass fiberoptic probe (Boston Scientific, Natick, Mass)[17]. Compared to the AQ-Flex nCLE probe, cystoscopy imaging using a Spyglass probe produced a suboptimal image that was further compromised by thick or cloudy fluid in some cysts. The cyst size was also an issue since the focal length of the Spyglass fiber (4 to 7 mm) was much larger than that of the nCLE probe (40 to 70 μm)[17].
Patients who are referred for EUS evaluation of large (≥ 2 cm) pancreatic cysts can undergo EUS-guided nCLE before the standard process of EUS-FNA. A 19-g FNA needle is preloaded with the AQ-Flex miniprobe. At least 2 mm to 3 mm of the nCLE probe should be advanced beyond the needle tip during pre-loading. The probe position is then secured by using a locking device that attaches the probe to the inlet of the needle biopsy channel.
After a comprehensive EUS examination of the pancreas, the cyst of interest is oriented for interrogation. A single pass of the preloaded 19-g FNA needle is then performed into the pancreatic cyst. The tip of the AQ-Flex miniprobe is advanced with the needle under EUS-guidance until there is contact with the intracystic epithelium. Fluorescein (2.5 to 5 mL of 10% fluorescein sodium) is intravenously injected immediately prior to CLE imaging.
The nCLE probe is gently positioned against the cyst wall (making contact without pressure) and multiple areas of the cyst wall are imaged in a fan-like distribution by using the elevator. The location of the cyst, position of the echoendoscope, and the size of the cyst limit the area covered. Aggressive maneuvering of the needle should be avoided to minimize risk of pancreatitis. While transitioning from one area of the cyst to another, it is preferable to withdraw the probe away from the cyst wall instead of grazing the cyst epithelium. Intracystic endomicroscopic videos are then captured for 2 to 5 min with permissible angulation of the 19-g FNA needle. Anecdotally longer video acquisition with excessive manipulation can increase risk of pancreatitis. Following this the AQ-Flex probe is gently withdrawn from the 19-g FNA needle. A syringe with negative suction is then attached to the proximal end of the needle for cyst aspiration. As per standard practice, the cyst fluid is sent for fluid analysis (CEA and amylase) and cytology. Prophylactic antibiotics are administered during and after the procedure.
All nCLE images are reviewed during the procedure (real time interpretation). However, comprehensive evaluation is feasible during post-procedure review of nCLE video files where representative images and video sequences can be selected for extraction and storage. Due to the high resolution of nCLE, real time video appears fast paced with rapid shifting of image sequences. A post-procedure play and pause approach counters this and allows for detailed review. A dedicated software provided by Mauna Kea Technologies (Cellvizio Viewer) can be downloaded from their website (compatible with both Mac and Windows operative software). This application provides multiple tools including measurement of structures, editing, and video format conversion.
The feasibility of EUS-guided nCLE was first demonstrated in animal models with depiction of in vivo histology from various abdominal organs (pancreas, spleen, liver and lymph node) after intravenous injection of fluorescein[18]. In this study, a total of 10 porcine models were examined with nCLE where the probe was inserted through the EUS-FNA needle. Organ biopsies were obtained for histologic evaluation and confirmation. Technical feasibility was demonstrated with in vivo image acquisition of histology grade resolution and suitable quality.
The first human pilot study demonstrated feasibility of EUS-guided nCLE for pancreatic lesions. A nCLE probe was used through a 19-g EUS-FNA needle in 16 cysts and 2 solid lesions of the pancreas. Technical feasibility was demonstrated in 17 of 18 cases[19]. A final diagnosis was established on either histologic analysis of a surgical specimen or positive cytology of a FNA specimen. Images of adequate quality were acquired in 10 patients. Post-procedure pancreatitis (requiring hospitalization) as an adverse event was observed in two patients (11.1%), tentatively attributable to longer nCLE image acquisition time.
The next study involving nCLE targeted development of descriptive criteria for image interpretation and classification of the nCLE findings for pancreatic masses and lymph nodes. The study included 11 patients (pancreatic masses: 4, CPLs: 3, and lymph nodes: 4)[20]. A non-malignant IPMN lesion was observed to display finger-like projections representing villous changes. Pancreatic malignancy demonstrated large dark clumps and leakage of fluorescein. Malignant lymph nodes were also noted to have large dark clumps and significant leakage of dye. In both the pancreatic mass and malignant lymph node, dye leakage was correlated with neovascularization characteristic of malignancy.
An international, multicenter pilot trial using in vivo CLE in the pancreas with endosonography of cystic tumors (INSPECT) was the next study to evaluate diagnostic potential and establish a safety profile[21]. A total of 66 patients with pancreatic lesions were evaluated of which 14 (21.2%) had surgical histopathology for confirmation (Table 1). Epithelial villous structures as revealed by nCLE were associated with neoplastic cystic lesions [sensitivity 59%, specificity 100%, positive predictive value (PPV) 100%, negative predictive value (NPV) 50%]. The study arrived at a few conclusions including the complementary role of nCLE imaging in diagnosis of cystic lesions and that the finding of villous or finger like structures is suggestive of IPMN type lesion. The rate of acute post procedural pancreatitis was 3% (one mild and the other moderate severity), a decrease compared to the author’s prior study mostly due to the limitation of imaging time to 10 min. Sampling error was recognized either due to mixed type of IPMN or imprecise probe placement. There were no reported adverse events to intravenous fluorescein.
Following this, the next clinical trial was DETECT (Diagnosis of Pancreatic Cysts: EUS, Through-the-Needle Confocal Laser Endomicroscopy and Cystoscopy Trial)[17]. The objective of this study was to assess the feasibility, safety, and diagnostic yield of a combination of cystoscopy using Spyglass and nCLE in the diagnosis of CPLs. This was a single center study where a preceding “cystoscopy” was performed using Spyglass followed by cyst interrogation with a nCLE probe. A total of 30 patients with pancreatic cystic lesions were studied where 2 (6%) had surgical histopathology. The authors studied the association of Spyglass-assisted cystoscopy and nCLE imaging with clinical diagnosis. In 18 high-certainty cases (2 independent investigators strongly agreed on the concordant diagnosis based on clinical presentation, image findings on EUS, CT, or MRI, fluid analysis, and cytology), nCLE alone had a sensitivity of 80%, specificity of 100%, PPV of 100%, NPV of 80%, and accuracy of 89% for diagnosis of mucinous cysts (Table 1). The sensitivity reached a 100% with the combination of Spyglass assisted cystoscopy and nCLE imaging. Rate of post procedure pancreatitis was 6.6% (2 of 30) and these patients required 4 to 5 d of hospitalization. No intravenous fluorescein-related adverse events were observed.
The most recent published results come from the Clinical evaluation of nCLE in the lymph nodes along with masses and cystic tumors of the pancreas (CONTACT) study. This is a multi-center study from France and was conducted in two phases[22]. The first phase involved identification of specific criteria for the characterization of cystic lesions in the pancreas, and retrospective validation of these criteria. Phase 2 (ongoing CONTACT 2 study) involves prospective validation of nCLE criteria for pancreatic cysts. During phase 1 of the study, a new nCLE pattern called “superficial vascular network” was identified which was a unique feature of SCA[22]. For nCLE-based diagnosis of SCA, the sensitivity, specificity, PPV and NPV were 69%, 100%, 100%, 82% and 87% respectively (Table 1). The criterion of superficial vascular network was validated in 31 patients. Among these 7 (22.5%) had surgical histopathology for confirmation of diagnosis. Rate of procedure related pancreatitis was 3.2% (1 of 31 patients). This adverse event was of mild severity. There were no complications related to intravenous fluorescein.
The data from CONTACT study was utilized to investigate the technical feasibility of EUS-guided nCLE[23]. The study aims also included assessment of EUS-nCLE related complications. The procedure was feasible in 131 (93% of 141) of patients. Significant technical limitation was observed for lesions in the head and uncinate process of the pancreas necessitating interrogation of the pancreatic cyst from the second portion of the duodenum. This being the largest number of patients evaluated by EUS-guided nCLE, post-procedural acute pancreatitis was observed in 2 (1.45%) patients. This is equal to current risk of acute pancreatitis following routine EUS-FNA with smaller caliber needles (22 g or 25 g)[24].
Multiple other studies with limited number of patients have validated common criteria for diagnosis of IPMNs, SCAs, MCNs and pseudocysts (Table 2). Notably, the overall specificity for diagnosis of IPMN type lesion when finger like papillae were observed was 100% in four of these studies[25-28]. The general consensus from these abstracts was that EUS-guided nCLE was safe, feasible, and impacted management of pancreatic cysts; albeit, the authors from various abstracts suggested that multicenter and/or studies with larger number of patients are necessary for validation of representative nCLE images.
| Study objectives | Patient, n | Pancreatitis and other complications | Accuracy data | Conclusions | |
| Napoleon et al[23] | To evaluate feasibility and assess complication rate of nCLE in CPLs | Total: 141 | Minor pancreatitis: 2 (1.45%) | NA | Main technical limitation observed when cyst interrogation requires approach through second part of the duodenum |
| CONTACT study | Technical feasibility: 93% (131 patients) | ||||
| DDW 2015 | Intracystic bleeding without extravasation - 10% | ||||
| Prospective study | |||||
| Kadayifci et al[28] | To assess the safety, feasibility and diagnostic value of EUS guided nCLE for CPLs | Total: 11 | No pancreatitis reported | The sensitivity, specificity, and accuracy for mucinous cyst (findings of papillae) were 57%, 100%, and 70% respectively | nCLE for pancreatic cysts was safe and feasible. nCLE has low sensitivity but high specificity for mucinous cysts |
| DDW 2015 | Procedure successful: 10 | ||||
| Retrospective | |||||
| Bertani et al[26] DDW 2015 | To validate prior described nCLE findings typical of IPMN lesions | Total: 9 | No pancreatitis reported | Finger-like projections were observed in 7 of 7 IPMN lesions | nCLE imaging identified common criteria for diagnosis of IPMN |
| Retrospective | |||||
| Krishna et al[25] | To validate prior described diagnostic nCLE imaging patterns | Total: 32 | Pancreatitis: 3.1% (1 patient) | Sensitivity, specificity, and accuracy for IPMN were 89%, 100%, and 96% respectively | Promising technology providing diagnosis of mucinous cysts |
| DDW 2015 | Inclusion: 26 | ||||
| Surgery: 7 (27%) | |||||
| Retrospective | |||||
| Sensitivity, specificity, and accuracy for SCA were 90%, 100%, and 96% respectively | |||||
| Sejpal et al[27] | To validate prior described nCLE findings for diagnosis of pancreatic cysts | Total: 19 | No pancreatitis reported | Sensitivity, specificity, and accuracy for IPMN were 80%, 100%, and 95% respectively | Possibly treating pseudocysts after nCLE examination bypass fluid analysis |
| DDW 2015 | |||||
| Retrospective | |||||
| Joshi et al[36] | To validate available nCLE criteria for diagnosis of CPLs | Total: 16 | No pancreatitis reported | Improved confidence in diagnosing type of cyst in 80% of patients | Can impact in management and avoiding unnecessary surgeries for pancreatic cysts |
| ACG 2014 | |||||
| Napoleon et al[37] | To investigate and describe nCLE characteristics of CPLs | Total: 31 | No pancreatitis reported | NA | nCLE images could help in the differentiation of IPMNs, MCN and SCA |
| CONTACT study | Inclusion: 16 | ||||
| DDW 2014 | |||||
| Prospective |
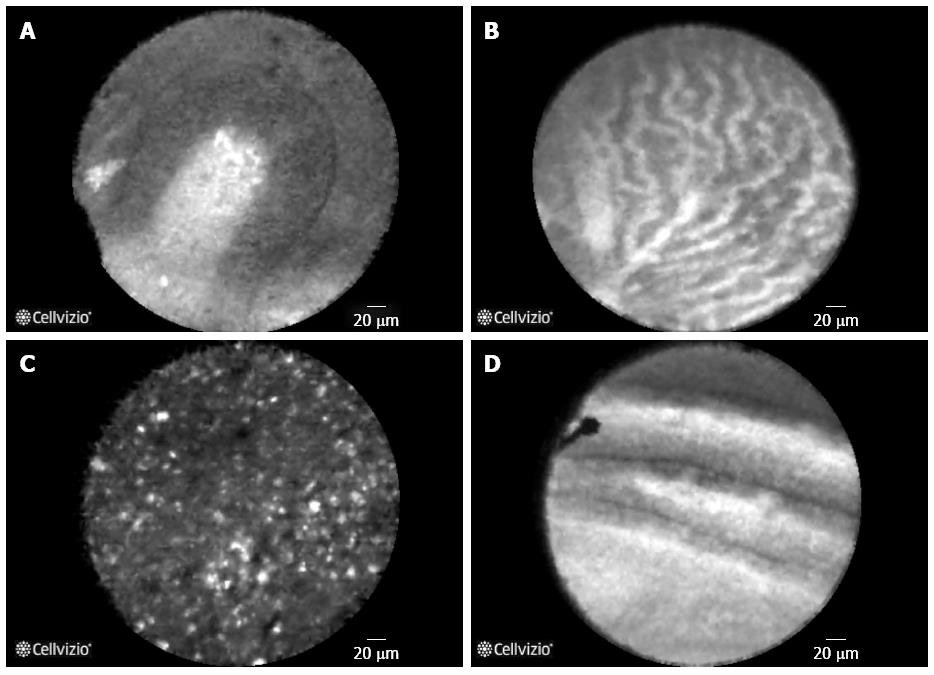
Based on currently published literature[17,19,21,22,29] and our experience, Table 3 summarizes the pancreatic structures visualized during EUS-guided nCLE examination of pancreatic cystic lesions. Table 4 summarizes the most common types of pancreatic cysts and the associated nCLE findings. Figure 1 are examples demonstrating the different types of pancreatic cysts. While the evidence for nCLE guided diagnosis of BD-IPMN and SCA has accrued in recent studies, further substantiation with larger studies and ex vivo[29] modeling is desired. The specificity for diagnosis of either BD-IPMN or SCA is high (nearing 100%) when finger like papillae or “superficial vascular network” pattern (respectively) are visualized. In the absence of visualizing these recognized image patterns, the sensitivity for diagnosis of the cystic lesion remains low (60% to 80%). For BD-IPMN lesions, distribution of the papillary epithelium is patchy and the limited intra-cystic mobility might restrict and prevent imaging the involved area of the cystic lesion. For SCA, the pattern of superficial vascular network was not observed in nearly 1/3rd of the cases. This could again be due to the limited range of movement of the nCLE probe, which is further compromised by absence of the vascular network in certain area of the cyst.
| Parenchymal structures | |
| Blood vessels | Thin or thick white bands; networking of blood vessels |
| Acinar cells | Dark lobular structures |
| Adipose cells | Grey oval structures |
| Pancreatic ductal epithelium | Thin grey bands |
| Fibrous strands | Ultrathin bright bands |
| Epithelial structures | |
| Villous structures | Finger-like papillary projections, dark ring with white core (cross section) |
| Wall (fibrous) | Paucicellular, avascular wall |
| Neoplasia | Dark aggregates of cells |
| Cyst luminal structures | |
| Inflammatory cells | Clusters of bright, floating, heterogeneous particles |
| Red blood cells | Small black particles |
| Debris | Bright white fixed spots or large dark round floating particles with varying sizes |
| Intraductal papillary mucinous neoplasm (Figure 1A) | |
| Finger like projections | Central fibrovascular core and overlying epithelium viewed in parallel |
| Dark rings | Central fibrovascular core and overlying epithelium viewed in transection |
| Parallel thick bands | Alternating papillae with central fibrovascular core and overlying epithelium |
| Absence of “superficial vascular network” | |
| Absence of “bright, floating, heterogeneous particles” | |
| Serous cystadenoma (Figure 1B) | |
| “Superficial vascular network” | Dense and tortuous appearing network of multiple blood vessels under cuboidal epithelium. Observed in both macrocystic and septa separating microcysts |
| Multiple blood vessels | |
| Absence of finger like projections | |
| Pseudocyst1 (Figure 1C) | |
| Clusters of bright, floating, heterogeneous particles | |
| Absence of finger like projections | |
| Mucinous cystadenoma1 (Figure 1D) | |
| Solitary epithelial bands | Epithelium (columnar, tall cells) lining the cysts |
| Large caliber blood vessels | |
| Clusters of bright particles | Epithelial cells and inflammatory elements |
The criteria for diagnosis of pseudocysts have not been formally validated in published literature. Our experience and current available evidence is summarized in Table 4. A detailed history, prior episodes of pancreatitis, review of prior cross-sectional imaging studies, fluid analysis, and cytology might augment diagnostic suspicion for suspected pancreatic pseudocysts. It is typically rare that a pseudocyst presents as a solitary cystic lesion in the absence of a suggestive history.
Diagnosing MCN also needs validation with clinical trials. The small number of patients identified in currently published studies and meeting presentations suggest that the presence of a single band like epithelium could be indicative of MCN. The characteristic “ovarian stroma” seen on histopathology has not been characterized by EUS-nCLE. Like pseudocysts, MCNs also demonstrate large caliber blood vessels, albeit without the distinctive vascular network of SCA.
Endomicroscopy features of other rare types of cystic lesions including lymphoepithelial cysts, cystic neuroendocrine tumors, retention cysts, and cystic degeneration of metastatic lesions need continued exploration.
A surgical histopathology as diagnostic gold standard was not universally available. Combining all three trials (INSPECT, CONTACT, and DETECT), only 20% (23 of 115) patients underwent surgical resection of the pancreatic lesion. With recent guidelines[5,13] stressing the role of watchful waiting in otherwise operative candidates (as per prior guidelines)[30], surgery for non-malignant pancreatic cystic lesions is not as frequently performed. A confirmatory diagnosis was thus not available and investigators had to resort to FNA cytology, imaging studies, patient follow-up, and consensus of experts.
Combining all three major trials (INSPECT, CONTACT, and DETECT), the rate of post-procedural pancreatitis was 4.3%. The highest risk was with the DETECT study (6.6%) especially since the procedure involved longer needle access time for Spyglass cystoscopy and nCLE imaging. The latest update from the CONTACT study evidences a much lower risk of acute pancreatitis. For the largest number of patients evaluated by EUS-guided nCLE (n = 141), post-procedural acute pancreatitis was observed in only 2 (1.45%) patients[23]. The prior reported risk of pancreatitis for 22-g and/or 25-g needles in cystic lesions is 2.4%[24]. The current nCLE miniprobe requires a 19-g needle. The outer diameter of a standard 19-g needle is 1.067 mm. Comparatively; a standard 22-g needle has an outer diameter of 0.718 mm. Thus a 19-g needle represents an approximate 48.6% increase in outer diameter over a 22-g needle. Although, prior studies comparing a 19-g to 22-g and 25-g needles for FNA of solid pancreatic lesions have not shown any increase in the risk of post-procedural pancreatitis, yet this doesn’t reflect the same risk when aspirating cystic lesions[31,32]. Furthermore, manipulation of the needle with the elevator of the linear echoendoscope combined with possible friction induced effect by the impact of the tip of the probe grazing the intracystic epithelium can also, theoretically increase risk of pancreatitis. The authors of the DETECT study recommended limiting both the needle access time as well as the amount of needle movement within the cyst[17].
In a randomized trial, the technical success rate for sampling pancreatic head masses was significantly lower for the 19-g needle than for the 22-g needle (80.8% vs 100%)[32]. In contrast, a recent study comparing the 19-g and 25-g needles (Expect, Boston Scientific Corporation, Natick, MA, United States) demonstrated that solid pancreatic lesions were successfully sampled irrespective of location, including patients crossed over from the 25-g cohort[31]. In the INSPECT and CONTACT trials, there were a total of 3 patients with cysts in the uncinate process. These can be technically challenging with a 19-g needle since this location necessitate access through the second part of the duodenum. In the latest update from the CONTACT study[23] involving evaluation of technical feasibility among 141 patients, 3 lesions were located in the uncinate and 64 in the head of the pancreas. Needle access through the second part of the duodenum was required in 4% of the patients. There were 3 (2%; 1 lesion in uncinate, 2 in head of the pancreas) technical failures of needle puncture and all of these involved attempts for needle access through the second part of the duodenum. In effect, FNA of the uncinate lesions with a 19-g needle from the second duodenum could represent a limitation.
Both the INSPECT and DETECT studies did not have independent observers and thus lacked testing for interobserver variability. In the phase I of the CONTACT study, four blinded independent observers underwent a training session and independently reviewed the recorded nCLE video sets. The interobserver agreement for the criterion of superficial vascular network was significant (κ = 0.77, 95%CI: 0.55-0.99)[22]. In the recently concluded DDW meeting, investigators utilized the data from CONTACT study for external retrospective validation of diagnostic nCLE criteria for pancreatic cysts[33]. Five independent gastroenterologists underwent a teaching session by an nCLE expert. Following this 31 nCLE sequences were reviewed. Interobserver agreements were kappa values of 0.71 (for SCA), 0.65 (for MCN), and 0.9 (for pseudocysts) respectively. Our experience with nCLE reveals that there is a short learning curve to facilitate image interpretation. There are two aspects for image analysis. The first, is that the endosonographer should get comfortable with maneuvering the 19-g needle for appropriate “image acquisition”. Following this, the endosonographer should interpret the recorded nCLE video. Familiarity with known patterns facilitates image acquisition since every minute expended during nCLE examination is “precious” in terms of exposing the patient for higher risk of procedure related pancreatitis. Our personal experience relates to improved and accelerated learning after listening to multiple sessions by nCLE experts at local and national level conferences.
While we need larger studies to validate nCLE findings for IPMNs, SCAs, MCNs and pseudocysts, the focus should also be on identifying additional image patterns or improve nCLE-imaging techniques to increase the sensitivity of diagnosis. Ex vivo examination of pancreatic cysts by higher resolution CLE probes might provide reference image patterns for in vivo image interpretation[29]. The images thus acquired will serve as reference standard (image atlas) for future EUS-based AQ-flex nCLE evaluation. This could potentially lead to identification of patterns suggestive of higher grades of dysplasia in IPMN and MCN. Furthermore, identification of subtypes of IPMN by nCLE imaging can lead to additional risk stratification given the fact that the morphological type is an independent predictor of patient's prognosis[34]. Enhanced CLE probe technology providing for a possible 22-g needle based device resulting in an approximate 49% reduction in needle outer diameter might improve needle maneuverability thus increasing sensitivity. This may also facilitate larger studies with wider acceptance among endosonographers. More importantly, the decreased size of the needle can hopefully reduce the risk of procedure-associated pancreatitis.
In our opinion, nCLE probe compatibility with a 22-g needle would perhaps provide the utmost advance in terms of diagnostic capability and patient safety. Identification of safe, additional fluorescent markers with preferential binding to areas of higher grades of dysplasia that can be detected by the nCLE probe might also provide a big impetus to further nCLE research[35].
In conclusion, EUS-guided nCLE appears to be a convincing minimally invasive process to diagnose and risk stratify pancreatic cystic lesions. In keeping with the gastroenterologist’s motto of “seeing is believing”, this technology is poised for continued and expanded research. The feasibility of visualization at the microscopic level enables in differentiating CPL, but with certain challenges. These include sampling error, interobserver variability, technical limitations, risk of pancreatitis, and the attendant learning curve. The usage, currently, is still limited to select tertiary referral centers with expectations of a broader acceptance among endosonographers with growing evidence of clinical applicability and technical progress.
P- Reviewer: Crippa S, Hanson JA, Maraveyas A S- Editor: Yu J L- Editor: A E- Editor: Ma S
| 1. | de Jong K, Nio CY, Hermans JJ, Dijkgraaf MG, Gouma DJ, van Eijck CH, van Heel E, Klass G, Fockens P, Bruno MJ. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol. 2010;8:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 376] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 2. | Laffan TA, Horton KM, Klein AP, Berlanstein B, Siegelman SS, Kawamoto S, Johnson PT, Fishman EK, Hruban RH. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol. 2008;191:802-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 724] [Cited by in RCA: 658] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 3. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 4. | Carrara S, Arcidiacono PG, Mezzi G, Petrone MC, Boemo C, Testoni PA. Pancreatic endoscopic ultrasound-guided fine needle aspiration: complication rate and clinical course in a single centre. Dig Liver Dis. 2010;42:520-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Tanaka M, Fernández-del Castillo C, Adsay V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB, Schmidt CM, Shimizu M, Wolfgang CL, Yamaguchi K, Yamao K; International Association of Pancreatology. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012;12:183-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1714] [Cited by in RCA: 1614] [Article Influence: 124.2] [Reference Citation Analysis (0)] |
| 6. | Park WG, Mascarenhas R, Palaez-Luna M, Smyrk TC, O’Kane D, Clain JE, Levy MJ, Pearson RK, Petersen BT, Topazian MD. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas. 2011;40:42-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Farrell JJ, Fernández-del Castillo C. Pancreatic cystic neoplasms: management and unanswered questions. Gastroenterology. 2013;144:1303-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 8. | Farrell JJ, Toste P, Wu N, Li L, Wong J, Malkhassian D, Tran LM, Wu X, Li X, Dawson D. Endoscopically acquired pancreatic cyst fluid microRNA 21 and 221 are associated with invasive cancer. Am J Gastroenterol. 2013;108:1352-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Ryu JK, Matthaei H, Dal Molin M, Hong SM, Canto MI, Schulick RD, Wolfgang C, Goggins MG, Hruban RH, Cope L. Elevated microRNA miR-21 levels in pancreatic cyst fluid are predictive of mucinous precursor lesions of ductal adenocarcinoma. Pancreatology. 2011;11:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 10. | Scheiman JM, Hwang JH, Moayyedi P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:824-848.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 295] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 11. | Valsangkar NP, Morales-Oyarvide V, Thayer SP, Ferrone CR, Wargo JA, Warshaw AL, Fernández-del Castillo C. 851 resected cystic tumors of the pancreas: a 33-year experience at the Massachusetts General Hospital. Surgery. 2012;152:S4-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 278] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 12. | Gaujoux S, Brennan MF, Gonen M, D’Angelica MI, DeMatteo R, Fong Y, Schattner M, DiMaio C, Janakos M, Jarnagin WR. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg. 2011;212:590-600; discussion 600-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 13. | Vege SS, Ziring B, Jain R, Moayyedi P. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819-822; quize12-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 759] [Article Influence: 75.9] [Reference Citation Analysis (1)] |
| 14. | Nakai Y, Isayama H, Shinoura S, Iwashita T, Samarasena JB, Chang KJ, Koike K. Confocal laser endomicroscopy in gastrointestinal and pancreatobiliary diseases. Dig Endosc. 2014;26 Suppl 1:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Wallace MB, Sharma P, Lightdale C, Wolfsen H, Coron E, Buchner A, Bajbouj M, Bansal A, Rastogi A, Abrams J. Preliminary accuracy and interobserver agreement for the detection of intraepithelial neoplasia in Barrett’s esophagus with probe-based confocal laser endomicroscopy. Gastrointest Endosc. 2010;72:19-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Kuiper T, Kiesslich R, Ponsioen C, Fockens P, Dekker E. The learning curve, accuracy, and interobserver agreement of endoscope-based confocal laser endomicroscopy for the differentiation of colorectal lesions. Gastrointest Endosc. 2012;75:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Nakai Y, Iwashita T, Park do H, Samarasena JB, Lee JG, Chang KJ. Diagnosis of pancreatic cysts: EUS-guided, through-the-needle confocal laser-induced endomicroscopy and cystoscopy trial: DETECT study. Gastrointest Endosc. 2015;81:1204-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Becker V, Wallace MB, Fockens P, von Delius S, Woodward TA, Raimondo M, Voermans RP, Meining A. Needle-based confocal endomicroscopy for in vivo histology of intra-abdominal organs: first results in a porcine model (with videos). Gastrointest Endosc. 2010;71:1260-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Konda VJ, Aslanian HR, Wallace MB, Siddiqui UD, Hart J, Waxman I. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc. 2011;74:1049-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Giovannini M, Caillol F, Poizat F, Bories E, Pesenti C, Monges G, Raoul JL. Feasibility of Intratumoral Confocal Microscopy under Endoscopic Ultrasound Guidance. Endosc Ultrasound. 2012;1:80-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Konda VJ, Meining A, Jamil LH, Giovannini M, Hwang JH, Wallace MB, Chang KJ, Siddiqui UD, Hart J, Lo SK. A pilot study of in vivo identification of pancreatic cystic neoplasms with needle-based confocal laser endomicroscopy under endosonographic guidance. Endoscopy. 2013;45:1006-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 143] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 22. | Napoléon B, Lemaistre AI, Pujol B, Caillol F, Lucidarme D, Bourdariat R, Morellon-Mialhe B, Fumex F, Lefort C, Lepilliez V, Palazzo L, Monges G, Filoche B, Giovannini M. A novel approach to the diagnosis of pancreatic serous cystadenoma: needle-based confocal laser endomicroscopy. Endoscopy. 2015;47:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Napoleon B, Pujol B, Palazzo L, Lucidarme D, Caillol F, Giovannini M. Su1712 Feasibility and Complications Rate of Needle-Based Confocal LASER Endomicroscopy (nCLE) in Pancreatic Cysts: Preliminary Results of a Multicentric Prospective Study. Gastrointest Endosc. 2015;81:AB387. [DOI] [Full Text] |
| 24. | de Jong K, Poley JW, van Hooft JE, Visser M, Bruno MJ, Fockens P. Endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions provides inadequate material for cytology and laboratory analysis: initial results from a prospective study. Endoscopy. 2011;43:585-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 25. | Krishna SG, Swanson B, Muscarella P, Bloomston M, Pidlaoan VP, El-Dika S, Walker JP, Arsenescu R, Hart P, Conwell D. Mo1461 Endoscopic Ultrasound (EUS)-Guided Needle Based Confocal LASER Endomicroscopy (nCLE) for Diagnosis of Cystic Pancreatic Lesions (CPLs): Implications for Management. Gastrointest Endosc. 2015;81:AB428-AB429. [DOI] [Full Text] |
| 26. | Bertani H, Pigò F, Mirante VG, Caruso A, Manno M, Mangiafico S, Conigliaro R, Barbera C. Tu1692 In-Vivo Identification of Intraductal Papillary Mucinous Neoplasia (IPMN) With Needle-Based Confocal Endomicroscopy. Gastrointest Endosc. 2015;81:AB561. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Sejpal DV, Haluszka O, Gress FG, Woods KE, George B, Vegesna AK. Tu1661 EUS Guided Needle Based Confocal LASER Endomicroscopy (nCLE): Preliminary Results From a Prospective, Multicenter Study of Pancreatic Cystic Lesions. Gastrointest Endosc. 2015;81:AB549. [DOI] [Full Text] |
| 28. | Kadayifci A, Atar M, Brugge WR. Su1711 Needle-Based Confocal LASER Endomicroscopy for Differential Diagnosis of Pancreatic Cystic Lesions. Gastrointest Endosc. 2015;81:AB386-AB387. [DOI] [Full Text] |
| 29. | Krishna SG, Swanson B, Conwell DL, Muscarella P. In vivo and ex vivo needle-based confocal endomicroscopy of intraductal papillary mucinous neoplasm of the pancreas. Gastrointest Endosc. 2015;82:571-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Tanaka M, Chari S, Adsay V, Fernandez-del Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K, Matsuno S. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006;6:17-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1539] [Cited by in RCA: 1441] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 31. | Ramesh J, Bang JY, Hebert-Magee S, Trevino J, Eltoum I, Frost A, Hasan MK, Logue A, Hawes R, Varadarajulu S. Randomized Trial Comparing the Flexible 19G and 25G Needles for Endoscopic Ultrasound-Guided Fine Needle Aspiration of Solid Pancreatic Mass Lesions. Pancreas. 2015;44:128-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Song TJ, Kim JH, Lee SS, Eum JB, Moon SH, Park do H, Seo DW, Lee SK, Jang SJ, Yun SC. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Carr-Locke DL, Bhutani MS, Haluszka O, Gress FG, Woods KE, Napoleon B. Tu1678 External Retrospective Validation of Needle-Based Confocal LASER Endomicroscopy (nCLE) Criteria for Pancreatic Cysts. Gastrointest Endosc. 2015;81:AB556. [DOI] [Full Text] |
| 34. | Furukawa T, Hatori T, Fujita I, Yamamoto M, Kobayashi M, Ohike N, Morohoshi T, Egawa S, Unno M, Takao S. Prognostic relevance of morphological types of intraductal papillary mucinous neoplasms of the pancreas. Gut. 2011;60:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 213] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 35. | Li H, Li Y, Cui L, Wang B, Cui W, Li M, Cheng Y. Monitoring pancreatic carcinogenesis by the molecular imaging of cathepsin E in vivo using confocal laser endomicroscopy. PLoS One. 2014;9:e106566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Li H; Pancreatic/Biliary. Am J Gastroenterol. 2014;109 Suppl 2:S59-S101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Napoleon B, Lemaistre AI, Pujol B, Caillol F, Mialhe-Morellon B, Giovannini M. Mo1431 In vivo Characterization of Pancreatic Cystic Tumors by Needle-Based Confocal LASER Endomicroscopy (nCLE). Proposition of a Comprehensive Classification. Gastrointest Endosc. 2014;79:AB434-AB435. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |